Abstract
Low birth weight (LBW) has consistently been associated with childhood attention- deficit hyperactivity disorder (ADHD), and a similar association has been found for childhood externalizing disorders, such as oppositional defiant disorder (ODD) and conduct disorder (CD), albeit to a lesser degree. Although the association between LBW and these disorders has been robustly replicated, few studies have adequately controlled for confounding variables, such as parental age at birth and prenatal tobacco use, examined the specificity of the risk of LBW for ADHD symptoms, or investigated potential nonlinear (i.e., quadratic) effects of birth weight. Additionally, the extent to which LBW confers risk for these disorders depending on childhood sex has rarely been examined. The current study examined associations between birth weight and ADHD, ODD, and CD symptom dimensions as well as the extent to which such associations are moderated by child sex, while also controlling for confounding variables. Significant interactions between sex and birth weight emerged across all analyses predicting ADHD and externalizing psychopathology, such that associations were stronger in males relative to females. Results remained when controlling for a number of confounds, including parental age, prenatal tobacco use, comorbid psychopathology, as well as other indicators of maternal and child health during the pre- and perinatal period. Both linear and quadratic associations emerged between birth weight and both hyperactivity and CD symptoms, whereas birth weight predicted inattention and ODD symptoms in a linear fashion. Future research should continue to investigate the impact of birth weight on ADHD and externalizing psychopathology, in particular the biological mechanisms underlying this association.
Keywords: Attention-Deficit Hyperactivity Disorder, Externalizing Psychopathology, Low Birth Weight, Sexual Selection Theory
There is substantial evidence of increased prevalence of ADHD among individuals born with low birth weight (LBW), defined by the World Health Organization (1992) as birth weight (BW) less than 2,500 grams (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Anderson et al., 2011; Groen-Blokhuis, Middeldorp, van Beijsterveldt, & Boomsma, 2011; Hack et al., 2009; Nigg & Breslau, 2007; Pettersson et al., 2014). Similarly, individuals diagnosed with ADHD have been found to be 2.5 to 4 times as likely to have been born LBW relative to their non-ADHD peers (Botting, Powls, Cooke, & Marlow, 1997; Mick, Biederman, Prince, Fischer, & Faraone, 2002; Szatmari, Saigal, Rosenbaum, Campbell, & King, 1990). The potential importance of BW as a causal contributor to ADHD closely aligns with the Developmental Origins of Health and Disease Hypothesis (DOHaD), which emphasizes the importance of early risk factors, such as LBW, in the etiology of subsequent psychopathology (Barker, 1998; D’Onofrio, Class, Lahey, & Larsson, 2014). However, DOHaD also emphasizes the importance of investigating the role of potential confounds that may account for such associations (e.g., measured and unmeasured genetic and environmental influences), particularly when examining mechanisms underlying associations between risk factors occurring early in development and later psychopathology.
To this end, several studies have utilized large population-based samples and a sibling comparison approach to control for potential unmeasured genetic confounds and test for a causal association between LBW and ADHD. In all, findings from these studies have demonstrated a link between LBW and ADHD even after controlling for shared genes, providing support for LBW as a causal risk factor in the development of ADHD (Class, Rickert, Larsson, Lichtenstein, & D’Onofrio, 2014; Groen-Blokhuis et al., 2011; Hultman et al., 2007; Pettersson et al., 2014). Further, several of these studies have examined the full dimensional distribution of normal range BW as a continuous variable (Hultman et al., 2007; Pettersson et al., 2014) instead of dichotomizing BW and examining individuals above or below a specific cut-point (Groen-Blokhuis et al., 2011; Mick, Biederman, Prince, et al., 2002). While such dichotomous approaches parallel guidelines of neonatal care regarding BW (World Health Organization, 1992), these methods can also artificially increase power and assume linear, additive effects of BW on behavioral outcomes. This may be problematic given that nonlinear (i.e., quadratic) effects may be present (Grissom & Reyes, 2013), such that youth both at lower and higher BW may be vulnerable to developmental problems, depending on the range of BW in a given sample.
Sex As a Moderator
In addition to the potential for non-linear effects, several questions remain regarding the role of LBW in ADHD in particular. First, and most primary are issues of potential moderation by sex. Several decades of research have established that externalizing disorders with an onset in early childhood (such as ADHD) tend to occur more commonly in males than in females (American Psychiatric Association, 2013; Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009; Copeland, Shanahan, Costello, & Angold, 2011). In fact, recent estimates indicate that males with ADHD outnumber females with the disorder at a ratio of approximately 3 to 1 (Visser et al., 2014). While several explanations for these sex differences in onset and prevalence have been proposed (i.e., differential risk thresholds, bias in diagnostic criteria; Gaub & Carlson, 1997) little work has explicitly evaluated whether early developmental risk factors have differential effects based on child sex (Murray et al., 2015).
Sexual selection theory integrates biological, psychological, and social risk factors and provides one possible framework for understanding the sex discrepancy in prevalence among early-onset externalizing disorders (Alcock & Crawford, 2008; Darwin, 1871; Geary, 2010; Martel, 2013). Most recently, Martel (2013) posited that sexual selection theory can adequately explain sex differences in prevalence observed in neurodevelopmental disorders. As she outlines, higher exposure to prenatal testosterone may cause males to be more susceptible to prenatal stressors (e.g., maternal stress, inadequate nutrition), which then may have downstream effects on both prenatal development (i.e., BW) and catecholamine neurotransmission (Hernandez et al., 1994; Kritzer & Creutz, 2008; Kuhn et al., 2010; Martel, Klump, Nigg, Breedlove, & Sisk, 2009; Morris, Jordan, & Breedlove, 2004). These alterations in catecholamine neurotransmission may then increase externalizing-relevant traits, such as disinhibition and sensation-seeking (Auyeung et al., 2009; Hampson, Ellis, & Tenk, 2008; Martel et al., 2009), thereby increasing risk for developing externalizing disorders among males specifically. Thus, under this model, prenatal risk factors, such as LBW, may show differential associations with childhood externalizing outcomes based on sex, such that LBW may evidence stronger effects among males relative to females.
Despite a strong theoretical foundation for such a hypothesis, few studies have formally tested sex as a moderator of the association between BW and externalizing psychopathology. A recent study utilizing a population at high risk for intrauterine growth problems (i.e., LBW, small for gestational age, etc.) did find an association between indices of intrauterine growth and attention problems, but only in females (Murray et al., 2015). These findings are in contrast to the hypothesis posited by sexual selection theory; however, further work is necessary to confirm this finding.
Role of Confounding Factors
In addition to formal tests of sex moderation, the potential influences of several health and environmental confounds on this relationship need to be evaluated. It is well established that BW is moderately correlated with gestational age, with both lower BW and earlier gestational age conferring risk for a number of psychological problems, including symptoms of ADHD and externalizing disorders (Anderson et al., 2011; Gustafsson & Kallen, 2011a; Johnson & Marlow, 2011; Vohr, 2014). Additionally, infants born at lower BW or earlier gestational age are more likely to have experienced prenatal or labor and delivery complications and are at higher risk for developing a variety of neonatal medical issues (Ancel et al., 2015; Raju, Mercer, Burchfield, & Joseph, 2014; Subramanian, Seo, Barton, & Montazami, 2014).
Similarly, prenatal substance use has been identified as a potential confound, as prenatal alcohol and tobacco exposure have been associated with both LBW (Bada et al., 2005; Bailey & Byrom, 2007; Okah, Cai, & Hoff, 2005) and ADHD (Linnet et al., 2005; Mick, Biederman, Faraone, Sayer, & Kleinman, 2002; Sagiv, Epstein, Bellinger, & Korrick, 2013; Thapar et al., 2003). Previous studies examining the association between LBW and ADHD have been inconsistent in controlling for prenatal substance use (Anderson et al., 2011; Botting et al., 1997; Class et al., 2014; Elgen, Sommerfelt, & Markestad, 2002; Elgen, Holsten, & Odberg, 2013; Hack et al., 2009; Halmoy, Klungsoyr, Skjaerven, & Haavik, 2012; Hatch, Healey, & Halperin, 2014; Szatmari et al., 1990). Additionally, more recent findings have indicated that prenatal tobacco exposure may not be causally associated with ADHD (D’Onofrio et al., 2008; Nigg & Breslau, 2007; Thapar et al., 2009), or may be predictive of comorbid externalizing spectrum psychopathology (i.e., ODD and CD), but not ADHD (Nigg & Breslau, 2007). Even so, given that LBW is a clear outcome of prenatal substance exposure (Bada et al., 2005; Bailey & Byrom, 2007; Bassi, Rosso, Moessinger, Blanc, & James, 1984; Okah et al., 2005), studies addressing any potential role of BW in ADHD and externalizing psychopathology likely also need to consider tobacco and alcohol exposure during pregnancy as a potential confounding factor (Nigg & Breslau, 2007).
