Abstract
To investigate the prevalence of phonatory symptoms, perceptual, acoustic and aerodynamic findings in patients with asthma compared to a control group. This study is a cross-sectional study. A total of 50 subjects, 31 asthmatic and 19 control subjects matched according to age and gender were enrolled in this study. All subjects were asked about the presence or absence of dysphonia, vocal fatigue, phonatory effort, cough, dyspnea, and respiratory failure. Perceptual evaluation, acoustic analysis and aerodynamic measurements were also performed. Patient’s self assessment using the Voice Handicap Index 10 was reported. The mean age of patients was 43.5 years with a female to male ratio of 2:1. There was a statistically significant difference in the prevalence of dysphonia between the two groups (32.3 vs. 5.3%, p value 0.025) with a non-significant higher prevalence of vocal fatigue and phonatory effort. The overall grade of dysphonia was significantly higher in asthmatics compared to controls (p value 0.002). Patients with asthma had also significantly higher degree of asthenia and straining (p value of 0.04 and 0.008, respectively) with borderline significant difference with respect to roughness. There was no significant difference in the means of any of the acoustic parameters between patients and controls except for Shimmer, which was higher in the asthmatic group (p value of 0.037). There was also no significant difference in the Maximum phonation time between the two groups. Dysphonia is significantly more prevalent in patients with asthma compared to controls.
Keywords: Asthma, Dysphonia, Voice disorders, Respiration, Acoustics
Introduction
Asthma is a common small airway disease that affects 4–5% of the general population [1]. It is characterized by impairment in expiration and inspiration with increased resistance to breathing. Patients suffer from airway obstruction with marked increase in the viscosity of the respiratory secretions. These changes can interfere with phonation since expelling of air is a major requisite for voice production. Adding to these variables, are the confounding effects of allergy, sinusitis and the adverse reactions of inhalers used in the treatment of asthmatic patients [2].
There are ubiquitous reports in the literature on upper airway diseases and laryngeal function, with a relatively small number of studies on the impact of lower airway diseases, specifically asthma, on voice [3–5]. Most of the reports on dysphonia in asthmatics have addressed dysphonia as a side effect to the usage of steroid inhalers in these patients. Based on a thorough review of the literature few reports have described the phonatory symptoms and only three have reported the acoustic and aerodynamic measurements in patients with asthma [3, 5–9]. The prevalence of dysphonia in these reports varied with more than 50% of patients reporting change in voice quality. The results on the acoustic analysis were conflicting with some reporting an increase in the perturbation parameters and others no significant differences in comparison to a controlled group. The discrepancies in the results of these reports can partially be attributed to differences in the group selection, severity of the disease, use of steroid inhalers and or the presence of confounding factors such allergic rhino-sinusitis which were not accounted for in the analysis.
The purpose of this study is to cast more light on vocal quality of patients with asthma. The authors investigated the prevalence of phonatory symptoms and reported the perceptual evaluation, acoustic and aerodynamic analysis of patients with asthma compared to an age and gender matched controlled group taking into consideration the confounding effect of allergy and smoking.
Methods
This study was approved by the Institutional Review Board. Informed consent was obtained from all individual participants included in this study.
A total of 50 subjects, 31 asthmatic and 19 control subjects matched according to age and gender were enrolled in this study. The diagnosis of asthma was based on the clinical assessment of the senior author of this manuscript, namely the presence of characteristic symptoms of “episodic breathlessness, wheezing, cough and chest tightness”, supported by lung function measurements [10]. Subjects with respiratory tract infection, laryngeal manipulation and or vocal fold pathologies were excluded from the study. Demographic data included age, gender, status of asthma i.e., controlled vs. uncontrolled, use of steroid inhalers, history of smoking and history of allergy. The control of disease was based on “Asthma Control Test” a validated questionnaire mentioned in the GINA 2012 report [10]. Allergic rhinitis was evaluated using a standardized validated questionnaire [11]. In view of the confounding effect of allergy and smoking, the authors made sure that the prevalence of either was similar in both patients and controls.
All subjects were asked about the presence or absence of dysphonia, vocal fatigue and increase in phonatory effort. Dysphonia was defined as change in voice quality, pitch, loudness, and or timbre. Vocal fatigue was defined as tiring of the voice after vocal loading or towards the end of the day. Vocal effort was defined as the effort needed to initiate phonation. Asthmatic patients were also asked about the presence or absence of cough, dyspnea and history of respiratory failure. The frequencies and means of the phonatory symptoms were computed.
All patients underwent perceptual evaluation of their voice by a senior speech language pathologist who graded their voice using the GRBAS classification [12]. A scoring system from 0 to 3 was used. The subjective evaluation included also the patient’s self assessment using the Voice Handicap Index 10, a simplified version of the Voice Handicap Index 30 [13, 14].
