Abstract
Objective
To determine the prevalence of sarcopenia in stroke survivors using different methodologies and compared a subset of the stroke group to age, sex, and BMI-matched non-stroke control counterparts.
Design
Cohort Study
Setting
Veterans Affairs Medical Center and University Hospital
Participants
Mild to moderately disabled chronic participants with stroke aged 40–84 yrs (n=190, 61% male, 57% African American, BMI: 29±1 kg/m2, X±SEM)
Interventions
Not applicable
Main Outcome Measures
DXA scans to assess appendicular lean mass (ALM). Rates of sarcopenia were determined using four established methods: 1) ALM/ht2; 2) European Working Group on Sarcopenia in Older Persons; 3) International Working Group on Sarcopenia; and 4) ALM/BMI.
Results
Sarcopenia prevalence in our stroke cohort ranged between 14% and 18%. The stroke survivor subset (n=38) matched one-for-one with control counterparts for race, sex, age±4 years and BMI±2.5 units had higher prevalence rates compared to their non-stroke counterparts (13.2% vs. 5.3%, P<0.0001). ALM/ht2 was related to six-minute walking speed (r=0.28, P<0.01) and VO2peak (l/min r=0.58, P<0.0001) for the stroke group.
Conclusions
Stroke survivors show an elevated prevalence of sarcopenia when considering age, sex, and race to non-stroke individuals.
Keywords: sarcopenia, frailty, exercise, muscles, stroke
The prevalence of stroke, a leading cause of disability in adults, is expected to substantially increase over the next two decades1. Approximately 50% of stroke patients suffer some degree of hemiparesis, rendering 30% unable to walk without an assistive device, and frequently resulting in long-term disability2. After a stroke, the progression and consequences of muscle wasting may be compounded by their relative inactivity, reduced strength, and lower fitness levels. Our previous findings indicate that the paretic thigh of stroke survivors has 20–24% lower muscle area and muscle volume and 17–25% higher intramuscular fat than the non-paretic thigh3,4. Furthermore, we have previously demonstrated that reduced muscle mass and greater severity of hemiparetic gait deficits are independent determinants of lower peak fitness5, suggesting that the primary components of sarcopenia after stroke translate directly into altered functional capacity and frailty.
Sarcopenia, originally described as a general loss of muscle mass with age6, is now more precisely characterized using clinical cutpoints that utilize lean mass measured by dual-energy x-ray absorptiometry and comparisons to a young reference population7. The definitions of sarcopenia continue to evolve by accounting for body size or body fat, strength and function8–11. Although the prevalence of sarcopenia has been reported in the literature using accepted cut point methodologies12, prevalence studies that are exclusive to stroke are limited. Given that sarcopenia increases the risk for subsequent injury and disability7,13,14 and predicts hospitalization and death15, it would be highly clinically relevant to arrive at a better understanding of sarcopenia prevalence in this at-risk population.
We tested the hypothesis that stroke survivors would have higher prevalence of sarcopenia than non-stroke controls. Thus, the aim of this study was to 1) determine the prevalence of sarcopenia in a large chronic stroke sample and 2) compare the prevalence of sarcopenia in a subset of our stroke group to age, sex and BMI-matched non-stroke controls.
Methods
Subject Selection
Stroke survivors (n=190, 116 men, 74 women) between 40–84 years and BMI between 16.5–44.6 kg/m2 (range underweight to morbid obesity) with residual hemiparetic deficits >six months after onset of ischemic stroke participated in the study. Subjects were recruited through local advertisements, flyers and stroke clinics at the University of Maryland School of Medicine and Baltimore VA Medical Center. All participants with stroke had mild to moderate hemiparetic gait deficits characterized by reduced stance or reduced stance with increased swing duration, and had completed conventional rehabilitation therapy. All subjects lived independently in the community and were able to walk six minutes with their usual assistive device (s) without human assistance. Ambulation was the primary means of mobility for participants with stroke. Use of an ankle-foot orthosis (AFO) or assistive device was documented. Evaluations included medical history, physical examination, fasting blood profile, and screening for dementia16 and depression17 to ensure adequate informed consent. Participants with stroke were excluded if they had unstable angina, congestive heart failure (NYHA II), severe peripheral arterial disease, end-stage organ disease, major post-stroke depression, dementia, severe receptive aphasia, orthopedic or chronic pain conditions. Healthy control counterparts had the same exclusion criteria as participants with stroke and were 46–80 years of age with a BMI between 21–42 kg/m2. All subjects were sedentary as defined by <20 min low intensity exercise 3x/week.