Parental age has also been proposed as a potential confound, particularly as infants born to relatively younger or older parents have been found to be at higher risk of LBW (Gustafsson & Kallen, 2011b; Reichman & Teitler, 2006) as younger mothers (i.e., teenagers) often do not receive adequate prenatal care and older mothers have an increased rate of multiple births (Martin et al., 2002). Additionally, advanced paternal age has been associated with sperm mutations and abnormalities, which may adversely affect fetal growth and development (Reichman & Teitler, 2006). Further, parental age (both younger and older) has also been associated with increased risk for neurodevelopmental disorders, including ADHD (Gardener, Spiegelman, & Buka, 2009; Gustafsson & Kallen, 2011a). Parental age has also not been consistently covaried in studies investigating associations between LBW and ADHD (Anderson et al., 2011; Botting et al., 1997; Breslau et al., 1996; Elgen et al., 2002; Elgen et al., 2013; Hack et al., 2009; Hatch et al., 2014; Strang-Karlsson et al., 2008; Szatmari et al., 1990), and when parental age is used as a covariate, studies typically use maternal age, but not paternal age (Halmoy et al., 2012; Mick, Biederman, Prince, et al., 2002).
Second, the specificity of LBW as a risk factor for ADHD versus externalizing problems needs to be more thoroughly evaluated. While ADHD is highly comorbid with externalizing problems, including ODD and CD (Biederman, Newcorn, & Sprich, 1991; Gau et al., 2010; Waschbusch, 2002), findings regarding the association between LBW and these comorbid disruptive behavior problems have been mixed. Some past work has indicated a similar pattern of association between LBW and childhood-onset CD (Thapar et al., 2005) and other externalizing behaviors (Lahat, Van Lieshout, Saigal, Boyle, & Schmidt, 2014) as that demonstrated for ADHD. In contrast, Nigg and Breslau (2007) reported a double dissociation, such that LBW predicted ADHD, but not ODD or CD, whereas prenatal tobacco exposure, and not LBW, was associated with ODD and CD (Nigg & Breslau, 2007). Further, LBW has been implicated in a variety of subsequent cognitive, emotional, and behavioral difficulties, including mood and anxiety problems (Burnett et al., 2011; Elgen et al., 2013; Nomura et al., 2007) as well as learning disorders (Aarnoudse-Moens et al., 2009; Breslau, Paneth, & Lucia, 2004; Roberts, Bellinger, & McCormick, 2007) – all of which are also commonly comorbid with ADHD (Biederman et al., 1991; Willcutt et al., 2012). Thus, more work is needed to evaluate whether LBW plays a specific role in ADHD apart from externalizing behavior problems, and if such associations remain when controlling for other commonly comorbid conditions.
The current study aimed to further explore associations between BW, ADHD, and externalizing psychopathology in a community-recruited sample. Based on the application of sexual selection theory to prevalence differences in childhood-onset externalizing psychopathology (Martel, 2013), we predicted that sex would moderate associations between BW and ADHD and externalizing outcomes, such that effects would be stronger in males relative to females. Further, we sought to test these hypotheses while also (1) examining both the linear and nonlinear effects of a continuous BW variable reflecting a normal range distribution of BW, (2) controlling for a number of confounding factors that may partially account for associations between BW and ADHD (i.e., familial confounds, prenatal tobacco and alcohol exposure, parental age), and (3) examining specificity of associations to ADHD symptoms.
METHODS
Participants
Participants included 915 children and adolescents ages 6 to 19 years (M=12.4, SD=4.3, 56.1% male). The sample included 431 singleton youth and 242 sibling pairs. Participants were recruited between 2002 and 2009 using mass mailings to parents in local school districts, public advertisements, and community outreach to local clinics (information posted in waiting rooms) to recruit a broad sample while avoiding potential biases inherent to clinic-referred samples. The majority of participants (over 75%), both with and without ADHD, were recruited using the school district mass mailings. A multi-stage, multi-informant assessment procedure was implemented to identify cases and non-cases among those who volunteered. Interested parents first completed a telephone screen to evaluate exclusionary criteria (see below). If eligible, the family was invited to complete the stage 2 diagnostic assessment, which included parent and teacher ratings on the DSM-IV ADHD Rating Scale (Reid et al., 1998) and the Conners’ Rating Scale – Revised Short Form (Conners et al., 1997). Parents also completed the Kiddie Schedule for Affective Disorders and Schizophrenia-E (KSADS-E; Kaufman et al., 1997) with a trained master’s level clinical interviewer to evaluate ADHD and other major psychiatric disorders for each child (i.e., disruptive behavior disorders, major depressive disorder, dysthymic disorder, generalized anxiety disorder, specific and social phobias, obsessive-compulsive disorder, post- traumatic stress disorder among others).
Diagnoses and symptom counts were determined using a best estimate procedure implemented by a board-certified child psychiatrist and a licensed child clinical psychologist. Both professionals used a symptom count “or” algorithm derived from parent and teacher reports (i.e., a symptom is counted as present if endorsed by either the parent or the teacher) as well as T-scores for both parent and teacher ratings on the Conners’ Cognitive Problems or Hyperactivity Problems subscales (T-scores>60 considered clinically significant). Clinical decisions concerning ADHD, ADHD subtype, and comorbid diagnoses (including all depressive and anxiety disorders) were made independently, using full DSM-IV-TR criteria. Agreement rates were acceptable for all ADHD subtype diagnoses (κ >.88) and all anxiety, mood, and disruptive behavior disorders (DBD) occurring at a 5% or higher base rate in the sample (all κ>.70).
Additionally, youth completed three subtests of the WISC-IV (Vocabulary, Block Design, Information) to derive an estimated full scale IQ and three subtests of the WIAT-III (Word Reading, Spelling, Math Reasoning) to estimate academic achievement. These data were also reviewed independently by our diagnostic team (i.e., a board-certified child psychiatrist and a licensed child clinical psychologist) to determine potential learning disorder diagnoses (i.e., significant discrepancies of >1.5 standard deviations between estimated full-scale IQ and academic achievement). Agreement rates were also acceptable for learning disorder diagnoses (κ = .85)
The current sample was comprised of 389 youth with ADHD and 384 non-ADHD comparison youth. All ADHD subtypes were included (169 youth with the primarily inattentive presentation, 8 youth with the primarily hyperactive-impulsive presentation, and 212 with the combined presentation). An additional 142 youth were classified as having sub-threshold (5 or fewer symptoms; n=66 youth) or situational (symptoms exhibited in only one setting, such as at school or at home; n=76 youth). These youth were excluded from group comparisons, but included in all primary analyses that relied on dimensional variables to maximize representation of the outcome domain. Twenty-seven percent of those diagnosed with ADHD were taking stimulant medication, consistent with expectations for community samples (Visser, Lesesne, & Perou, 2007). In addition to ADHD, we retained information about prior diagnoses of disruptive behavior disorder (ODD, CD) and history of lifetime major depression, any anxiety disorder (generalized anxiety disorder, specific or social phobia, separation anxiety disorder, obsessive- compulsive disorder, post-traumatic stress disorder), and learning disorder for subsequent analyses. Exclusion criteria included the following criteria: low IQ (full-scale IQ<75); parent- reported head injury with loss of consciousness, history of seizures, or autism spectrum disorder; current major depressive episode; lifetime bipolar disorder or psychosis; or current substance abuse or dependence.
