Abstract
BACKGROUND
The incidence of Hepatocellular Carcinoma (HCC) has doubled over the past two decades, becoming the fifth most common cancer worldwide. Orthotopic liver transplant (OLT) is the gold standard treatment for those with HCC meeting eligibility criteria, although recurrence rates of HCC after OLT still remain an under-studied obstacle.
METHODS
We performed a single-center, retrospective, longitudinal study with the aim of determining the predominant baseline and follow-up variables associated with HCC recurrence. We gathered pre and post-transplant data and conducted univariate and multivariate analysis to assess variables predicting HCC recurrence after OLT.
RESULTS
Between 2003 and 2015, 141 patients underwent OLT for HCC. We identified 9 (6.4%) cases of documented HCC recurrence. Univariate analysis indicated that the difference in serum alpha-fetoprotein (AFP) levels (most recent prior to transplant subtracted from maximum level) was lower in the HCC recurrence group (median 3ng/mL vs 0ng/mL, p=0.052), as well as the pre-transplant serum cholesterol level (median 158mg/dL vs 113mg/dL, p=0.019) and days between HCC neo-adjuvant treatment initiation and transplantation (median 122 vs. 0, p=0.045). Multivariate analysis revealed that a low pre-transplant serum cholesterol level (<100 mg/dL) was independently associated with HCC recurrence (HR 11.0, p=0.004).
CONCLUSION
The risk of HCC recurrence after OLT was low, at 6.4%, in this cohort. Low pre-transplant serum cholesterol was the strongest predictor of recurrence and may help clinicians risk-stratify patients for appropriate post-transplant monitoring and follow-up.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third leading cause of cancer mortality.1 Its incidence is disproportionately increased in areas with high rates of HBV infection and aflatoxin exposure, such as sub-Saharan Africa and parts of Asia as well as in males across the world. In the U.S. it is associated more closely with the increase in HCV cases due to widespread HBV vaccination. The large majority of cases arise in a cirrhotic liver in Western populations with notable causes of cirrhosis including HBV/HCV, alcoholic liver disease, and nonalcoholic steatohepatitis (NASH). HCC may arise from mature hepatocytes or progenitor cells in the setting of cirrhosis and may begin as highgrade, dysplastic nodules. The constant inflammation and regeneration of cells leading to cirrhosis may cause accumulation of mutations of which the end result is genomic instability, a hallmark of HCC. Since patients with cirrhosis are at an increased risk they are imaged every six months, as the prognosis of an advanced HCC is poor.2
In those patients meeting eligibility criteria, the gold standard of treatment for HCC is orthotopic liver transplantation (OLT); but the primary concern post-transplant is recurrence of the cancer. This concern has led to inclusion criteria for liver transplant in order to increase the chance of survival and to better allocate the available organs (Milan criteria).3 Despite the standard use of these criteria, the rate of recurrence is estimated currently to be 15–20% and occurs typically at an extrahepatic site4. To monitor for this, patients undergo imaging post-transplant, and their serum alpha-fetoprotein (AFP) levels are monitored serially. There remains a need for research that improves the ability to determine patients who are high-risk of recurrence beyond the use of the Milan criteria. Recently published literature points to AFP, neutrophil to lymphocyte ratio (NLR), and tumor differentiation as risks of recurrence apart from the current markers of size and number of tumors.5,6 The aim of this study was to utilize detailed baseline data to determine risk factors for recurrence of HCC post OLT in our population beyond the standard patient demographics and tumor burden.