The Institutional Review Board of the University of Maryland and the Baltimore VA Research & Development Committee approved all methods and procedures. Each participant provided written informed consent.
Exercise and Functional Tests
Exercise testing with open circuit spirometry was conducted to measure VO2peak using a graded treadmill test18. A standardized six-minute walk test recorded the distance traveled, with participants with stroke walking at their comfortable self-selected walking speed using their usual assistive devices to determine gait speed. Seventeen participants with stroke did not have VO2peak testing and 22 were missing the six-minute walk test.
Body Composition and Sarcopenia
Height (cm) and weight (kg) were measured. Fat mass, lean tissue mass and %body fat were determined by DXA (Prodigy and iDXA LUNAR GE).
Four definitions for sarcopenia were applied to classify sarcopenia7–11. These include the definition as appendicular lean mass/height2, ALM/ht2 <7.26 kg/m2 in men and <5.45 kg/m2 in women7. Second, sarcopenia as agreed to by the European Working Group on Sarcopenia in Older People (EWGSOP)8, includes gait speed <0.8 m/s and ALM <7.23 kg/m2 in men and ALM <5.67 kg/m2 in women. Third, the International Working Group on Sarcopenia (IWG) defines sarcopenia as gait speed <1.0 m/s and ALM/ht2 ≤ 7.23 kg/m2 in men and ≤ 5.67 kg/m2 in women9. Fourth, ALM divided by BMI was calculated to discriminate the presence or absence of weakness with cutpoints as follows: ALM/BMI <0.789 in men and ALM/BMI <0.512 in women11. Finally, in order to eliminate potential confounders of sarcopenia, a matched pair analysis was performed wherein stroke survivors were matched with non-stroke control counterparts on the basis of race, sex, age ± 4 years and BMI ± 2.5 kg/m2.
Statistical Analyses
Descriptive statistics were analyzed using SPSS (PASW Statistics, Version 18, Chicago, IL). Differences by sex and between stroke and non-stroke control counterparts for all variables were determined using unpaired Student t-tests and variances compared using Levine’s test for equality of variances. Relationships between variables were determined by linear regression analyses with calculation of Pearson product moment correlation coefficients. Statistical assumptions are verified through tests of normality by the Kolmogorov-Smirnov Test and random sampling of subjects. Sample size analyses conducted assuming a significance of 5% and 80% power indicate that the effect size (f2) for the regression analyses ranged from 0.085–0.136, suggesting the need for sample sizes ranging from 27–143 stroke subjects, which is within the 190 subjects. Data are presented as means±SEM. P values <0.05 are statistically significant.
Results
Physical Characteristics (Table 1)
Table 1.