Measures
ADHD and Comorbid Disruptive Behavior Problems
Parent and teacher ratings on the ADHD Rating Scale (Reid et al., 1998) constituted the main outcome measures. Parents and teachers rated the frequency of all 18 DSM ADHD symptoms on a 4-point Likert Scale (never, sometimes, often, very often). Internal consistencies were adequate for ratings of inattention (parent α=.91; teacher α=.92) and hyperactivity (parent α=.89; teacher α=.90). Additionally, parent-reported ODD and CD symptoms were assessed with the KSADS-E (α=.89 ODD; α=.84 CD), whereas teachers rated these behaviors using the same metric as the ADHD Rating Scale. Items rated by teachers as occurring “often” or “very often” were counted as symptoms (α=.87 ODD; α=.83 CD). To reduce the number of statistical tests required to achieve our aims while maximizing data collected from multiple informants, scores were averaged across informant for each symptom dimension (e.g., inattention, hyperactivity, ODD, and CD), based on recent work indicating that average composite scores best predicted ADHD diagnosis when compared to other methods of combining across informants (Martel, Schimmack, Nikolas, & Nigg, 2015). Cross-informant correlations for these symptom dimensions ranged from r=.44 to r=.56. These average composites were retained as the four primary outcomes in subsequent regression models.
Parental Psychopathology
Primary parents indicated positive family history of a variety of conditions as part of the developmental history questionnaire (see below). Items were scored to determine parental ADHD (yes/no) and externalizing disorder (yes/no) status. Parental ADHD diagnosis was positive if the child’s biological mother and/or father had a confirmed or suspected diagnosis of ADHD (or ADD). Parental externalizing disorder was positive if the child’s biological mother and/or father had a confirmed or suspected diagnosis of alcoholism or other form of substance abuse/dependence or a history of delinquency, conduct, or legal problems.
BW, Substance Exposure, and Pre/Perinatal Characteristics
A comprehensive developmental history questionnaire was completed for each youth by their primary caregiver (89.7% maternal report and 10.3% paternal report) to assess various components of early developmental risk. This included several questions regarding the health and behavior of the mothers during pregnancy and delivery as well as questions regarding the health of the children during delivery and after birth. Previous research has indicated that parental report of pregnancy and birth outcomes is reliable, even up to 30 years after delivery, and parental reports of pregnancy and birth outcomes have been deemed acceptable for use in research studies (Catov et al., 2006; Lumey, Stein, & Ravelli, 1994; Olson, Shu, Ross, Pendergrass, & Robison, 1997; Tomeo et al., 1999). Parents provided retrospective report regarding their child’s BW (in ounces, later converted to grams), gestational age, and whether or not the child’s biological mother used tobacco during pregnancy. Although parents may under-report prenatal tobacco use, the rate of prenatal smoking in the current sample (16%) is similar to, albeit somewhat higher than, the rate of prenatal smoking in larger epidemiological studies (estimated at approximately 12%; see Hamilton, Martin, Sutton, Centers for Disease, & Prevention, 2004; Martin et al., 2002), providing some evidence that the estimated prevalence of prenatal tobacco exposure in the current sample is similar to that in the general population.
Parents also indicated the presence or absence of a variety of health conditions during pregnancy (e.g., gestational diabetes, preeclampsia, placenta previa), labor and delivery characteristics (e.g., type of delivery, length of labor), and perinatal medical problems (e.g., use of an incubator, meconium aspiration, and umbilical cord prolapse). These health conditions were combined into three variables: (1) prenatal medical problems, (2) labor and delivery medical problems, and (3) perinatal medical problems. Given that the occurrence of each specific health condition was low (.1% to 10% of the sample affected), these variables were dichotomized as to whether or not the child experienced any of the health conditions during the prenatal, labor and delivery, and perinatal periods. Finally, parents indicated their ages at the birth of each participating child.
Data Analysis
Descriptive statistics and group differences (i.e., ADHD vs. non-ADHD) were first examined using a series of chi-square and independent t-tests. Next, our primary analyses used hierarchical linear regression models to examine the effects of sex, linear BW, quadratic BW, the interaction between linear BW and sex, and the interaction between quadratic BW and sex on composite scores of inattention, hyperactivity, ODD, and CD. All models included age, ethnicity, and income as covariates. Ethnicity was dummy coded and entered as four separate variables (African-American, Caucasian, Latino, and other). Additionally, primary analyses also included the effects of parental ADHD status (when examining inattention and hyperactivity symptoms) and externalizing disorder status (when examining ODD and CD symptoms) as a proxy method for controlling unmeasured familial factors (i.e., shared genes between parents and children) that may account for associations between BW and ADHD/DBD symptoms.
Following our primary tests of moderation, we conducted a series of follow-up analyses to further examine (1) the association of gestational age, as opposed to BW, with ADHD/DBD symptoms and potential moderation of those relationships by sex, (2) the role of potential confounding factors in the association between BW and ADHD/DBD symptoms (i.e., maternal and paternal age at birth, prenatal tobacco, prenatal, labor, and perinatal medical problems), (3) the specificity of effects to ADHD, and (4) the association between BW and ADHD/DBD within a sample of normal BW individuals.
Similar to the primary analyses, the follow-up analyses used hierarchical linear regression with additional covariates entered into the analyses. Analyses examining gestational age as a predictor directly mirrored those examining BW (i.e., controlling for parental ADHD or externalizing disorder status as well as examination of sex moderation and linear and quadratic effects). For the remainder of the follow-up analyses, only the independent variables that were significant in the primary analyses were also included in the follow-up analyses. For example, quadratic BW and its interaction with sex were not found to be significant in the primary analysis predicting inattention symptoms; thus these two terms were excluded from the follow-up analyses.
Several analyses were used to examine the role of potential confounding variables. Analyses were completed separately for confounding variables due to concerns about multi- collinearity among the confounding variables and possible suppression effects. These included models controlling for (1) prenatal tobacco exposure, (2) maternal and paternal age (both linear and quadratic effects) and (3) other prenatal, labor, and perinatal medical problems. Additionally, to examine the specificity of effects to ADHD versus other externalizing disorders, symptoms of ODD and CD were added as covariates in the analyses examining inattention and hyperactivity as the dependent variables, and symptoms of inattention and hyperactivity were added as covariates in the analyses examining ODD and CD as the dependent variables. Further, learning disorder, anxiety, and depression diagnoses were added as covariates in analyses examining the specificity of effects to externalizing versus internalizing disorders. Finally, primary analyses were conducted with a sub-sample of individuals that excluded individuals born LBW (<2500g) to examine whether the association between LBW and ADHD/DBD symptoms is driven by individuals at the low end of the BW distribution.
All analyses were conducted in MPlus 7.0 (Muthen & Muthen, 1998-2013). Non-independence issues arising from the use of sibling data were managed via the CLUSTER option in Mplus. Further, missing data were handled using full information maximum likelihood procedures in MPlus, and were minimal with the exception of income (9.6% missing). Standardized parameter estimates were computed and are presented along with their 95% confidence intervals, which were computed using a delta standard errors method.
RESULTS
Demographic and Descriptive Statistics
Demographic and descriptive statistics are presented in Table 1. Diagnostic procedures were effective in discriminating ADHD from non-ADHD youth, as ADHD symptoms and comorbid problems were significantly higher among youth with ADHD relative to those without. As expected, the ADHD group included more males, relative to the comparison group. Income was also significantly lower among families of ADHD youth. Therefore, age and income level were covaried in all subsequent analyses. While groups were not statistically different in regard to ethnicity, the proportion of African-Americans was somewhat higher among non-ADHD youth, whereas the proportion of youth identifying as multi-ethnic was marginally higher in the ADHD relative to the non-ADHD group. Additionally, ethnic minority youth had significantly higher rates of LBW than Caucasian youth (χ2=7.54, p=.006). Thus, ethnicity was included as a covariate as well.
Table 1.
