Abstract
Background
Propranolol has been shown previously to restore bone marrow function and improve anemia after lung contusion/hemorrhagic shock. We hypothesized that daily clonidine administration would inhibit central sympathetic outflow and restore bone marrow function in our rodent model of lung contusion/hemorrhagic shock with chronic stress.
Methods
Male Sprague-Dawley rats underwent six days of restraint stress after lung contusion/hemorrhagic shock during which the animals received clonidine (75μg/kg) after the restraint stress. On post-injury day seven, we assessed urine norepinephrine, blood hemoglobin, plasma granulocyte colony stimulating factor (G-CSF), and peripheral blood mobilization of hematopoietic progenitor cells (HPC), as well as bone marrow cellularity and erythroid progenitor cell growth.
Results
The addition of clonidine to lung contusion/hemorrhagic shock with chronic restraint stress, significantly decreased urine norepinephrine levels, improved bone marrow cellularity, restored erythroid progenitor colony growth, and improved hemoglobin (14.1±0.6 vs. 10.8±0.6 g/dL). The addition of clonidine to lung contusion/hemorrhagic shock with chronic restraint stress significantly decreased HPC mobilization and restored G-CSF levels.
Conclusions
After lung contusion/hemorrhagic shock with chronic restraint stress, daily administration of clonidine restored bone marrow function and improved anemia. Alleviating chronic stress and decreasing norepinephrine is a key therapeutic target to improve bone marrow function after severe injury.
Keywords: trauma, G-CSF, erythroid progenitor cells, anemia, rodent
Introduction
Severe injury induces bone marrow dysfunction in rodents and humans, which is associated with a hypercatecholamine state1–4. After trauma, the initial catecholamine surge is a necessary and appropriate response to injury, but this surge can be detrimental when prolonged. A prolonged hypercatecholaminemia is associated with pathophysiologic changes, including cardiac, metabolic and psychiatric disorders5–7. The molecular pathways by which hypercatecholaminemia leads to the manifestations of these diseases is poorly understood and the loss of autonomic balance alters homeostasis in the host 7. Previous work has demonstrated that increased concentrations of norepinephrine (NE) levels after injury can persist up to six days in rodents and up to 10 days in humans 1, 8–10. We have demonstrated in rodents after a model of lung contusion/hemorrhagic shock, that hypercatecholaminemia, particularly an increase in NE, was associated with decreased bone marrow cellularity, decreased growth of an erythroid progenitor cell colony, and prolonged mobilization of hematopoietic progenitor cells8.
Previous investigation of the role of NE in bone marrow dysfunction after trauma focused on propranolol, a non-selective beta blocker, that competitively blocks NE from binding to its receptors. Use of propranolol after lung contusion/hemorrhagic shock with chronic restraint stress in a rodent model decreased plasma granulocyte-colony stimulating factor (G-CSF) and decreased mobilization of hematopoietic progenitor cells (HPC)11–13. Currently, there are no data examining the suppression of NE within the central nervous system and its’ effect on bone marrow function. Clonidine, an alpha 2-adrenergic agonist, acts as a central sympatholytic that specifically inhibits the release of NE but not epinephrine or dopamine14–18. In hypertensive patients, treatment with clonidine decreases urine NE levels up to 90% 19. If NE is a key mediator of prolonged bone marrow dysfunction after trauma, then clonidine administration could be a cost-effective intervention to improve post-injury outcomes. This study investigated the effects of daily clonidine administration in a rodent model of lung contusion/hemorrhagic shock with chronic restraint stress that better attempts to recapitulate the human condition. NE levels, mobilization of hematopoietic progenitor cells, bone marrow erythroid function, and hemoglobin levels were determined as part of the post-injury evaluation in these animals. Using our model, we hypothesized that daily clonidine administration would inhibit central sympathetic outflow and restore bone marrow function.
Material and methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing 300 to 315g (n=6–8/group) were housed in pairs with free access to food and water during daily night/day cycles of 12 hours each. Female animals were excluded due to estrous cycle variability and its impact after hemorrhagic shock. The animals were housed and handled in accordance with the Guide for the Care and Use of Laboratory Animals and the protocol had been approved by the Institutional Animal Care and Use Committee prior to initiation (Protocol #201408271).