PATIENT AND METHODS
This was a single-center, retrospective study assessing the pre and post-transplant factors associated most strongly with HCC recurrence after OLT. Approval was obtained from the local IRB and electronic medical records of patients who underwent OLT for HCC as well as by review of incidental findings of HCC on the explant between 2003 and 2015. A total of 141 patients were identified, based on an assessment of ICD-9 codes and the transplant registry database. Groups were divided and compared based on if recurrence of HCC post-transplant was documented in the medical record either by the transplant center or other providers caring for the patient. Data were collected for a number of pre and post-transplant variables identified in the literature as being potential risk factors for HCC recurrence, as well as common sociodemographic characteristics collected routinely for liver transplant studies. The primary underlying liver disease was noted and defined as HBV, HCV, NASH, cryptogenic cirrhosis, alcoholic liver disease, autoimmune hepatitis, cholestatic liver disease, primary HCC, and other. The MELD score was calculated from the patient’s most recent lab values prior to transplant (INR, creatinine, bilirubin) using the Mayo Clinic’s MELD Model calculator for health care professionals. It was also noted if the patient received a MELD exception for HCC. Pre-transplant laboratory values collected included the neutrophil to lymphocyte ratio (NLR), total serum cholesterol (mg/dL), and serum alpha fetoprotein (AFP). The values for the aforementioned labs recorded were the most recent prior to OLT; we also recorded the maximum value of serum AFP at any point prior to OLT. The number of days prior to transplant for which the HCC was diagnosed was determined by magnetic resonance imaging (MRI) or computed tomography (CT), and the subsequent opinion of the radiologist. Size criteria for the HCC tumors were stratified as follows: incidental finding on explant, within Milan criteria, initially beyond Milan criteria and successfully down-staged to within Milan, and beyond Milan criteria. Greatest tumor size on imaging was defined based on if the tumor was discovered incidentally on explant, the maximum diameter of the tumor was less than 5 centimeters, or the maximum diameter was greater than 5 centimeters. Most patients received pre-transplant, locoregional therapy, which included transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), and liver resection. The particular therapy, number of treatments received (for TACE), and the number of days prior to transplant that neo-adjuvant therapy was initiated were recorded as well. The surgical pathology report was reviewed for every case and tumor number, maximum tumor diameter, presence of vascular invasion, and tumor differentiation was recorded. Vascular invasion were divided as: none, microvascular, or macrovascular. Tumor differentiation was stratified as: well, moderate, poor, or unable to determine due to complete necrosis, and was also gathered from the explant pathology report. Biopsy proven episodes of acute rejection with a Rejection Activity Index (RAI) of at least 4 were noted as well as the subsequent treatment for the rejection episode.
The primary outcome collected for this study was the recurrence of HCC. If the HCC did recur, the number of days between transplant and diagnosis of recurrence was recorded as well as the site of recurrence. Patient and graft survival was also collected as an outcome and defined as: alive with no recurrence, alive with recurrence, deceased with recurrence, deceased with no recurrence, and deceased with cause of death unknown. The number of days between OLT and patient death or last follow up was also recorded.
Statistical analysis included standard descriptive statistics, including percentages for categorical variables and means, standard deviations, medians and interquartile ranges for continuous data. Univariate analysis was conducted using the two-sample independent t-test for continuous data and the chi square test for categorical data. Time to event analysis was conducted using Cox regression, reporting both unadjusted and adjusted hazard ratios for covariates of interest (with 95% confidence intervals). Backward elimination was utilized to remove variables from Cox models that failed to be predictive or associated with HCC recurrence. A two-sided p-value of <0.05 was considered statistically significant. All data was analyzed using SPSS version 20.0 (IBM Corp, Armonk, NY).
RESULTS
Between 2003 and 2015, 141 individuals underwent OLT for HCC. The median age was 58 years, 78% were male, and 80% were Caucasian (Table 1). Hepatitis C was the most common underlying diagnosis (67%), followed by NASH (13%) and cryptogenic cirrhosis (7%). MELD exemption points were given to 36% of patients. Neo-adjuvant therapy to treat HCC prior to transplant was utilized in 58% of patients, with 54% receiving TACE, 4% receiving radioembolization and 1% receiving liver resection. Pre-transplant radiographic findings demonstrated that the greatest tumor size prior to transplant was categorized as follows: >5cm in 2% of patients, <5cm in 93% of patients and an incidental finding on explant in 5% of patients. Pre-transplant tumors were found to be within Milan Criteria (85%), outside Milan but down-staged (6%), and outside Milan and not down-staged (4%).
TABLE 1.