Body Composition and Physical Function (n=190)
| Women X±SEM |
Men X±SEM |
Total Group X±SEM |
Total Group Range |
|
|---|---|---|---|---|
|
| ||||
| Age (yr) | 62 ± 1 | 63 ± 1 | 63 ± 1 | (40 – 84) |
| Weight (kg) | 77.4 ± 1.9 | 85.2 ± 1.4* | 82.2 ± 1.2 | (40 – 134) |
| BMI (kg/m2) | 29.1 ± 0.7 | 28.3 ± 0.5 | 28.6 ± 0.4 | (16 – 45) |
| VO2peak (L/min) (n=173) | 0.91 ± 0.05 | 1.40 ± 0.05 | 1.21 ± 0.04 | (0.29 – 3.29) |
| VO2peak (ml/kg/min) (n=173) | 11.8 ± 0.6 | 16.2 ± 0.5 | 14.5 ± 0.4 | (4.6 – 34.2) |
| Gait speed (m/s) (n=168) | 0.56 ± 0.04 | 0.72 ± 0.04* | 0.66 ± 0.03 | (0.07 – 1.51) |
| Body fat (%) | 43.4 ± 0.7 | 30.5 ± 0.6† | 35.6 ± 0.6 | (11 – 58) |
| Fat mass (kg) | 34.1 ± 1.3 | 26.6 ± 0.9† | 29.5 ± 0.8 | (7.67 – 64.2) |
| Lean mass (kg) | 40.6 ± 0.8 | 55.3 ± 0.7† | 49.5 ± 0.8 | (27.0 – 75.6) |
| Paretic leg lean mass (kg) | 6.58 ± 0.18 a | 8.96 ±0.16† b | 8.05 ± 0.15 b | (3.9 – 13.1) |
| Non-Paretic leg lean mass (kg) | 6.73 ± 0.17 | 9.32 ± 0.15† | 8.32 ± 0.15 | (4.4 – 14.4) |
| Appendicular lean mass (kg) | 17.8 ± 0.4 | 25.0 ± 0.4† | 22.2 ± 0.4 | (11.2 – 36.9) |
| ALM/ht2 (kg/m2) | 6.69 ± 0.15 | 8.29 ± 0.12† | 7.66 ± 0.11 | (4.60 – 11.89) |
BMI= body mass index; ALM=appendicular lean mass
Men vs. Women,
P <0.005;
P <0.0001
Paretic vs. Non-Paretic,
P<0.05;
P<0.0001
Subjects with stroke are 61% male and 39% female. The group is also racially mixed with 42% Caucasian (n=78), 57% African-American (n=108), and the remaining 2% (n=4) other nationalities. Latency since stroke averaged 51 ± 7 months (range 6 – 375 months). Twenty-three percent of participants with stroke had diagnosed type 2 diabetes upon study entry. Approximately 57% of participants with stroke used a single point cane, 14% used a quad cane, 14% used a walker, and 15% did not use any assistive device for ambulation and 50% used an AFO. Men have higher body weight than women (P<0.005) but BMI is not different. Women have higher percent body fat and fat mass (P<0.0001) and lower total lean mass, paretic and non-paretic lean mass (P<0.0001) than men. ALM and ALM/ht2 are higher in men than women (P<0.0001). VO2peak (l/min) is 1.3 fold-higher in men than women (P<0.0001) but is not significantly different when expressed per body weight (ml/kg/min). Gait speed is also ~1.3 fold higher in men than women (P<0.005). Paretic leg lean mass by DXA is lower than non-paretic leg lean mass in the total group (P<0.0001), and in both men (P<0.0001) and women (P<0.05).
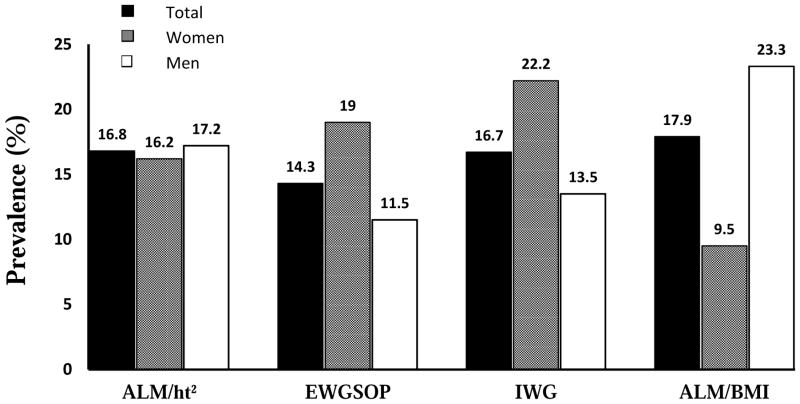
The total prevalence of sarcopenia, independent from sex, ranged from 14.3% – 17.9%, depending on the method used for the diagnosis (Figure 1). The lowest prevalence in the total group is found when using the EWGSOP criteria (14.3%) and the highest prevalence is found when using the ALM/BMI criteria (17.9%). When stratified by sex, the prevalence of sarcopenia is greater in women than men using the criteria of EWGSOP (19 vs.11.5%) and the IWG (22.2 vs.13.5%) but higher in men than women by ALM/BMI (23.3 vs. 9.5%) criteria. By the ALM/ht2 criteria, there is the smallest difference by sex (men vs. women: 17.2 vs. 16.2%).