Demographic and descriptive statistics.
| Control | ADHD | p | |
|---|---|---|---|
| N | 384 | 389 | |
| % Male | 45.6 | 66.3 | <.001 |
| % Caucasian | 74.2 | 72.8 | .65 |
| % African-American | 13.0 | 8.6 | .06 |
| % Latino | 4.4 | 6.6 | .17 |
| % Multi-ethnic | 6.0 | 9.9 | .04 |
| Age | 12.8 (2.9) | 11.9 (3.0) | .001 |
| Income+ | 73.4 (50.0) | 60.4 (36.0) | <.001 |
| Inattention Sx | 1.5 (1.4) | 6.9 (2.8) | <.001 |
| Hyperactivity Sx | .96 (1.2) | 3.8 (3.1) | <.001 |
| % ODD | 12.1 | 41.7 | <.001 |
| % CD | 2.1 | 9.3 | <.001 |
| % Mood Disorder | 8.8 | 18.2 | <.001 |
| % Anxiety Disorder | 14.5 | 24.0 | <.001 |
| % Learning Disorder | 4.7 | 18.1 | <.001 |
| Birth Weight (g) | 3443.1 (590.2) | 3352.7 (520.3) | <.001 |
| % Prenatal Tobacco Exposure | 4.0 | 15.4 | <.001 |
| % Prenatal Alcohol Exposure | 7.1 | 9.9 | .12 |
| Maternal Age at Birth | 29.1 (5.2) | 28.5 (5.9) | .18 |
| Paternal Age at Birth | 31.8 (6.0) | 30.3 (6.3) | .03 |
Note. ODD=oppositional defiant disorder, CD=conduct disorder. Birth weight reported in grams. +Income reported in thousands. % anxiety disorder indicated presence of any or multiple anxiety disorders.
Consistent with past work, mean BW was significantly lower among ADHD youth relative to non-ADHD youth (Cohen’s d=.38, p<.001), and a higher proportion of ADHD youth were exposed to prenatal tobacco relative to non-ADHD youth. While maternal age did not significantly differ across the groups (nor did prenatal alcohol exposure), paternal age at birth was significantly younger for ADHD youth compared to non-ADHD comparisons.
BW and ADHD/DBD Symptoms
Regression parameters from the primary analyses, as well as all follow-up analyses, are presented in Tables 2-5 for inattention, hyperactivity, ODD, and CD symptoms, respectively. Both child sex (β = -.27, [-.34, -.20], p<.001) and BW (β = -.46, [-.65, -.26], p<.001; total R2=.19) significantly predicted inattention, such that males had higher inattention scores and higher BW predicted lower inattention scores. Additionally, the interaction between sex and BW was significantly different from zero (β =.34, [.13, .55], p=.002). Simple slopes analysis revealed that lower BW was related to significantly higher inattention scores in males, but not in females (see Figure 1). The quadratic effect of BW, as well as the interaction with sex, was not significant (ps>.15).
Table 2.
Regression parameters (βs & 95% confidence intervals) for models examining the effects of sex and gestational age on symptoms of inattention, hyperactivity, oppositional defiant disorder, and conduct problems
| Inattention | Hyperactivity | Oppositional Defiant | Conduct Problems | |
|---|---|---|---|---|
| Sex | -.30 (-.36, -.24) | -.27 (-.33, -.21) | -.18 (-.24, -.12) | -.11 (-.17, -.04) |
| Gestational Age | -.10 (-.25, .05) | -.12 (-.30, .05) | -.16 (-.36, .04) | -.11 (-.25, .04) |
| Linear Interaction | .05 (-.12, .22) | .06 (-.11, .22) | .18 (-.01, .36) | .14 (0, .28) |
Note: bold font indicates p<.05; All models included child age, ethnicity, and family income as covariates; the analyses for inattention and hyperactivity included parental ADHD diagnosis as a covariate and the analyses for oppositional defiant disorder and conduct disorder included parental externalizing disorder diagnoses as covariates.
Table 5.
Regression parameters (βs & 95% confidence intervals) for models examining the effects of sex and birth weight on symptoms of oppositional defiant disorder
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Familial | Tobacco | Parental Age | Medical Problems | Ext Comorbidity | Int Comorbidity | |
| Sex | -.14 (-.21, -.06) | -.17 (-.23, -.11) | -.15 (-.22, -.08) | -.15 (-.22, -.07) | -.02 (-.07, .04) | -.15 (-.21, -.09) |
| Linear birth weight | -.40 (-.59, -.20) | -.36 (-.54, -.17) | -.45 (-.65, -.25) | -.40 (-.60, -.20) | -.17 (-.34, -.01) | -.34 (-.53, -.15) |
| Linear interaction | .32 (.14, .51) | .27 (.09, .45) | .37 (.18, .57) | .33 (.14, .52) | .16 (.01, .32) | .27 (.09, .346) |
| Quadratic birth weight | .15 (-.03, .33) | --- | --- | --- | --- | --- |
| Quadratic interaction | -.13 (-.30, .04) | --- | --- | --- | --- | --- |
Note: bold font indicates p<.05; All models included child age, ethnicity, and family income as covariates; Model 1 was the primary analysis and included parental externalizing disorder diagnoses as covariates; Model 2 included prenatal tobacco exposure as a covariate; Model 3 included maternal and paternal age at birth as a covariate; Model 4 included prenatal, labor and delivery, and perinatal medical problems as covariates; Model 5 included child symptoms of inattention and hyperactivity as covariates; Model 6 included child diagnoses of depression, anxiety, and learning disorder as covariates; Quadratic birth weight was the quadratic interaction were not included in models 2-5 as these terms were not significant in the primary analysis.
Figure 1.
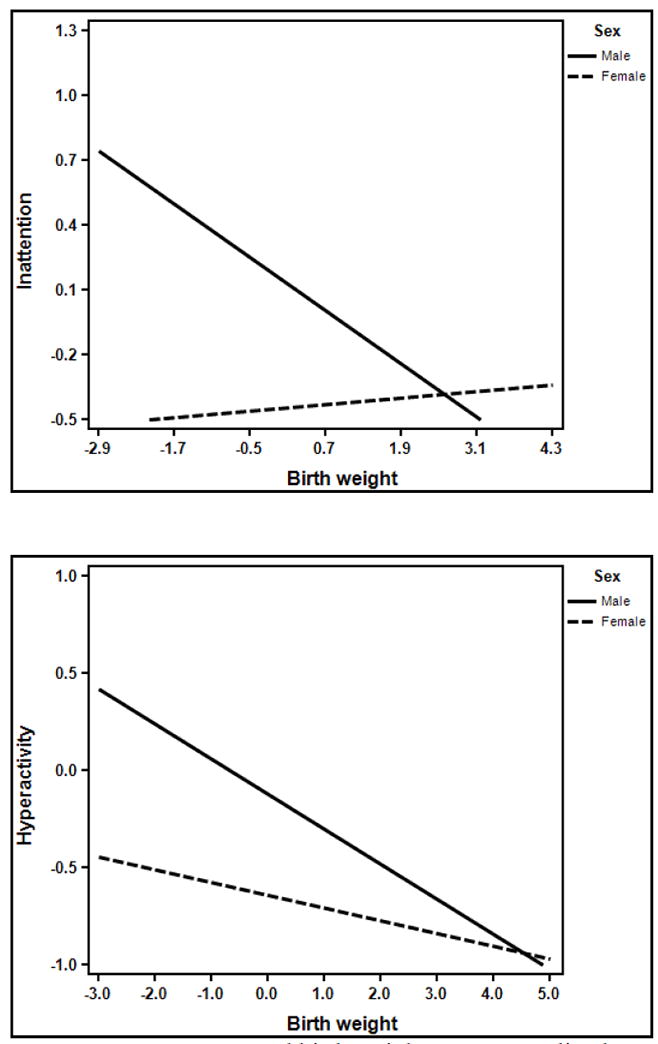
Moderation by sex of the association between birth weight and inattention and hyperactivity, respectively.
Note: Symptom scores and birth weight were normalized.
Similarly, both child sex (β = -.22, [-.29, -.15], p<.001) and BW (β = -.33, [-.52, -.4], p<.001) significantly predicted composite scores of hyperactivity, again such that males had higher hyperactivity scores and higher BW predicted lower hyperactivity (total R2=.23). However, the quadratic effect of BW on hyperactivity was also significant (β = .20, [.04, .36], p=.016), and revealed a curvilinear association, such that higher BW predicted lower hyperactivity scores up until a point (1.3 SD), but that beyond that point, higher BW was associated with increased hyperactivity. The interaction between sex and BW was marginally (β = .18, [-.01, .37], p=.07), and again revealed a trend to stronger effects of BW in males relative to females (see Figure 1). Sex did not moderate the quadratic association between BW and hyperactivity (p=.19).