Baseline pre-transplant recipient characteristics
| Characteristic | Number (%) | |
|---|---|---|
| Median Age in Years (IQR) | 58 (54, 63) | |
|
| ||
| Race | Caucasian | 113 (80%) |
| African-American | 18 (13%) | |
| Other | 10 (7%) | |
|
| ||
| Male Sex | 110 (78%) | |
|
| ||
| Primary Underlying Disease | Hepatitis B | 5 (4%) |
| Hepatitis C | 94 (67%) | |
| Non-Alcoholic Steatohepatitis | 18 (13%) | |
| Cryptogenic Cirrhosis | 10 (7%) | |
| Alcoholic Liver Disease | 5 (4%) | |
| Autoimmune Hepatitis | 2 (1%) | |
| Cholestatic Disease | 2 (1%) | |
| Other | 5 (4%) | |
|
| ||
| Received MELD Exemption | 50 (36%) | |
|
| ||
| Pre-Transplant HCC Neo-Adjuvant Therapy | 82 (58%) | |
| Transcatheter arterial Chemoembolization (TACE) | 76 (54%) | |
| Radioembolization | 5 (4%) | |
| Resection | 1 (1%) | |
|
| ||
| Number of times received TACE | 0 | 66 (47%) |
| 1 | 51 (36%) | |
| 2 | 20 (14%) | |
| 3 | 3 (2%) | |
| 4 | 1 (1) | |
|
| ||
| Size Criteria of Tumor | Incidental Finding on Explant | 7 (5%) |
| Within Milan Criteria | 115 (85%) | |
| Beyond Milan and Down-Staged | 8 (6%) | |
| Beyond Milan and not Down-Staged | 5 (4%) | |
|
| ||
| Greatest Tumor Size | Incidental | 7 (5%) |
| ≤5cm | 126 (93%) | |
| >5cm | 2 (2%) | |
Table 2 displays baseline biochemical characteristics. Median lab values (IQR) obtained most recently prior to the liver transplant included NLR (2 [2, 4]), total serum cholesterol (157 mg/dL [125, 194]) and AFP (9 ng/mL [4, 6]). Maximum value any time prior to transplant for AFP was 17 ng/mL (7, 82). The median MELD score at time of transplant was 13 points [10, 18]. In those patients who were documented to develop HCC recurrence, the median number of days between OLT and HCC recurrence was 588 days (272, 1160).
TABLE 2.
Biochemical and HCC tumor characteristics
| Baseline and Follow-Up Characteristics | Median (IQR) |
|---|---|
|
| |
| NLR most recent to transplant | 2 (2, 4) |
| MELD at transplant | 13 (10, 18) |
| Maximum AFP (ng/mL) prior to transplant | 17 (7, 82) |
| Most recent AFP (ng/mL) prior to transplant | 9 (4, 26) |
| Most recent serum cholesterol (mg/dL) prior to transplant | 157 (125, 194) |
| Days prior to transplant neo-adjuvant therapy started | 109 (0, 289) |
| Days prior to transplant HCC was diagnosed* | 232 (109, 403) |
| Number of days between OLT and HCC recurrence | 588 (272, 1160) |
Or date of referral to transplant center if unknown
Post-transplant pathologic findings after OLT that were assessed included tumor grade/differentiation, vascular invasion, and tumor number (Table 3). Tumor grade/differentiation was recorded as nuclear grade 1–2 and well differentiated (33%), nuclear grade 2–3 and moderately-poorly differentiated (41%), nuclear grade 4 and poorly differentiated (2%) and unknown grade due to complete necrosis (24%). Tumor number ranged from 0–9, with 82 patients (63%) having 1 tumor and 19 (15%), 18 (14%), 4 (3%), and 4 (3%) having 2, 3, 4, and ≥5 tumors, respectively. The majority of patients did not have vascular invasion (94%); those with microvascular or macrovascular invasion accounted for 4% and 2%, respectively. Interleukin-2 receptor antagonist (IL2RA) induction immunosuppression therapy was utilized in 19 patients (13%). Episodes of acute rejection post-transplant, as defined by a biopsy proven RAI of 4 or worse, occurred in 38 (27%) of the patients.
Table 3.