Figure 1.
The prevalence of sarcopenia in the total group, in women, and in men with criteria of appendicular lean mass (ALM)/ht2, the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group (IWG), and ALM/body mass index.
The prevalence of sarcopenia in the total group increases with age using all the criteria except the IWG (Table 2). The highest prevalence in individuals over the age of 60 years is 23% using the ALM/BMI criteria and is twice that of individuals younger than 60 years (10%). In a breakdown by age and sex, the prevalence of sarcopenia is slightly greater in women and men older than 60 years (both 18%) than those younger than 60 years by ALM/ht2 (15 and 16%, respectively) By the EWGSOP, prevalence is similar between age groups in men (12vs. 11%) but about 6% higher in older than younger women. In contrast, the prevalence of sarcopenia with the IWG criteria is slightly higher in both men and women younger than 60 than those older than 60 years of age. With the ALM/BMI criteria, the prevalence increases with age in men but declines in women.
Table 2.
Sarcopenia Status
| Women | Men | Total Group | |
|---|---|---|---|
| Stroke | |||
| ALM/ht2 (n=190) | |||
| < 60 yrs (n=77) | 15% (n=5) | 16% (n=7) | 16% (n=12) |
| ≥ 60 yrs (n=113) | 18% (n=7) | 18% (n=13) | 18% (n=20) |
| EWGSOP (n=168) | |||
| < 60 yrs (n=72) | 16% (n=5) | 12% (n=5) | 14% (n=10) |
| ≥ 60 yrs (n=96) | 21% (n=7) | 11% (n=7) | 15% (n=14) |
| IWG (n=168) | |||
| < 60 yrs (n=72) | 23% (n=7) | 15% (n=6) | 18% (n=13) |
| ≥ 60 yrs (n=96) | 21% (n=7) | 13% (n=8) | 16% (n=15) |
| ALM/BMI (n=190) | |||
| < 60 yrs (n=77) | 13% (n=4) | 10% (n=4) | 10% (n=8) |
| ≥ 60 yrs (n=113) | 9% (n=3) | 33% (n=21) | 23% (n=26) |
ALM=appendicular lean mass; EWGSOP: European Working Group on Sarcopenia in Older People; IWG=International Working Group; BMI= body mass index
The non-stroke control counterparts are well matched to the stroke group (n=38 per group) in terms of age (controls: 63 ± 1 years, range 46–80 years and stroke: 63 ± 1 years, range 49–81 years) and BMI (controls: 32 ± 1 kg/m2, range 21–42 kg/m2 and stroke: 32 ± 1 kg/m2, range 21–41 kg/m2). None of the control subjects had type 2 diabetes and 31% of the matched stroke group had diagnosed type 2 diabetes. After matched pair analysis, 5.3% of the control counterparts have sarcopenia while 13.2% of the stroke group have sarcopenia (P<0.0001). Lean mass of both the paretic and non-paretic leg are lower than lean mass of the right side of the control counterparts (both P<0.0001), suggesting that the frequency of the sarcopenia did not result only from the loss of muscle mass of the affected side.
ALM/ht2 is related to gait speed (r=0.28, P<0.0001) and VO2peak (l/min r=0.58, P<0.0001 and ml/kg/min r=0.26, P<0.0005) independent of sex in the stroke population. Likewise, ALM/BMI is related to gait speed (r=0.34, P<0.0001) and VO2peak (l/min r=0.47, P<0.0001 and ml/kg/min r=0.48, P<0.0001) independent of sex.
Discussion
The present study is the first to show that the prevalence of sarcopenia in stroke varies between 14% and 18%. Further, we show differences in sarcopenia by sex. When matched to non-stroke control counterparts, stroke survivors may be at an elevated risk for sarcopenia when considering age, sex, and race.