Experimental Design
Animals were allocated randomly into one of seven groups (Table 1). The groups (n=6–8/group) included: 1) naïve control; 2) chronic restraint stress (CS) alone; 3) CS with administered clonidine (CS+clonidine); 4) lung contusion followed by hemorrhagic shock (LCHS); 5) LCHS with administered clonidine (LCHS + clonidine), 6) LCHS followed by CS (LCHS/CS); and 7) LCHS/CS with administered clonidine (LCHS/CS + clonidine). In the LCHS + clonidine group, clonidine (Letco Medical, Decatur, AL#684754), 75μg/kg was injected intraperitoneally 10 minutes after LCHS and daily for six days. In the groups subject to CS or LCHS/CS, clonidine was given immediately after the CS period. Naïve control animals underwent daily handling. All groups were sacrificed on day seven after LCHS or CS and peripheral blood, urine, and bone marrow were harvested.
Table 1.
Experimental groups defined. CS= chronic restraint stress; LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock, and chronic restraint stress.
| N | Lung contusion | Hemorrhagic shock | Chronic restraint stress | Clonidine administration | |
|---|---|---|---|---|---|
| Naïve | 6 | - | - | - | - |
| CS | 6 | - | - | + | - |
| CS+clonidine | 6 | - | - | + | + |
| LCHS | 8 | + | + | - | - |
| LCHS+clonidine | 6 | + | + | - | + |
| LCHS/CS | 8 | + | + | + | - |
| LCHS/CS+clonidine | 6 | + | + | + | + |
Tissue Injury and Hemorrhagic Shock
As described previously, after intraperitoneal administration of 50 mg/kg pentobarbital, a unilateral lung contusion was created with a percussive nail gun (Sears Brand, Chicago, IL) applied directly to a 12 mm metal plate that was placed in the right axilla of the rat8. Ten min after lung contusion, the right femoral artery and internal jugular vein were exposed and cannulated with Polyethylene 50 tubing (PE-50) (BD Biosciences, San Jose, CA). Both cannulas secured using 4–0 silk sutures placed proximal and distal to the insertion site were flushed with heparinized saline (10units/ml). The femoral artery tubing was then connected to a continuous blood pressure monitoring device (BP-2 Digital Blood Pressure Monitor; Columbus Instruments, Columbus, Ohio) for measurement of mean arterial pressure and heart rate. Blood was then withdrawn from the internal jugular to maintain a mean arterial pressure of 30–35 mmHg for 45 min. After the period of shock animals were reinfused their shed blood, and the cannulas were removed.
Chronic Restraint Stress (CS)
CS was performed daily for six days and consisted of two h of restraint in a nose cone rodent cylinder (Kent Scientific Corporation, Torrington, CT, USA) that restricted their movements. Every 30 min during the two h of restraint, loud, ringing, beeping, constant alarms were played for two min, and the animals were repositioned to prevent habituation.
Norepinephrine and G-CSF Measurements
Urine NE levels were determined using a quantitative ELISA kit (IBL America, Minneapolis, MN), and plasma G-CSF levels were assessed using a standard ELISA (R&D Systems, Minneapolis, MN, USA). Samples were processed in duplicate according to the manufacturer’s instructions.
Flow Cytometry
Peripheral blood collected on day seven was used to identify HPC that were counted by flow cytometry using CD-71 and c-kit antibodies. CD-71, also known as transferrin receptor-1, is a marker for one of the earliest precursors of erythroid progenitor cells, burst- forming unit-erythroid (BFU-E) cells.20 C-kit, also known as CD 117, is a tyrosine protein kinase receptor also expressed on early progenitor cells, such as colony-forming units-granulocyte-, erythrocyte-, monocyte- megakaryocyte (CFU-GEMM), BFU-E, and colony-forming unit-erythroid (CFU-E).21 Briefly, whole blood was incubated with mouse anti-rat CD71 (BD Biosciences, San Jose, CA) antibody conjugated with fluorescein isothiocyanate and rat anti-mouse CD117 (c-kit) (Southern Biotech, Birmingham, AL, USA) antibody conjugated to phycoerythrin (BD Biosciences). After washing and lysing the red blood cells, the samples were run on BD LSR II flow cytometer equipped with FACSDiva software (BD Biosciences) to quantitate CD117 and CD71 positive cells. CD117 and CD71-positive cells included CFU-GEMM, BFU-E, and CFU-E.