Child sex (β = -.14, [-.21, -.04], p<.001) and BW (β = -.40, [-.59, -.20], p<.001) also predicted ODD scores in a similar fashion (i.e., higher ODD scores among males relative to females, and a negative association between BW and ODD scores, total R2=.14). Quadratic effects of BW (and their interaction with child sex) were not reliably different from zero (ps>.12). The interaction between sex and BW was also statistically significant (β = .32, [.14, .51], p=.001), and again revealed that the negative association between BW and ODD scores was stronger in males relative to females (see Figure 2).
Figure 2.
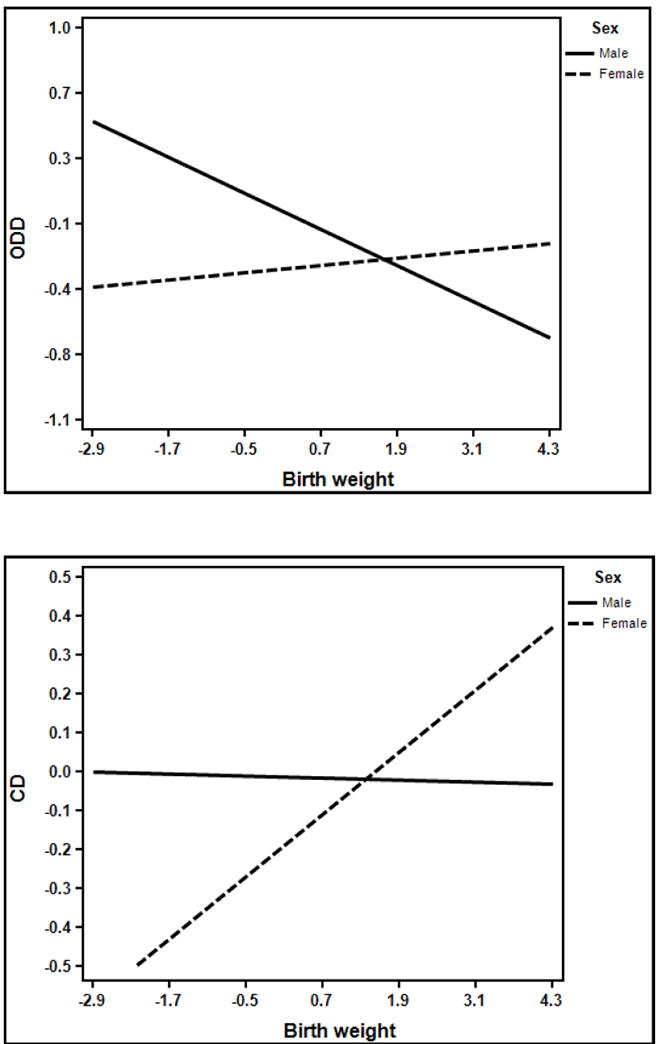
Moderation by sex of the association between birth weight and ODD and CD, respectively.
Note: Symptom scores and birth weight were normalized.
BW predicted CD scores in both a linear (β = -.21, [-.42, -.001], p=.05) and quadratic fashion (β = .26, [.07, .44], p=. 006, total R2=.09). Similar to hyperactivity, higher BW predicted a decrease in CD scores up until a point (-0.16 SD), but beyond that, increased BW predicted increased CD symptoms. Notably, both effects were also moderated by sex (linear interaction β = .26, [.07, .45], p=.009; quadratic interaction β = -.20, [-.39, -.02], p=.03). Examination of the linear interaction revealed that higher BW as associated with increased CD symptoms in females but not in males, unlike models predicting ADHD and ODD. However, this was qualified by the quadratic BW x sex interaction, which revealed that for males, low BW predicted increased CD symptoms only to a point, but that beyond that, increased BW predicted increased CD symptoms (see Figure 2).
Role of Gestational Age
We next examined gestational age, sex and their interaction in predicting ADHD/DBD symptoms to determine if degree of preterm birth may be driving associations between BW and externalizing psychopathology. Regression parameters are presented in Table 2. In all cases, sex was negatively associated with child externalizing symptoms, but gestational age was not. Additionally, the interaction between gestational age and sex was not significant for any of the outcomes, with the exception of CD symptoms. As before, younger gestational age was associated with increased CD symptoms for males but not females.
Role of Confounding Variables
Next, we examined whether potential moderation by sex would remain after controlling for various confounding factors. Results are presented in Tables 3-6. Regression parameters from the analyses controlling for prenatal tobacco exposure, linear and quadratic parental age, and prenatal, labor, and perinatal medical problems were largely the same as those in the primary analyses examining moderation by sex. Two exceptions to this were the linear interaction for hyperactivity when controlling for prenatal tobacco and the quadratic interaction for CD symptoms when controlling for parental age were no longer significant.
Table 3.
Regression parameters (βs & 95% confidence intervals) for models examining the effects of sex and birth weight on symptoms of inattention
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Familial | Tobacco | Parental Age | Medical Problems | Ext Comorbidity | Int Comorbidity | |
| Sex | -.27 (-.34, -.20) | -.29 (-.35, -.23) | -.27 (-.34, -.21) | -.27 (-.34, -.20) | -.21 (-.27, -.16) | -.27 (-.33, -.22) |
| Linear birth weight | -.46 (-.65, -.26) | -.43 (-.62, -.23) | -.43 (-.64, -.23) | -.48 (-.68, -.29) | -.26 (-.46, -.07) | -.37 (-.57, -.18) |
| Linear interaction | .34 (.13, .55) | .32 (.12, .52) | .32 (.11, .53) | .36 (.15, .58) | .19 (-.01, .38) | .28 (.08, .48) |
| Quadratic birth weight | .15 (-.05, .34) | --- | --- | --- | --- | --- |
| Quadratic interaction | -.08 (-.29, .14) | --- | --- | --- | --- | --- |
Note: bold font indicates p<.05; All models included child age, ethnicity, and family income as covariates; Model 1 was the primary analysis and included parental ADHD diagnosis as a covariate; Model 2 included prenatal tobacco exposure as a covariate; Model 3 included maternal and paternal age at birth as a covariate; Model 4 included prenatal, labor and delivery, and perinatal medical problems as covariates; Model 5 included child symptoms of ODD and CD as covariates; Model 6 included child diagnoses of depression, anxiety, and learning disorder as covariates; Quadratic birth weight and the quadratic interaction were not included in models 2-5 as these terms were not significant in the primary analysis.
Table 6.
Regression parameters (βs & 95% confidence intervals) for models examining the effects of sex and birth weight on symptoms of conduct disorder
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Familial | Tobacco | Parental Age | Medical Problems | Ext Comorbidity | Int Comorbidity | |
| Sex | -.05 (-.12, .02) | -.05 (-.12, .02) | -.04 (-.12, .04) | -.06 (-.13, .01) | .02 (-.06, .09) | -.05 (-.12, .03) |
| Linear birth weight | -.21 (-.42, -.01) | -.21 (-.42, -.01) | -.26 (-.49, -.03) | -.21 (-.43, 0) | -.11 (-.32, .11) | -.19 (-.41, .02) |
| Linear interaction | .26 (.07, .45) | . 25 (.06,.45) | .33 (.12, .55) | .26 (.06, .45) | .17 (-.02 .37) | .24 (.04, .44) |
| Quadratic birth weight | .26 (.07, .44) | .25 (.07, .43) | .24 (.04, .45) | .26 (.07, .45) | .22 (.03, .40) | .26 (.07, .45) |
| Quadratic interaction | -.20 (-.39, -.02) | -.20 (.38, -.02) | -.17 (-.37, .04) | -.20 (-.39, -.01) | -.17 (-.36, .02) | -.20 (-.39, -.01) |
Note: bold font indicates p<.05; All models included child age, ethnicity, and family income as covariates; Model 1 was the primary analysis and included parental externalizing disorder diagnoses as covariates; Model 2 included prenatal tobacco exposure as a covariate; Model 3 included maternal and paternal age at birth as a covariate; Model 4 included prenatal, labor and delivery, and perinatal medical problems as covariates; Model 5 included child symptoms of ODD and CD has covariates; Model 6 included child diagnoses of depression, anxiety, and learning disorder as covariates.