Sarcopenia is shown to increase with age19 with loss of muscle mass associated with an increased risk of incident disability and all-cause mortality13. The increase in mortality risk with sarcopenia is independent of age, sex, education, activities of daily living, and disease19. Sarcopenia also leads to higher health care costs in the US20. Risk factors for sarcopenia include advancing age, low levels of physical activity, inadequate nutrition, and co-morbid disease (i.e. type 2 diabetes mellitus and end-stage organ diseases)13.
We find a 14–18% prevalence rate in the group as a whole. Our findings in stroke survivors generally fall within the sarcopenia prevalence range (1–29%) reported in other populations12; however, prevalence rates can depend on the age of the subjects and the diagnostic criteria. In the Leiden Longevity Study in the Netherlands21, there was a 0% to ~45% prevalence rate depending on the criteria. Community-dwelling men in the Hertforshire Cohort Study in the United Kingdom22 have a prevalence of <7% using the EWGSOP definition. In a report of individuals aged 65 and older in Belgium, prevalence rates of sarcopenia varied between 9% and 18% 23. In comparison to our study, the prevalence of sarcopenia for our comparable age group of individuals with stroke (those 60 years and older), is slightly higher, ranging between 15 and 23%. Furthermore, results from our study are in line with reports whose subjects have a mean age that is higher than ours of 63 years suggesting that individuals with stroke are at a higher risk for sarcopenia at a younger age. For example, in persons older than 80 years in the BELFRAIL study in Belgium, the 12.5% prevalence of sarcopenia by EWGSOP24 is slightly lower than the prevalence in our stroke cohort despite the fact that our mean age is almost 20 years younger. Furthermore, in the iISIRENTE study, Italians aged 80 and older have a ~22% sarcopenia prevalence using the EWGSOP criteria19. In another study, women and men older than 80 years have prevalence rates of sarcopenia of ~32% and 17%, respectively25. Comparing these results to ours suggests that the consequences of stroke likely outweigh the aging associated increase in sarcopenia.
The sarcopenia definition from Cawthon et al.11 allowed us to examine the prevalence of sarcopenia that discriminates weakness. With this method, we find the greatest variance in prevalence between men and women stroke survivors, e.g. about a two-fold greater prevalence in men than women. Despite not having measured grip strength to determine if this influences the results, we have previously shown that paretic leg strength is significantly lower than the non-paretic leg4,26. Presumably grip strength is likewise reduced on the paretic side. Further, we have previously show that hemiparesis is associated with lower gait speed in subjects with stroke.27 Thus, it is reasonable to suggest that any calculation of sarcopenia prevalence involving grip strength and gait speed would be influenced in stroke.
In addition to the physical inactivity and disuse observed after stroke, we can speculate some possible skeletal muscle mechanisms for the elevated prevalence of sarcopenia observed in this stroke population. In this larger sample, we confirm our previous findings3 of significantly lower lean tissue mass in the paretic compared to the non-paretic leg by DXA which also supports our findings of lower muscle area and muscle volume by computed tomography on the paretic leg.3,4 Increased infiltration of adipose tissue in skeletal muscle can occur during the atrophy process in stroke and is higher in paretic than non-paretic skeletal muscle3,4. Not only could the increased intramuscular fat impact the amount of lean mass, higher intramuscular fat is associated with slow gait speed28; thus, two contributors to sarcopenia definitions could be affected. Sarcopenia in stroke could also potentially be the result of increased skeletal muscle protein breakdown or a decrease in skeletal muscle protein synthesis. Our findings of 40% higher myostatin mRNA levels in the paretic vs. nonparetic vastus lateralis skeletal muscle suggest a protein synthesis/breakdown imbalance26. Yet, myostatin levels are modifiable and our data indicates that resistive training results in a 49% reduction in myostatin in stroke paretic skeletal muscle26. Inflammatory processes could also play a role in sarcopenia in stroke as tumor necrosis factor-α mRNA levels is increased on the paretic leg compared to controls29.