Bone Marrow cellularity
Bone marrow was flushed from the left femur using a 19-gauge needle with a syringe containing 1ml Iscove’s Modified Dulbecco’s Medium (IMDN) (Lonza, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Flowery Branch, GA, USA). Cell viability was determined by 0.4% Trypan blue staining, and cellularity was assessed on a hemocytometer.
Assays of Bone Marrow Erythroid Progenitor Colonies
Growth of bone marrow erythroid progenitor colonies was determined for colony-forming units-granulocyte-, erythrocyte-, monocyte- megakaryocyte (CFU-GEMM), burst-forming unit-erythroid (BFU-E), and colony-forming unit-erythroid (CFU-E) as described previously13, 22. Briefly, bone marrow mononuclear cells in a concentration of 1x106 cells/mL of IMDM were plated in duplicate with Methocult media (Stemcell, Vancouver, BC, Canada). Plates were supplemented with 1.3 U/mL rhEpo and 6 U/mL rhIL-3 for BFU-E and CFU-E colonies or 3 U/mL rhGM-CSF for CFU-GEMM. Plates were then incubated at 37°C in 5% CO2 incubator. A blinded reader assessed colony growth of CFU-E on day seven, BFU-E colonies on day 14, and CFU-GEMM on day 17.
Hemoglobin Measurements
Heparinized blood samples were obtained via cardiac puncture and used for the analysis of hemoglobin (VetScan HM5, Abaxis, CA, USA). The levels were measured on day seven.
Statistical Analysis
The data were analyzed statistically using Graph Pad Prism Version 6 (GraphPad Software, Inc, San Diego, CA, USA) as t-tests followed by one-way analysis of variance (ANOVA) and Tukey-Kramer for multiple comparisons. The data are expressed as mean ± standard deviation. Significance was defined as *p < 0.05 vs. naïve controls **p < 0.05 vs. untreated counterpart.
Results
The Effect of Clonidine on Urine NE Levels
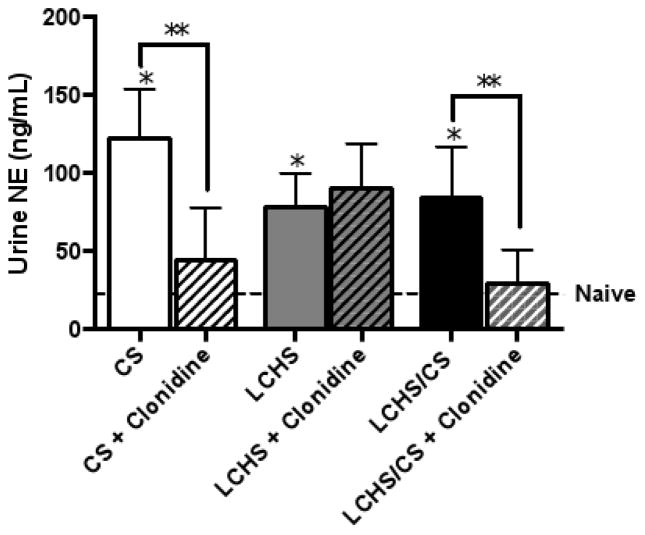
CS alone, LCHS, and LCHS/CS all resulted in a significant increase in urine NE levels on day seven as compared to naïve controls (Figure 1) (*p=0.006, *p=0.0369, and *p=0.0357). This illustrated that CS, as well as tissue injury and shock, are significant stimulators of NE. When clonidine was administered to all three rodent models, only CS and LCHS/CS resulted in suppressed urine NE levels versus their untreated counterpart (Figure 1) (*p=0.0144 and *p=0.0281). In contrast, LCHS+clonidine did not change urine NE levels compared to LCHS (Figure 1).
Figure 1.
Daily clonidine administration decreased urine norepinephrine (NE) levels seven days after CS and LCHS/CS. CS= chronic restraint stress; LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock, and chronic restraint stress; *p <0.05 vs. naïve and **p <0.05 vs. untreated counterpart. (n=6–8/group).