Post-Transplant recipient and tumor characteristics
| Characteristic | Number (%) | |
|---|---|---|
| Tumor Nuclear Grade/Differentiation | ||
| Grade 1–2/well differentiated | 39 (33%) | |
| Grade 2–3/poorly-moderately differentiated | 48 (41%) | |
| Grade 4/poorly differentiated | 2 (2%) | |
| Unknown due to complete tumor necrosis | 28 (24%) | |
| Vascular Invasion | None | 121 (94%) |
| Micro | 5 (4%) | |
| Macro | 3 (2%) | |
| Tumor Number | Incidental finding on explant | 4 (3%) |
| 1 | 82 (63%) | |
| 2 | 19 (15%) | |
| 3 | 18 (14%) | |
| 4 | 4 (3%) | |
| ≥5 | 4 (3%) | |
| IL2RA Induction Therapy | 19 (13%) | |
After OLT, 9 (6.4%) of 141 patients had documented recurrence of HCC. There were 3 recurrences found in the transplanted liver, 3 in the peritoneum, 2 in local lymph nodes and 1 in bone (lumbar vertebrae). Univariate analysis revealed that AFP difference between maximum and most recent (p=0.052), lower total pre-transplant serum cholesterol (p=0.019) and fewer days between neo-adjuvant therapy imitation and transplant (p=0.045) were associated with recurrence. A trend for HCC recurrence was demonstrated with an increased maximum tumor diameter, with the median maximum tumor diameter being 3 cm (2, 5) in the recurrence cohort and 2 cm (2, 3) in the non-recurrence cohort (p=0.082). Post-transplant predictors of recurrence included moderately-poorly graded/differentiated neoplasms (p=0.032), greater tumor number (p=0.040) and vascular invasion (p=0.002). A trend was observed with the use of IL2RA induction therapy (p=0.076, Table 4).
Table 4.
Univariate analysis assessing risk factors for HCC recurrence
| Characteristic | No HCC Recurrence (N=132) |
Recurrence of HCC (N=9) |
p-Value | |
|---|---|---|---|---|
| Median Age (IQR) | 58 (54, 63) | 60 (51, 66) | 0.709 | |
|
| ||||
| Race | Caucasian | 79% | 100% | 0.314 |
| African-American | 13% | 0% | ||
|
| ||||
| Male Sex | 78% | 89% | 0.430 | |
|
| ||||
| Median MELD at transplant (IQR) | 13 (10, 17) | 14 (12, 22) | 0.245 | |
|
| ||||
| Median maximum AFP (ng/mL) prior to transplant (IQR) | 17 (7, 74) | 14 (9, 30) | 0.747 | |
|
| ||||
| Median most recent AFP (ng/mL) prior to transplant (IQR) | 9 (4, 26) | 11 (9, 30) | 0.430 | |
|
| ||||
| Median most recent cholesterol (mg/dL) pre-transplant (IQR) | 158 (128, 194) | 113 (78, 155) | 0.019 | |
|
| ||||
| Median AFP difference (maximum – most recent, IQR) | 3 (0, 20) | 0 (0, 2) | 0.052 | |
|
| ||||
| Median days prior to transplant neo-adjuvant therapy initiated (IQR) | 122 (0, 291) | 0 (0, 20) | 0.045 | |
|
| ||||
| Median max tumor diameter (cm, IQR) | 2 (2, 3) | 3 (2, 5) | 0.082 | |
|
| ||||
| Tumor Nuclear Grade/Differentiation | 0.032 | |||
| Grade 1–2/well differentiated | 29% | 11% | ||
| Grade 2–3/poorly-moderately differentiated | 32% | 67% | ||
| Grade 4/poorly differentiated | 1% | 11% | ||
| Unknown due to complete tumor necrosis | 22% | 0% | ||
|
| ||||
| Vascular Invasion | None | 96% | 67% | 0.002 |
| Micro | 3% | 22% | ||
| Macro | 2% | 11% | ||
|
| ||||
| Tumor Number | Incidental finding on explant | 3% | 0% | 0.040 |
| 1 | 62% | 78% | ||
| 2 | 16% | 0% | ||
| 3 | 14% | 11% | ||
| 4 | 3% | 0% | ||
| >5 | 2% | 11% | ||
|
| ||||
| IL2RA Induction Therapy | 12% | 33% | 0.076 | |
Multivariable analysis demonstrated that total cholesterol <100mg/dL (HR=11.0, 95% CI=2.1–54.9, p=0.004), maximum tumor size (HR=0.17, 95% CI=0.02–1.64, p=0.125) and a poorly-moderately differentiated neoplasm (HR=6.01, 95% CI=0.69–52.2, p=0.104) produced the most parsimonious model for predicting recurrence risk. Total pre-transplant cholesterol <100mg/dl was the only statistically significant predictor of HCC recurrence risk (p=0.004, Figure 1).