Study Limitations
Limitations of this study include the lack of grip strength measures, stratification by comorbid conditions (type 2 diabetes), and investigator blinding to stroke and control groups, and a generally lower age range than some population studies. Another limitation is that the participants with stroke and the control cohorts were not matched on diabetes status which could confound the comparison between these two groups. A larger sample size may provide greater power for examining the relationships between sarcopenia and fitness. However, this is a chronically disabled and unique study population in terms of examining sarcopenia. The subjects studied represent a racially and sex mixed group, which is statistically similar to stroke survivors in the U.S.30 Moreover, a broad range of body composition and gait speed represent strengths. It is also important to recognize that the control counterparts were sedentary as were the participants with stroke. Sedentary behavior is associated with sarcopenia in community-dwelling men and women 60–86 years of age31. The measurement of gait speed and its relationship with ALM/ht2 indicates that functional impairment is one factor related to sarcopenia in stroke, although deficits could be related to other central motor and sensory factors such as spasticity.
Conclusions
Future studies could be directed at interventions such as exercise to reduce sarcopenia in stroke survivors as well as include an in-depth examination of the mechanisms for the muscle atrophy and weakness observed in this disabled population. In conclusion, stroke survivors show an elevated prevalence of sarcopenia when considering age, sex, and race to non-stroke individuals, which are related to reduced fitness in this vulnerable population.
Acknowledgments
This work was presented at the International Stroke Conference, American Heart Association, 2014.
Our appreciation is extended to those stroke subjects who participated in this study.
Financial Support: This work was supported by: VA Senior Research Career Scientist Award, VA Merit Awards (Veterans Affairs), National Institutes of Health (R01-AG030075 and the Claude D. Pepper Older Americans Independence Center, P30AG028747), and the Baltimore VA Geriatric Research, Education, and Clinical Center (GRECC), and VA Rehabilitation Research & Development Maryland Exercise and Robotics Center of Excellence (B3688R; RR&D MERCE).
Abbreviations
- BMI
body mass index
- DXA
dual-energy x-ray absorptiometry
- ALM
appendicular lean mass
- EWGSOP
European Working Group on Sarcopenia in Older Persons
- IWG
International Working Group on Sarcopenia
- VO2peak
peak oxygen consumption
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013 Aug;44(8):2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 2.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003 May-Jun;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002 Dec;83(12):1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabilitation and neural repair. 2011 Nov;25(9):865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan ASDC, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: role of muscle mass and gait deficit severity. J Stroke Cerebrovasc Dis. 2000;9:185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 6.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993 Feb;123(2 Suppl):465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010 Jul;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011 May;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011 Jul;12(6):403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014 Nov;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010 Jan;13(1):1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- 14.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007 May;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi L, Ferrucci L, Cherubini A, et al. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2015 Sep 2; doi: 10.1093/gerona/glv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979;88(2):174–181. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 18.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke; a journal of cerebral circulation. 1997 Feb;28(2):326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 19.Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012 Jan;67(1):48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004 Jan;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 21.Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age. 2013 Jun;35(3):871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013 May;42(3):378–384. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudart C, Reginster JY, Slomian J, Buckinx F, Locquet M, Bruyere O. Prevalence of sarcopenia: the impact of different diagnostic cut-off limits. Journal of musculoskeletal & neuronal interactions. 2014 Dec;14(4):425–431. [PubMed] [Google Scholar]
- 24.Legrand D, Vaes B, Mathei C, Swine C, Degryse JM. The prevalence of sarcopenia in very old individuals according to the European consensus definition: insights from the BELFRAIL study. Age Ageing. 2013 Nov;42(6):727–734. doi: 10.1093/ageing/aft128. [DOI] [PubMed] [Google Scholar]
- 25.Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2014 Apr;69(4):438–446. doi: 10.1093/gerona/glt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011 Feb;42(2):416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88(1):115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Addison O, Drummond MJ, LaStayo PC, et al. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. The journal of nutrition, health & aging. 2014 May;18(5):532–538. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafer-Macko CE, Yu S, Ryan AS, Ivey FM, Macko RF. Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke. 2005;36(9):2021–2023. doi: 10.1161/01.STR.0000177878.33559.fe. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015 Jan 27;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 31.Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int. 2015 Feb;26(2):571–579. doi: 10.1007/s00198-014-2895-y. [DOI] [PubMed] [Google Scholar]