The Impact of Clonidine on Mobilization of Hematopoietic Progenitor Cells
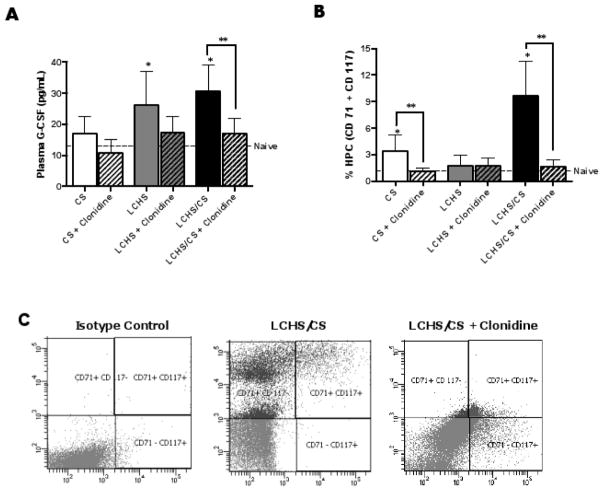
Both LCHS and LCHS/CS increased plasma G-CSF levels by 51 and 58% (*p<0.04 each) while CS alone increased plasma G-CSF levels by 23% (*p=0.006) when compared to naïve controls (Figure 2A). In contrast, the addition of clonidine to all three rodent models resulted in the suppression of plasma G-CSF levels. When the LCHS/CS animals received clonidine daily, the plasma G-CSF levels were decreased by 44% (**p<0.004) compared to LCHS/CS (Figure 2A). The addition of clonidine to CS alone and LCHS resulted in a trend of decreased plasma G-CSF levels by 41 and 34%, respectively (Figure 2A) (p=0.0961 and p=0.0995).
Figure 2.
Figure 2A–B. Seven days of clonidine administration decreased hematopoietic progenitor cell mobilization after CS and LCHS/CS. 2A. Clonidine administration significantly decreased plasma G-CSF levels after LCHS/CS. 2B. Clonidine administration significantly decreased peripheral blood mobilization of CD71+ and CD117+ progenitor cells seven days after CS and LCHS/CS. 2C. Representative flow cytometry analysis of the number of progenitor cells (CD 71+/CD 117+) in the peripheral blood seven days after isotype, LCHS/CS, LCHS/CS + clonidine in one rodent. CS= chronic restraint stress; LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve and **p <0.05 vs. untreated counterpart. (n=6–8/group)
After severe trauma, NE and G-CSF have been demonstrated to be associated with prolonged HPC mobilization1, 8, 12, 23–25. Seven days after CS alone, HPC mobilization was significantly prolonged (Figure 2B). Similar to previous work, the addition of CS to LCHS increased HPC mobilization by 84% (*p=0.003) compared to naïve (Figure 2B)8. When CS and LCHS/CS animals received daily clonidine, the prolonged mobilization of HPC was markedly attenuated by 66 and 84% (**p=0.0416 and **p=0.0057) compared to CS and LCHS/CS (Figure 2B and 2C). Not surprisingly, daily use of clonidine after LCHS without chronic stress had no impact on HPC mobilization, because HPC mobilization was not prolonged after LCHS alone (Figure 2B).