Figure 1.
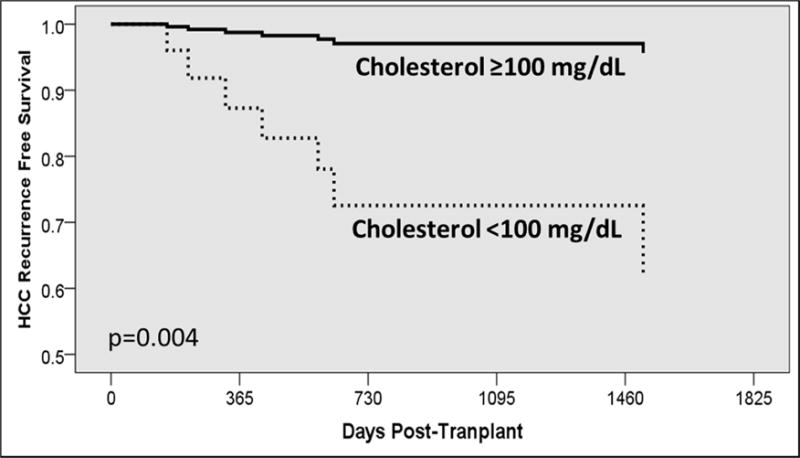
Kaplan-Meier curve estimating HCC-free survival, stratified by pre-transplant cholesterol
DISCUSSION
The results of this analysis provide evidence that a low (<100mg/dL) pre-transplant total serum cholesterol strongly predicts post-transplant HCC recurrence, suggesting that information beyond that of tumor quantity and size may be useful to risk-stratify patients requiring liver transplant for HCC. Currently, the Milan Criteria adopted in 1996 uses tumor size and number obtained via radiographic imaging to assess risk of HCC recurrence and to determine eligibility criteria. Although the Milan Criteria has been well demonstrated to be a working predictor of risk, it is limited by the sole use of radiographic findings, and some groups consider the Milan Criteria to be overly conservative.7,8 Additionally, a study by Hanouneh et al demonstrated that patients excluded from liver transplant because they did not meet the current Milan or the more liberal UCSF criteria can still have acceptable post-transplant outcomes due to favorable biology of the HCC. The authors concluded that tumor growth rate may be a useful tool in selecting patients with HCC outside of Milan criteria for liver transplantation.9 Likewise, another study concluded that the current Milan and UCSF criteria were insufficient in predicting patients at high and low risk for recurrence of HCC after OLT.10
It is important to differentiate between pre and post-transplant variables when determining risk. Understanding which pre-transplant variables, including laboratory inquiries (AFP, cholesterol, NLR,) and radiographic findings are most appropriate for predicting accurately risk of HCC recurrence, determining patient eligibility for transplant and setting initial follow-up protocols to assess for recurrence are needed. Post-transplant variables, including pathology assessed on the explanted liver and peri-operative clinical data may also help to understand the biology of the tumor and aid in determining a suitable, patient-centered, long-term follow-up protocol.11 Future large-scale studies with long-term follow up must assess both pre and post-transplant clinical and biochemical variables in order to better understand which sets of variables are most relevant in predicting risk.