Specificity of Effects to ADHD
Notably, all main effects and significant sex moderation effects persisted when controlling for comorbid externalizing symptoms, with one exception (i.e., the interaction between sex and birth weight predicting hyperactivity was no longer significant). Thus, the impact of BW appeared to be robust across ADHD and externalizing outcomes and revealed no unique association with ADHD. Similarly, all effects of BW as well as the moderation of effects by sex also generally persisted when controlling for comorbid internalizing disorders and learning disorder, negating the possibility that these associations are due to the relation between BW and other comorbid psychopathology.
Association of BW and ADHD/DBD Symptoms in Normal BW Individuals
Similar to past work, being classified as LBW (<2500 grams, n=55), very LBW (<1500 grams, n=6) weight, or extremely LBW (<1000 grams, n=2) was associated with increased risk for ADHD (OR=1.28, [1.03, 1.63], p=.04). Accordingly, primary analyses described above were rerun while excluding these individuals to ensure outliers at low levels of BW did not dictate current results. All effects remained significant, indicating that individuals at the low end of the BW distribution are not driving these findings, and that normal variations in BW are important for understanding individual variation in ADHD and externalizing psychopathology.
DISCUSSION
The current study examined BW as a statistical predictor of ADHD and comorbid externalizing symptoms, as well as the moderation of the effect of BW by sex. Consistent with our hypotheses, BW significantly predicted ADHD and externalizing disorder symptoms, and these associations showed consistent evidence of moderation by sex. In all cases, lower BW was associated with higher ADHD and externalizing symptoms in males, but not in females. These findings are consistent with sexual selection theory, which predicts males are more susceptible to early life adversity and this results in the manifestation of symptoms of externalizing disorders. Furthermore, there were both linear and nonlinear effects on the associations between BW and symptoms of hyperactivity and CD, suggesting that BW at both ends of the distribution (low and high) confers risk. In contrast, only a linear association was observed for symptoms of inattention and ODD.
Notably, associations between BW and ADHD and comorbid externalizing behaviors (as well as moderation effects) remained even after adjusting for a number of potential confounds. First, findings remained when adjusting for associations between parental ADHD and externalizing problems and child symptom scores, suggesting that this association is independent of familial effects. These findings are consistent with past work indicating that BW may be an early causal risk factor for later psychopathology, including ADHD and externalizing problems (Class et al., 2014; D’Onofrio et al., 2014; Groen-Blokhuis et al., 2011; Hultman et al., 2007; Lahat et al., 2014; Nigg & Breslau, 2007; Pettersson et al., 2014; Thapar et al., 2005). Importantly, results examining gestational age indicated that while gestational age was moderately correlated with BW (r=.29, p<.01), it did not drive BW findings in the current sample.
Second, findings persisted when controlling for a variety of confounding variables including prenatal, labor and delivery, and perinatal medical problems, prenatal tobacco use, and parental age, all of which have been found to be associated with low BW, complicating the interpretation and implications of the significant association between BW and ADHD and externalizing symptoms. However, a variety of prenatal, labor and delivery, and perinatal medical problems were controlled for in the current analyses, providing support for an association of BW with ADHD and externalizing symptoms independent of exposure to such medical problems. Additionally, while recent work has indicated that the impact of prenatal tobacco exposure on ADHD and externalizing psychopathology may be due to confounded genetic and/or familial factors (Skoglund, Chen, Franck, Lichtenstein, & Larsson, 2015; Thapar et al., 2009; Wiggs, Elmore, Nigg, & Nikolas, 2016), the effects of BW do seem to be causal and important for ADHD and externalizing psychopathology. Still, prenatal tobacco use contributed significantly in the model for ODD and was marginally significant in the model for hyperactivity, indicating prenatal tobacco exposure may have causal effects for these specific behaviors in addition to the effects of BW. In a similar fashion, results were largely the same when parental age was co-varied, although child sex no longer moderated the effect of quadratic BW for CD. Although the findings remained the same with parental age, it is important to note that the quadratic effect of maternal age was significant for both inattention and hyperactivity, indicating future research examining factors conferring risk for ADHD should investigate the role of both younger and older maternal age.
Lastly, findings remained when considering the overlap among ADHD and comorbid externalizing problems as well as when considering the overlap among ADHD, internalizing, and learning disorders. These results indicate that the association between BW and ADHD also extends to externalizing behavior problems, which is consistent with some work demonstrating causal effects for externalizing psychopathology broadly (Lahat et al., 2014; Thapar et al., 2005), but stands in contrast to past work suggesting some specificity of effects of BW to ADHD (Nigg & Breslau, 2007).
Most notably, BW effects were moderated by child sex across all four symptom dimensions, with male sex consistently conferring greater risk. The moderation of BW effects by child sex has been previously investigated in a population-based sibling analysis; however, results from that study did not indicate sex moderation (Class et al., 2014). The use of sibling designs remains essential for parsing genetic confounds in work aiming to understand the impact of environmental exposures on the development of psychopathology (D’Onofrio et al., 2014). Yet, much of this work has focused on categorical ADHD outcomes (rather than dimensional symptom scores), which may account for our discrepant findings. That is, the population-based sibling designs may be more limited when examining moderation of effect of BW by sex across the full dimension of ADHD symptom severity. Future work utilizing similar sibling analyses may indeed benefit by exploring dimensional measures of ADHD when investigating the impact of BW and moderation of effects by sex.
The finding that male sex confers greater risk in LBW individuals aligns with sexual selection theory, which suggests that males are more sensitive to environmental perturbations during the prenatal period, which may lead to lower BW, and that this sensitivity is mediated by higher prenatal testosterone (Martel, 2013). Significant moderation of associations between BW and externalizing psychopathology by sex suggests that males may be more vulnerable to the detrimental effects of lower BW, compared to females, and that one potential mechanism may involve increased neural vulnerability associated with prenatal testosterone exposure (Hernandez et al., 1994; Kritzer & Creutz, 2008; Kuhn et al., 2010; Martel et al., 2009; Morris et al., 2004). However, more research is critical to explore how prenatal hormonal surges may contribute to sex differences in timing of sensitivity to environmental influences. Previous work has indicated higher levels of prenatal testosterone decrease the rate of fetal development in males, which in turn increases the ability of males to respond to prenatal cues about the environment (Geary, 2010; Geschwind & Galaburda, 1985; Glover, 2011; Morris et al., 2004; Talge et al., 2007). Additionally, individuals with particular genetic alleles known to alter dopaminergic neurotransmission systems, such as the 10-repeat allele of DAT1, may have increased susceptibility to prenatal environmental cues, as testosterone is believed to modulate the dopaminergic system (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Karg, Burmeister, Shedden, & Sen, 2011; Martel et al., 2011; Nigg, Nikolas, & Burt, 2010; Nikolas, Friderici, Waldman, Jernigan, & Nigg, 2010).
The current research has some limitations. First, the prenatal variables used in the analyses (prenatal tobacco use and BW) were retrospective parental reports, which may be subject to retrospective recall bias. Although retrospective parental report of BW has been found to be reliable (Catov et al., 2006; Lumey et al., 1994; Olson et al., 1997; Tomeo et al., 1999), there is concern that prenatal substance exposure may be under-reported when using retrospective parental report, limiting the ability to account for this in analyses. While one study has suggested that reporting rates of prenatal substance exposure may actually be higher when using retrospective (vs. prospective) methods (Jacobsen, et al., 1999), it is unclear how much bias is operating in the current study. Notably, the rate of prenatal tobacco use in the current sample (~16%) was similar to, if not larger than, the rate in large epidemiological samples (~12%). An additional limitation related to prenatal tobacco exposure was the dichotomization of prenatal tobacco exposure. Dichotomizing this variable may limit the interpretability of the effect of prenatal tobacco exposure on ADHD and externalizing symptoms, as it is likely the magnitude of effect is influenced by the amount of tobacco exposure. Future research would benefit from a more comprehensive measure of prenatal tobacco exposure when examining the association between BW and ADHD/DBD symptoms. Additionally, the current analyses did not include prenatal exposure to illicit drugs due to the low base rate (n=23) in the current sample. Future research would benefit from including exposure to other prenatal substances, such as alcohol and illicit drugs, in addition to tobacco.