The Effects of Clonidine on Bone Marrow Erythroid Function
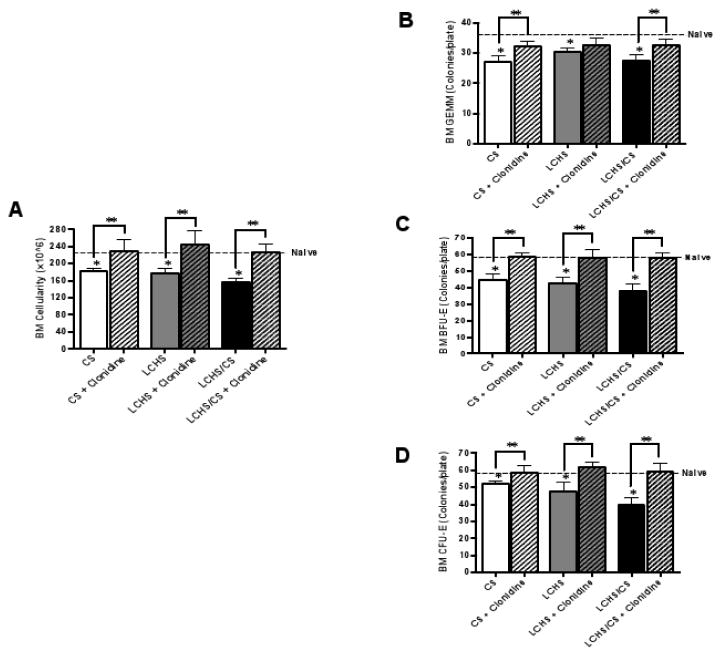
Bone marrow cellularity and erythroid progenitor colony growth were determined to evaluate bone marrow function. Seven days after CS and LCHS, bone marrow cellularity was decreased by 19 and 21% (*p=0.0111 and *p=0.0084). Seven days after LCHS/CS, there was a 30% suppression (*p=0.0009) in bone marrow cellularity compared to naive (Figure 3A). When CS, LCHS, and LCHS/CS animals received daily clonidine, bone marrow cellularity improved by 21, 27, and 31% (**p=0.0081, 0.0012, <0.0001) as compared to their untreated counterparts (Figure 3A). The addition of clonidine to CS, LCHS, and LCHS/CS animals also restored bone marrow cellularity to that of naïve animals (Figure 3A).
Figure 3.
Figure 3A–D. Seven days of clonidine administration restored bone marrow function after CS, LCHS and LCHS/CS. 3A. Clonidine administration significantly improved bone marrow cellularity in all three rodent models. 3B–D. The addition of clonidine restored bone marrow CFU-GEMM, BFU-E, and CFU-E colony growth after CS, LCHS, and LCHS/CS. BM=bone marrow; CS= chronic restraint stress; LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve and **p <0.05 vs. untreated counterpart. (n=6–8/group)
On day seven, CS, LCHS, and LCHS/CS resulted in decreased growth of bone marrow CFU-GEMM, BFU-E, and CFU-E erythroid progenitor colonies (Figure 3B–D). In contrast, when CS, LCHS, and LCHS/CS animals were administered daily clonidine, bone marrow CFU-GEMM, BFU-E, and CFU-E colony growth improved by 10–39% (**p<0.0001 to 0.0086) when compared to their untreated counterparts (Figure 3B–D). The addition of clonidine to CS, LCHS, and LCHS/CS animals restored BFU-E and CFU-E colony growth to that of naïve animals (Figure 3C, D).
The Impact of Daily Clonidine on Hemoglobin
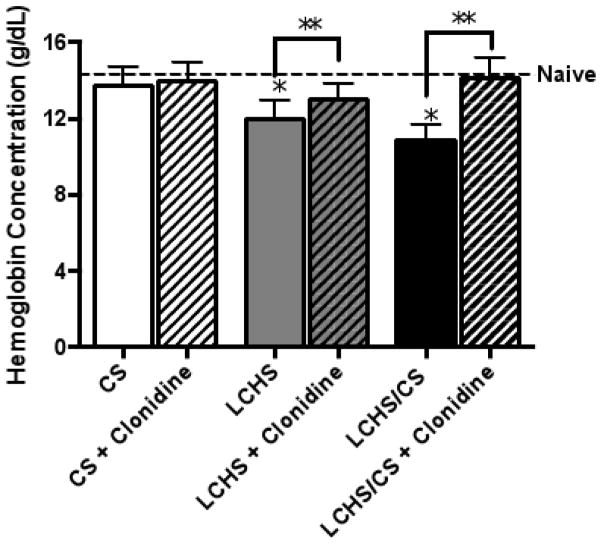
Seven days after CS alone, there was no significant impact on hemoglobin when compared to naïve controls (13.7 ± 1.0 vs. 14.3 ± 0.3 g/dL). After LCHS alone, there was a mild persistent anemia compared to naïve controls (12.0 ± 0.9 vs. 14.3 ± 0.3 g/dL) (*p=0.0006) (Figure 4). As shown previously, LCHS/CS was associated with a persistent, injury-associated anemia with a hemoglobin of 10.8 ± 0.8 g/dL(*p<0.0001)8.
Figure 4.