Pre-transplant variables found to be associated with recurrence included total serum cholesterol, differences in AFP values pre-transplant, number of days prior to transplant that neo-adjuvant therapy was started and maximum tumor size; total serum pre-transplant cholesterol was found to be significant in multivariate analysis. A number of previous studies have investigated pre-transplant data and suggested that some variables may assist in predicting risk of HCC recurrence. One longitudinal cohort study demonstrated that serum levels of AFP, total pre-transplant cholesterol and NLR were significant predictors of HCC recurrence, while another study found serum levels of AFP, glutamate dehydrogenase and alkaline phosphatase to be early predictors of HCC recurrence.7,12 Both of these studies support serum AFP levels as a significant predictor (significant univariate and initial multivariate predictor in our study), while the former study found pre-transplant cholesterol as a significant predictor, which is analogous to our study. Because of the findings in our study as well as the findings in previous studies, the current selection criteria should be re-assessed, and biologic markers should be added to offer a better prediction model, which may allow for patients who do not meet Milan criteria to be eligible for transplant based on good prognostic biology.11
Post-transplant biomarkers that have been found to be predictors of lower recurrence-free survival include an increased serum C-reactive protein (CRP).13 Although we did not investigate CRP, our study did find that a moderately-poorly differentiated neoplasm and any type of vascular invasion to be post-transplant variables that were significant in our initial multivariate analysis of HCC recurrence predictors. Furthermore, a single institution, retrospective cohort study from the University of Washington found recurrences with single tumor size >4.5 cm, macro-invasion, and bi-lobar tumors to be significant predictors.14 This study did not investigate the effects of cholesterol on their patient population but did demonstrate that macrovascular invasion and a >4.5cm single tumor size were significant predictors. Taken in context with our results, it appears that several post-transplant characteristics are strong predictors of recurrence, which may assist clinicians in determining the optimal post-transplant follow-up protocol.
Serum levels of total cholesterol pre-transplant can predict the extent of liver damage; indeed, lower pre-operative serum cholesterol levels have predicted worse post-operative outcomes after partial hepatectomy in patients with HBV or HCV related HCC. The liver is the primary organ responsible in synthesizing cholesterol, thus a more severely damaged liver produces less cholesterol. This mechanistic understanding of cholesterol homeostasis supports our finding regarding a lower serum pre-transplant cholesterol level predicting recurrence of HCC after OLT and thus worse outcomes.15 Support for a lower serum cholesterol being correlated to aggressive tumor biology is also demonstrated in mouse models, where mice with active tumorigenesis had lower total serum cholesterol levels when compared to wild-type mice; likely because of the increased uptake of cholesterol by the actively dividing cancer cells.16 Furthermore, a study investigating gastrointestinal cancer and serum cholesterol levels suggested that tumor cells use and store increased amounts of cholesterol, thus a lower serum cholesterol may be associated with an aggressively proliferating tumor.17 Likewise, a cohort study with 97,000 participants from Japan reported that liver cancer mortality was greatest among those participants with the lowest LDL serum cholesterol levels.18 Another cohort study investigated the association between serum cholesterol and total cancer risk, and demonstrated that only liver cancer demonstrated an association between low serum cholesterol and cancer risk.19 These prior studies help explain the biology behind aggressive liver tumors and their association with lowering total serum cholesterol, thus reinforcing the findings of our study.
Limitations of this study include the low recurrence of HCC in this cohort of patients, which may reflect low baseline risk or lack of follow-up reporting. Further single institution studies would help provide a better understanding of this limitation as well as continued follow-up of patients to observe if later recurrence is more common. Second, because ours is a retrospective study, information obtained may be prone to misclassification due to differences in charting citations and procedures. Additionally, under reporting of some data was an issue, especially in the post-transplant pathologic reports for the explanted liver.
In conclusion, pre-transplant serum cholesterol (<100 mg/dL) was the strongest predictor of HCC recurrence after liver transplant. The results, taken in conjunction with previous literature, support adding biochemical markers to the current radiographic criteria clinicians use to assess transplant eligibility and may also help risk-stratify patients for appropriate post-transplant monitoring and follow-up.