An additional limitation is that the variables used to control for familial effects (parental ADHD and parental externalizing disorders) were parental reports of lifetime ADHD or externalizing disorder diagnosis, as opposed to dimensional measures of ADHD and externalizing symptoms. Also, outcome variables utilized for CD were parent and teacher reports of symptoms, although teenaged youth are considered to be the best reporters of their delinquent behavior. Future research efforts may benefit from including self-reports of externalizing behavior from teenaged youth. Finally, although quadratic effects of BW were found for CD and hyperactivity, these effects may be driven by other prenatal variables (i.e., maternal obesity, gestational diabetes, etc.) that were not accounted for in the current analyses. Despite these limitations, this study was strengthened by the systematic investigation of the effect of BW on several distinct but related symptom dimensions, while also controlling for various confounding factors and examining the moderation of effects by child sex.
In sum, the present study demonstrated that BW is a robust predictor of ADHD and externalizing psychopathology, and that effects are substantially stronger in males than in females. This work extends previous research by examining BW as a continuous variable in a population-based sample while controlling for several potential genetic and environmental confounds. Furthermore, this study examined the moderation of BW by sex and found results consistent with sexual selection theory, suggesting future research examining the biological mechanisms of ADHD and externalizing disorders may benefit by investigating the effects of varying levels of prenatal testosterone exposure on the infant brain, and how this might influence vulnerability to other environmental perturbations. Finally, the current findings highlight the utility of BW as an early marker of risk for ADHD and externalizing psychopathology.
Overall, the current study contributes to the current literature regarding the causal association between BW and symptoms of ADHD, as it is the first to systematically examine the effect of BW and its moderation by sex on symptoms of ADHD and other comorbid externalizing disorders, while controlling for other broad comorbid disorders, familial confounds, parental age, and prenatal substance abuse.
Table 4.
Regression parameters (βs & 95% confidence intervals) for models examining the effects of sex and birth weight on symptoms of hyperactivity
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Familial | Tobacco | Parental Age | Medical Problems | Ext Comorbidity | Int Comorbidity | |
| Sex | -.22 (-.29, -.15) | -.26 (-.32, -.20) | -.23 (-.30, -.16) | -.24 (-.31, -.17) | -.15 (-.20, -.09) | -.23 (-.29, -.16) |
| Linear birth weight | -.33 (-.52, -.14) | -.27 (-.46, -.09) | -.37 (-.56, -.18) | -.34 (-.53, -.14) | -.14 (-.30, .02) | -.27 (-.46, -.08) |
| Linear interaction | .18 (-.11, .37) | .12 (-.07, .30) | .20 (.01, .40) | .20 (0, .39) | .02 (-.13 .17) | .11 (-.07, .30) |
| Quadratic birth weight | .20 (.04, .36) | .08 (.02, .14) | .12 (.05, .18) | .19 (.02, .35) | .09 (.03, .15) | .10 (.04, .16) |
| Quadratic interaction | -.10 (-.26, .05) | --- | --- | --- | --- | --- |
Note: bold font indicates p<.05; All models included child age, ethnicity, and family income as covariates; Model 1 was the primary analysis and included parental ADHD diagnosis as a covariate; Model 2 included prenatal tobacco exposure as a covariate; Model 3 included maternal and paternal age at birth as a covariate; Model 4 included prenatal, labor and delivery, and perinatal medical problems as covariates; Model 5 included child symptoms of ODD and CD as covariates; Model 6 included child diagnoses of depression, anxiety, and learning disorder as covariates; The quadratic interaction was not included in models 2-5 as this term was not significant in the primary analysis.
GENERAL SCIENTIFIC SUMMARY.
The current study suggests that low birth weight is a risk factor for the development of externalizing behavior in children, such as inattention, hyperactivity, oppositional defiant behavior, and conduct problems, and that this risk is moderated by sex such that males are more likely to experience increased symptoms if born low birth weight. Further, the current study indicates this risk remains after taking into consideration a number of factors including family history of externalizing behavior problems, prenatal tobacco exposure, parental age at birth, and exposure to prenatal, labor and delivery, and neonatal medical difficulties.
Acknowledgments
This study was funded by an NIH grant (R01-MH070004-01A2) awarded to Joel T. Nigg, Ph.D. Allison M. Momany and Jaclyn M. Kamradt were supported by NIH grant GM108540.
Footnotes
The ideas presented in this manuscript have not been previously disseminated. The data in this manuscript have been used in previous manuscripts (e.g. Wiggs, Elmore, Nigg, & Nikolas, 2016), but the analyses in previous manuscripts did not specifically examine the moderation of the association between birth weight and Attention-Deficit Hyperactivity Disorder by sex.
References
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Alcock J, Crawford C. Evolutionary question for evolutionary pscyhologists. In: Crawford C, Krebs D, editors. Foundations of evolutionary psychology. New York, NY: Erlbaum; 2008. pp. 25–46. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Ancel PY, Goffinet F, Group E-W, Kuhn P, Langer B, Matis J, Kaminski M, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW Victorian Infant Collaborative Study, G. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36(1):57–73. doi: 10.1080/87565641.2011.540538. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. PsycholSci. 2009;20(2):144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, Higgins R, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25(10):631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- Bailey BA, Byrom AR. Factors predicting birth weight in a low-risk sample: the role of modifiable pregnancy health behaviors. Matern Child Health J. 2007;11(2):173–179. doi: 10.1007/s10995-006-0150-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and health in later life. 2. Edinburgh ; New York: Churchill Livingstone; 1998. [Google Scholar]
- Bassi JA, Rosso P, Moessinger AC, Blanc WA, James LS. Fetal growth retardation due to maternal tobacco smoke exposure in the rat. Pediatr Res. 1984;18(2):127–130. doi: 10.1203/00006450-198402000-00002. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: a Biology x Sex x Environment interaction model of antisocial and borderline traits. Dev Psychopathol. 2009;213:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148(5):564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38(8):931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Brown GG, DelDotto JE, Kumar S, Ezhuthachan S, Andreski P, Hufnagle KG. Psychiatric sequelae of low birth weight at 6 years of age. J Abnorm Child Psychol. 1996;24(3):385–400. doi: 10.1007/BF01441637. [DOI] [PubMed] [Google Scholar]
- Breslau N, Paneth NS, Lucia VC. The lingering academic deficits of low birth weight children. Pediatrics. 2004;114(4):1035–1040. doi: 10.1542/peds.2004-0069. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Anderson PJ, Cheong J, Doyle LW, Davey CG, Wood SJ. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychol Med. 2011;41(12):2463–2474. doi: 10.1017/S003329171100081X. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Newman AB, Kelsey SF, Roberts JM, Sutton-Tyrrell KC, Garcia M, Ness RB, et al. Accuracy and reliability of maternal recall of infant birth weight among older women. Ann Epidemiol. 2006;16(6):429–431. doi: 10.1016/j.annepidem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Class QA, Rickert ME, Larsson H, Lichtenstein P, D’Onofrio BM. Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry. 2014;205(5):355–361. doi: 10.1192/bjp.bp.113.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25(6):487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Costello EJ, Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2011;50(3):252–261. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Lahey BB, Larsson H. Testing the Developmental Origins of Health and Disease Hypothesis for Psychopathology Using Family-Based Quasi-Experimental Designs. Child Dev Perspect. 2014;8(3):151–157. doi: 10.1111/cdep.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. London, England: John Murray; 1871. [Google Scholar]