Seven days of daily clonidine administration improved hemoglobin levels after LCHS and LCHS/CS. CS= chronic restraint stress; LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve and **p <0.05 vs. untreated counterpart. (n=6–8/group)
Daily clonidine administration after CS had no significant effect on hemoglobin. After LCHS, the use of daily clonidine improved hemoglobin levels (LCHS+clonidine:13.1 ± 0.8 vs. LCHS:12.0 ± 0.9 g/dL)(**p=0.0492). Daily administration of clonidine after LCHS/CS resulted in the greatest improvement in hemoglobin (LCHS/CS+clonidine:14.1 ± 0.6** vs. LCHS/CS:10.8 ± 0.6 g/dL)(**p=0.0001). Seven days of clonidine administration after LCHS/CS resolved the persistent, injury-associated anemia.
Discussion
After severe injury, trauma patients experience substantial bone marrow dysfunction that is associated with a hypercatecholamine state and prolonged HPC mobilization1, 2, 4, 11, 26. Clinically, this bone marrow dysfunction is important because it manifests as a persistent, injury-associated anemia that can last for several weeks after trauma2, 26, 27. Our previous work revealed that urine NE levels in severely injured trauma patients remained increased for two weeks after injury1. This persistent hypercatecholamine state was associated with prolonged HPC mobilization to the peripheral blood in humans25. In addition, increased NE levels attenuate growth of bone marrow erythroid progenitor cells, both in vitro and in vivo1, 5, 8, 28. By blocking beta-adrenergic receptors, daily propranolol use prevented prolonged HPC mobilization and improved the growth of bone marrow erythroid progenitors 12, 13, 22, 26, 26. Our study examined the effects of clonidine on bone marrow function after trauma. Our study demonstrated that the daily use of clonidine after lung contusion/hemorrhagic shock with CS, a clinically relevant rodent model of persistent anemia, prevented prolonged HPC mobilization, improved bone marrow function, and thus restored hemoglobin levels.
Severe trauma triggers a persistent hypercatecholamine state that is associated with increases in plasma levels of G-CSF and mobilization of bone marrow HPCs which disrupts function of the homeostatic bone marrow1, 5, 8, 12, 23. In three different injury models (CS, LCHS, and LCHS/CS), urine NE levels increased three to six fold. These findings correlate with previous rodent work by Bible et al.8, where the addition of CS to LCHS injury increased urine NE levels six days post injury. Clonidine has been shown to decrease urinary NE levels, and our work has verified this result after both CS and LCHS/CS15, 19, 21. In the absence of chronic stress, clonidine administration after LCHS had no effect on urinary NE levels. Daily chronic stress, whether alone or in addition to LCHS, appears to be the driving force behind the effects of NE-mediated hypercatecholaminemia. We hypothesize that clonidine best works in environment of constant stimulation of sympathetic outflow such as CS and LCHS/CS models of rodents. This concept would explain why there was no decrease in NE levels after LCHS alone. One mechanism through which clonidine suppresses NE output is at the level of the central nervous system by targeting alpha-2 adrenergic receptors, located in the locus coeruleus within the medial prefrontal cortex17–19. Kubota et al.17 demonstrated that when rodents were treated with clonidine, there was a suppression of basal rates of NE release from the brain by binding to alpha-2 adrenergic receptors in the central nervous system. In addition, Martin et al.15 and Dorman et al.30 demonstrated that clonidine decreased plasma and urine NE levels by 54–90% one day after administration in patients with alcohol amnestic disorder and in patients undergoing major abdominal surgery. It is likely that a decrease in central NE outflow was responsible for some of the NE-mediated effects seen in the bone marrow of our rodent model of traumatic lung injury model with CS.