Acknowledgments
Sources of financial support:
The research efforts conducted for this analysis were supported through a graft funded by the NIDDK (K23DK099440)
Abbreviations and Acronyms
- AFP
Alpha fetoprotein (ng/mL)
- HCC
Hepatocellular carcinoma
- IL2RA
Interleukin-2 receptor antagonist
- OLT
Orthotopic liver transplant
- MELD
Model for End-Stage Liver Disease
- Txp
Transplant
- NASH
Nonalcoholic steatohepatitis
- NLR
Neutrophil to Lymphocyte ratio
- UCSF
University of California, San Francisco
- TACE
Transcatheter arterial chemoembolization
- Neo-Adjuvant therapy
TACE/radioembolization
- IQR
Interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology Biomarkers Prev. 2010 Aug;19(8):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Kumar Vinay, Abbas Abul K, Fausto Nelson, Robbins Stanley L, Cotran Ramzi S. Robbins and Cotran pathologic basis of disease. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 3.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011 Oct;17(Suppl 2):S44–57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 4.Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013 Feb;26(2):109–18. doi: 10.1111/j.1432-2277.2012.01562.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizumi T, Harimoto N, Itoh S, Okabe H, Kimura K, Uchiyama H, Ikegami T, Ikeda T, Maehara Y. Living Donor Liver Transplantation for Hepatocellular Carcinoma within Milan Criteria in the Present Era. Anticancer Res. 2016 Jan;36(1):439–45. [PubMed] [Google Scholar]
- 6.Guerrini GP, Pinelli D, Di Benedetto F, Marini E, Corno V, Guizzetti M, Aluffi A, Zambelli M, Fagiuoli S, Lucà MG, Lucianetti A, Colledan M. Predictive value of nodule size and differentiation in HCC recurrence after liver transplantation. Surg Oncol. 2015 Sep 14; doi: 10.1016/j.suronc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 9.Hanouneh IA, et al. Rate of tumor growth predicts recurrence of hepatocellular carcinoma after liver transplantation in patients beyond Milan or UCSF criteria. Transplant Proc. 2011;43(10):3813–3818. doi: 10.1016/j.transproceed.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Dai Z, Wang Z, Fan J, Zhou J. Prognostic Indicators for Tumor Recurrence after Liver Transplantation in Hepatocellular Carcinoma and Related Molecular Targeted Therapy. Oncology. 2011;81(suppl 1):116–122. doi: 10.1159/000333273. [DOI] [PubMed] [Google Scholar]
- 11.Fahrner R, Dondorf F, Ardelt M, Dittmar Y, Settmacher U, Rauchfuß F. Liver transplantation for hepatocellular carcinoma - factors influencing outcome and disease-free survival. World Journal of Gastroenterology. 2015;21(42):12071–12082. doi: 10.3748/wjg.v21.i42.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piras-Straub K, Khairzada K, Gerken G, Saner F, Treckmann J, Paul A, Canbay A, Herzer K. Glutamate dehydrogenase and alkaline phosphatase as very early predictors of hepatocellular carcinoma recurrence after liver transplantation. Digestion. 2015;91:117–127. doi: 10.1159/000370212. [DOI] [PubMed] [Google Scholar]
- 13.Kornberg A, et al. Postoperative peak serum C-reactive protein is a predictor of outcome following liver transplantation for hepatocellular carcinoma. Biomarkers. 2015:1–8. doi: 10.3109/1354750X.2015.1118548. [DOI] [PubMed] [Google Scholar]
- 14.Chan EY, Larson AM, Fix OK, Yeh MM, Levy AE, Bakthavatsalam R, Halldorson JB, Reyes JD, Perkins JD. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: Implications for surveillance studies and new adjuvant therapies. Liver Transplant. 2008;14:956–965. doi: 10.1002/lt.21449. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Lau WY, Zhang B, Zhang Z, Huang Z, Luo H, Chen X. Preoperative total cholesterol predicts postoperative outcomes after partial hepatectomy in patients with chronic hepatitis B- or C-related hepatocellular carcinoma. Surgery. 2014;155:263–70. doi: 10.1016/j.surg.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Gemma Llaverias, Christiane Danilo, Isabelle Mercier, Kristin Daumer, Franco Capozza, Williams Terence M, Sotgia Federica, Lisanti Michael P, Frank Philippe G. Role of Cholesterol in the Development and Progression of Breast Cancer. Am J Pathol. 2011 Jan;178(1):402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dessi S, et al. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73(2):253. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Saito N, Sairenchi T, Irie F, et al. Low serum LDL cholesterol levels are associated with elevated mortality from liver cancer in Japan: the Ibaraki Prefectural health study. Tohoku J Exp Med. 2013;229(3):203–211. doi: 10.1620/tjem.229.203. [DOI] [PubMed] [Google Scholar]
- 19.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int J Cancer. 2009;125:2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]