- Elgen I, Sommerfelt K, Markestad T. Population based, controlled study of behavioural problems and psychiatric disorders in low birthweight children at 11 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;87(2):F128–132. doi: 10.1136/fn.87.2.F128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgen IB, Holsten F, Odberg MD. Psychiatric disorders in low birthweight young adults. Prevalence and association with assessments at 11 years. Eur Psychiatry. 2013;28(7):393–396. doi: 10.1016/j.eurpsy.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS-F, Ni H-C, Shang C-Y, Soong W-T, Wu Y-Y, Lin L-Y, Chiu Y-N. Psychiatric comorbidity among children and adolescents with and without persistent attention-deficit hyperactivity disorder. Australian and New Zealand Journal of Psychiatry. 2010;44(2):135–143. doi: 10.3109/00048670903282733. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Geary DC. Male, female: The evolution of human sex differences. Washington, DC, US: American Psychological Associaiton; 2010. [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization Biological mechanisms, associations, and pathology: I A hypothesis and a program for research. Arch Neurol. 1985;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry. 2011;52(4):356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci. 2013;31(6):406–414. doi: 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen-Blokhuis MM, Middeldorp CM, van Beijsterveldt CE, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. J Am Acad Child Adolesc Psychiatry. 2011;50(12):1247–1254 e1242. doi: 10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Gustafsson P, Kallen K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Developmental Medicine and Child Neurology. 2011a;53(3):263–268. doi: 10.1111/j.1469-8749.2010.03820.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson P, Kallen K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Developmental Medicine and Child Neurology. 2011b;53(3):263–268. doi: 10.1111/j.1469-8749.2010.03820.x. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30(2):122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmoy A, Klungsoyr K, Skjaerven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71(5):474–481. doi: 10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Sutton PD Centers for Disease, C., & Prevention, N. C. f. H. S. Births: preliminary data for 2003. Natl Vital Stat Rep. 2004;53(9):1–17. [PubMed] [Google Scholar]
- Hampson E, Ellis CL, Tenk CM. On the relation between 2D:4D and sex- dimorphic personality traits. Arch Sex Behav. 2008;37(1):133–144. doi: 10.1007/s10508-007-9263-3. [DOI] [PubMed] [Google Scholar]
- Hatch B, Healey DM, Halperin JM. Associations between birth weight and attention-deficit/hyperactivity disorder symptom severity: indirect effects via primary neuropsychological functions. J Child Psychol Psychiatry. 2014;55(4):384–392. doi: 10.1111/jcpp.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, Paez X, Gottberg E, Baptista T. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neurosci Lett. 1994;171(1-2):172–174. doi: 10.1016/0304-3940(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Torrang A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: a prospective Swedish twin study. J Am Acad Child Adolesc Psychiatry. 2007;46(3):370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. JNeurosci. 2008;28(38):9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Van Lieshout RJ, Saigal S, Boyle MH, Schmidt LA. Small for gestational age and poor fluid intelligence in childhood predict externalizing behaviors among young adults born at extremely low birth weight. Dev Psychopathol. 2014:1–8. doi: 10.1017/S0954579414000662. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen T. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116(2):462–467. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Ravelli AC. Maternal recall of birthweights of adult children: validation by hospital and well baby clinic records. Int J Epidemiol. 1994;23(5):1006–1012. doi: 10.1093/ije/23.5.1006. [DOI] [PubMed] [Google Scholar]
- Martel MM. Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychol Bull. 2013;139(6):1221–1259. doi: 10.1037/a0032247. [DOI] [PubMed] [Google Scholar]
- Martel MM, Klump K, Nigg JT, Breedlove SM, Sisk CL. Potential hormonal mechanisms of attention-deficit/hyperactivity disorder and major depressive disorder: a new perspective. Horm Behav. 2009;55(4):465–479. doi: 10.1016/j.yhbeh.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nikolas M, Jernigan K, Friderici K, Waldman I, Nigg JT. The dopamine receptor D4 gene (DRD4) moderates family environmental effects on ADHD. J Abnorm Child Psychol. 2011;39(1):1–10. doi: 10.1007/s10802-010-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Schimmack U, Nikolas M, Nigg JT. Integration of symptom ratings from multiple informants in ADHD diagnosis: a psychometric model with clinical utility. Psychol Assess. 2015;27(3):1060–1071. doi: 10.1037/pas0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM, Sutton PD. Births: final data for 2001. Natl Vital Stat Rep. 2002;51(2):1–102. [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23(1):16–22. doi: 10.1097/00004703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. NatNeurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Murray E, Matijasevich A, Santos IS, Barros AJ, Anselmi L, Barros FC, Stein A. Sex differences in the association between foetal growth and child attention at age four: specific vulnerability of girls. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12422. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. Sixth ed. Los Angeles, CA: Muthen & Muthen; 1998-2013. [Google Scholar]
- Nigg J, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(3):362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- Nigg J, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):863–873. doi: 10.1016/j.jaac.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolas M, Friderici K, Waldman I, Jernigan K, Nigg JT. Gene x environment interactions for ADHD: synergistic effect of 5HTTLPR genotype and youth appraisals of inter-parental conflict. Behav Brain Funct. 2010;6:23. doi: 10.1186/1744-9081-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Wickramaratne PJ, Pilowsky DJ, Newcorn JH, Bruder-Costello B, Davey C, Weissman MM. Low birth weight and risk of affective disorders and selected medical illness in ffspring at high and low risk for depression. Compr Psychiatry. 2007;48(5):470–478. doi: 10.1016/j.comppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okah FA, Cai J, Hoff GL. Term-gestation low birth weight and health- compromising behaviors during pregnancy. Obstet Gynecol. 2005;105(3):543–550. doi: 10.1097/01.AOG.0000148267.23099.b7. [DOI] [PubMed] [Google Scholar]
- Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. Am J Epidemiol. 1997;145(1):58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- Pettersson E, Sjolander A, Almqvist C, Anckarsater H, D’Onofrio BM, Lichtenstein P, Larsson H. Birth weight as an independent predictor of ADHD symptoms: a within-twin pair analysis. J Child Psychol Psychiatry. 2014 doi: 10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TN, Mercer BM, Burchfield DJ, Joseph GF., Jr Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096. doi: 10.1097/AOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- Reichman NE, Teitler JO. Paternal age as a risk factor for low birthweight. Am J Public Health. 2006;96(5):862–866. doi: 10.2105/AJPH.2005.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R, DuPaul GJ, Power TJ, Anastopoulos AD, Rogers-Adkinson D, Noll MB, Riccio C. Assessing culturally different students for attention deficit hyperactivity disorder using behavior rating scales. J Abnorm Child Psychol. 1998;26(3):187–198. doi: 10.1023/a:1022620217886. [DOI] [PubMed] [Google Scholar]
- Roberts G, Bellinger D, McCormick MC. A cumulative risk factor model for early identification of academic difficulties in premature and low birth weight infants. Matern Child Health J. 2007;11(2):161–172. doi: 10.1007/s10995-006-0158-z. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Epstein JN, Bellinger DC, Korrick SA. Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. JAtten Disord. 2013;17(1):47–57. doi: 10.1177/1087054711427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H. Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry. 2015;77(10):880–886. doi: 10.1016/j.biopsych.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Strang-Karlsson S, Raikkonen K, Pesonen AK, Kajantie E, Paavonen EJ, Lahti J, Andersson S, et al. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: the Helsinki study of very-low-birth- weight adults. Am J Psychiatry. 2008;165(10):1345–1353. doi: 10.1176/appi.ajp.2008.08010085. [DOI] [PubMed] [Google Scholar]
- Subramanian KS, Seo SC, Barton AM, Montazami S. Extremely Low Birth Weight Infant. 2014 Retrieved from http://emedicine.medscape.com/article/979717-overview.
- Szatmari P, Saigal S, Rosenbaum P, Campbell D, King S. Psychiatric disorders at five years among children with birthweights less than 1000g: a regional perspective. Developmental Medicine and Child Neurology. 1990;32(11):954–962. [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V, Early Stress TR Prevention Science Network, F., Neonatal Experience on, C., & Adolescent Mental, H. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48(3-4):245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Hay D, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, Fowler T, Rice F, Turic D, Whittinger N, O’Donovan M, et al. Catechol O-methyltransferase gene variant and birth weight predict early-onset antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2005;62(11):1275–1278. doi: 10.1001/archpsyc.62.11.1275. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Harold G, et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Buka SL, et al. Reproducibility and validity of maternal recall of pregnancy- related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Blumberg SJ, et al. Trends in the parent-report of health care provider- diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46. e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119(Suppl 1):S99–106. doi: 10.1542/peds.2006-2089O. [DOI] [PubMed] [Google Scholar]
- Vohr BR. Neurodevelopmental outcomes of extremely preterm infants. Clin Perinatol. 2014;41(1):241–255. doi: 10.1016/j.clp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive-impulsive- attention problems and conduct problems. Psychol Bull. 2002;128(1):118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Wiggs K, Elmore AL, Nigg JT, Nikolas MA. Pre- and Perinatal Risk for Attention-Deficit Hyperactivity Disorder: Does Neuropsychological Weakness Explain the Link? J Abnorm Child Psychol. 2016 doi: 10.1007/s10802-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Lahey BB, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) Geneva: WHO; 1992. [Google Scholar]