HPC mobilization has been shown to be mediated by both NE and G-CSF23. In steady-state hematopoiesis, G-CSF regulates the level of neutrophils in the bone marrow and the blood, but G-CSF is not essential for erythroid differentiation and growth in the bone marrow31. In our rodent model, the lung contusion is a stimulus for the mobilization of HPC to the injured tissue to aid in repair. Seven days after LCHS and CS, our previous rodent data showed that plasma G-CSF remained persistently increased and that there was a prolonged HPC mobilization12. These results were confirmed, and in the absence of injury in the CS alone model, there was no increase in plasma G-CSF. Those animals who received daily clonidine after LCHS/CS had a decrease in plasma G-CSF levels. This is different from previous work, where daily propranolol administration decreased plasma G-CSF levels in LCHS in addition to LCHS/CS12. This observation can be explained in part by the work of Katayama et al.23 who demonstrated that when mice received G-CSF directly into central nervous system via intracerebroventricular infusion versus when given systemically by subcutaneous injection, HPC mobilization was less after central nervous system administration rather than systemic injection. The G-CSF-induced HPC mobilization can also be terminated with NE depletion in mice treated with 6-hydroxydopamine23. These findings indicate that G-CSF release may be dependent on NE release for HPC mobilization to occur and that the effects of clonidine on G-CSF are mediated by the degree that clonidine suppresses NE release. Once clonidine depleted the central outflow of NE from the brain, G-CSF-induced HPC mobilization after LCHS/CS was decreased. Thus, by decreasing plasma levels of G-CSF with clonidine use, the continued retention of hematopoietic progenitor cells in the bone marrow allows these cells to differentiate along the erythroid pathway and restore growth of erythroid colonies.
Both in rodents and humans, severe traumatic injury suppresses the growth of erythroid progenitor cell and induces anemia1–3, 5, 8, 13, 32. Previous injury models in rodents have shown that although hemorrhagic shock and injury decrease growth of bone marrow erythroid colonies, the presence of high NE or chronic stress continue to persistently suppress bone marrow function 1, 8, 13, 28. In our study, CS alone decreased bone marrow cellularity and growth of bone marrow erythroid progenitor colonies. The use of daily clonidine after CS not only restored bone marrow cellularity but also preserved growth of bone marrow erythroid progenitor colonies. The benefit of daily clonidine administration was most important in the combined LCHS/CS model, which had improved bone marrow cellularity and growth of bone marrow erythroid progenitor cells. This observation is supported by Baranski et al.22 and Mohr et al.17, who showed similar results with the use of propranolol after LCHS.
In the LCHS/CS model, the decrease in bone marrow cellularity and growth of bone marrow erythroid progenitor colonies along with the prolonged HPC mobilization is associated with persistent, injury-associated anemia. Similarly after critical illness, recovery of erythropoiesis is impaired for up to six months after discharge from the intensive care unit27. Our study demonstrated that daily clonidine administration after LCHS/CS improved hemoglobin, similar to our previous work where daily propranolol treatment after LCHS improved hemoglobin13. Ours is the first study to describe clonidine as a potential therapy for persistent, injury-associated anemia after LCHS/CS. In both rodent studies, propranolol and clonidine decreased the hypercatecholamine state through different mechanisms but were able to improve bone marrow function. Peripherally, propranolol competitively blocks NE from binding to bone marrow beta-adrenergic receptors. Centrally, clonidine depletes NE release from the brain by competitively binding to alpha -2 adrenergic receptors, decreasing NE outflow. Improved bone marrow function was manifested by improved bone marrow cellularity, improved growth of bone marrow erythroid progenitor cells and decreased HPC mobilization which leads to an improvement in hemoglobin. A decrease in NE is hypothesized to improve bone marrow function by both decreasing plasma levels of G-CSF and allowing HPCs to remain in the bone marrow to differentiate along the erythroid pathway as well as preventing the dose-related, adverse effects of NE on growth of bone marrow erythroid colonies.
In summary, this study confirms the important role NE plays in bone marrow dysfunction after traumatic injury and chronic stress. Using a clinically relevant rodent model of lung contusion/hemorrhagic shock, and chronic stress, the use of daily clonidine depleted the central outflow of NE and maintained bone marrow function. Future therapeutic goals should focus on decreasing the prolonged hypercatecholamine state associated with chronic stress that is seen after severe traumatic injury to improve bone marrow function and decrease anemia.
Acknowledgments
This research was supported by the National Institutes of Health. AMM was supported by NIH NIGMS grant R01 GM105893-01A1. TJL was supported by a training grant in burn and trauma research T32 GM-08431. PAE was supported by P30 AG028740 from the National Institute on Aging and by the NIH NIGMS grant R01 GM113945-01. Finally, AMM and PAE were supported by P50 GM111152-01 (NIGMS).
Footnotes
Presented at the 11th Annual Academic Surgical Congress in Jacksonville, FL, February 2–4, 2016.
This submission has not been published elsewhere and the authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect. 2004;5:385–93. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 2.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston DH, Gentile PS, Malangoni MA. Bone marrow failure after hemorrhagic shock. Circ Shock. 1990;30:255–63. [PubMed] [Google Scholar]
- 4.Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–9. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 6.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–7. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald PJ. Noradrenaline transmission reducing drugs may protect against a broad range of diseases. Auton Autacoid Pharmacol. 2015;34:15–26. doi: 10.1111/aap.12019. [DOI] [PubMed] [Google Scholar]
- 8.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg. 2015;79:91–6. doi: 10.1097/TA.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein J, Breslow MJ. The stress response of critical illness. Crit Care Clin. 1999;15:17–33. doi: 10.1016/s0749-0704(05)70037-3. [DOI] [PubMed] [Google Scholar]
- 10.Weissman C. The metabolic response to stress: an overview and update. Anesthesiology. 1990;73:308–27. doi: 10.1097/00000542-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Beiermeister KA, Keck BM, Sifri ZC, ElHassan IO, Hannoush EJ, Alzate WD, et al. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69:338–43. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015;158:595–601. doi: 10.1016/j.surg.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohr AM, ElHassan IO, Hannoush EJ, Sifri ZC, Offin MD, Alzate WD, et al. Does beta blockade postinjury prevent bone marrow suppression? J Trauma. 2011;70:1043–9. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cubeddu LX, Hoffmann IS, Davila J, Barbella YR, Ordaz P. Clonidine reduces elevated cerebrospinal fluid catecholamine levels in patients with essential hypertension. Life Sci. 1984;35:1365–71. doi: 10.1016/0024-3205(84)90393-x. [DOI] [PubMed] [Google Scholar]
- 15.Martin PR, Ebert MH, Gordon EK, Kopin IJ. Urinary catecholamine metabolites and effects of clonidine in patients with alcohol amnestic disorder. Clin Pharmacol Ther. 1983;33:19–27. doi: 10.1038/clpt.1983.3. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PA, De Quattro V, Foti A, Curzon G. Effects of clonidine on central and peripheral nerve tone in primary hypertension. Hypertension. 1986;8:611–7. doi: 10.1161/01.hyp.8.7.611. [DOI] [PubMed] [Google Scholar]
- 17.Kubota T, Hirota K, Yoshida H, Takahashi S, Ohkawa H, Anzawa N, et al. Inhibitory effect of clonidine on ketamine-induced norepinephrine release from the medial prefrontal cortex in rats. Br J Anaesth. 1999;83:945–7. doi: 10.1093/bja/83.6.945. [DOI] [PubMed] [Google Scholar]
- 18.Scheinin M, Schwinn DA. The locus coeruleus. Site of hypnotic actions of alpha 2-adrenoceptor agonists? Anesthesiology. 1992;76:873–5. doi: 10.1097/00000542-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Martin PR, Ebert MH, Gordon EK, Linnoila M, Kopin IJ. Effects of clonidine on central and peripheral catecholamine metabolism. Clin Pharmacol Ther. 1984;35:322–7. doi: 10.1038/clpt.1984.37. [DOI] [PubMed] [Google Scholar]
- 20.Dong HY, Wilkes S, Yang H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol. 2011;35:723–32. doi: 10.1097/PAS.0b013e31821247a8. [DOI] [PubMed] [Google Scholar]
- 21.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. [PubMed] [Google Scholar]
- 22.Baranski GM, Offin MD, Sifri ZC, Elhassan IO, Hannoush EJ, Alzate WD, et al. beta-blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–31. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Cook KM, Sifri ZC, Baranski GM, Mohr AM, Livingston DH. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2013;216:57–64. doi: 10.1016/j.jamcollsurg.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–31. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 26.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, et al. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77:54–60. doi: 10.1097/TA.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–12. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 28.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, et al. Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res. 2010;163:e85–90. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina PE. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005;24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- 30.Dorman T, Clarkson K, Rosenfeld BA, Shanholtz C, Lipsett PA, Breslow MJ. Effects of clonidine on prolonged postoperative sympathetic response. Crit Care Med. 1997;25:1147–52. doi: 10.1097/00003246-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–67. doi: 10.1016/j.cytogfr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234:224–32. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]