All patients underwent acoustic analysis using VISI-PITCH IV by Kay Pentax (Montvale, NJ, USA) [15]. The following acoustic variables were measured, Average Fundamental frequency, Habitual pitch, Jitter, shimmer, Noise to Harmony ratio, Voice Turbulence Index.
The aerodynamic measurements included the Vital Capacity, FEV1, FEV1/FVC, maximum phonation time MPT, and the phonatory quotient PQ. The Vital capacity and FEV1 were retrieved from the pulmonary function tests performed on patients with asthma. The maximum phonation time was measured by asking the patient to take a deep breath and phonate for as long as he or she can. The Real Time module of VISI-PITCH IV by Kay Pentax was used for this task. The phonatory quotient defined by the ratio of Vital capacity to Maximum phonation time was computed for patients.
Results
Demographic Data
The mean age of patients with asthma was 43.5 years with a female to male ratio of 2:1. Close to 50% had allergic rhinitis and 29% were smokers. Fifty eight per cent were using steroid inhalers at the time of enrollment. See Table 1.
Table 1.
Demographics
| Patients (N = 31) | Controls (N = 19) | |
|---|---|---|
| Age | 43.5 ± 16.9 | 39.0 ± 13.5 |
| Gender | ||
| Females | 21 (67.7%) | 12 (63.2%) |
| Males | 10 (32.3%) | 7 (36.8%) |
| Smoking | 9 (29%) | 6 (31.6%) |
| Allergy rhinitis | 15 (48.4%) | 8 (42.1%) |
| Controlled asthma | 12 (41.4%) | N/A |
| Uncontrolled asthma | 17 (58.6%) | N/A |
| Steroid intake | 18 (58.1%) | N/A |
Respiratory and Phonatory Symptoms in Patients and Controls
There was a significant difference in the prevalence of respiratory symptoms, namely cough and dyspnea between the two groups. Eighty percent of asthmatic patients had cough compared to only 5.6% of controls (p value <0.05). Similarly, 87% of patients with asthma had mild to moderate dyspnea compared to 5.6% of controls (p value <0.05).
With respect to phonatory symptoms, there was a statistically significant difference only in the prevalence of dysphonia between the two groups (32.3 vs. 5.3%, p value 0.025). See Table 2.
Table 2.
Respiratory and phonatory symptoms in patients vs. controls
| Patients (N = 31) | Controls (N = 19) | p value | |
|---|---|---|---|
| Cough | 24 (80%) | 1 (5.6%) | <0.05 |
| Dyspnea | 27 (87.1%) | 1 (5.6%) | <0.05 |
| Respiratory failure | 0 (0%) | 0 (0%) | 0.197 |
| Dysphonia | 32.3% | 5.3% | 0.025 |
| Vocal fatigue | 41.9% | 21.1% | 0.13 |
| Phonatory effort | 32.3% | 10.5% | 0.081 |
Statistically significant values are in bold
Correlation Between Phonatory Effort, Vocal Fatigue, Dysphonia, and Cough and Dyspnea
There was a weak and non-significant correlation between the phonatory symptoms namely: Phonatory effort, Vocal fatigue and Dysphonia, and the respiratory symptoms: Cough and Dyspnea in both Patients and the Controls (all r values were below r < 0.3).
Perceptual Evaluation and Voice Handicap Index-10
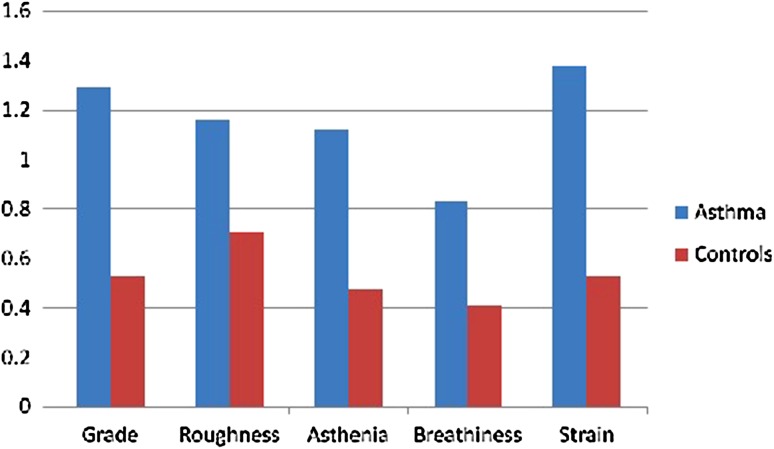
There was a significant difference in three out of the five perceptual parameters between the two groups. The overall grade of dysphonia was significantly higher in asthmatic compared to controls (p value of 0.002). Patients with asthma had also significantly higher degree of asthenia and straining with borderline significant difference with respect to roughness (p values of 0.04, 0.008 and 0.052 respectively). See Table 3 and Fig. 1
Table 3.
Means for grade, roughness, asthenia, breathiness, and strain in patients (N = 25)* and controls* (N = 17)
| Patients (N = 25) | Controls (N = 17) | p value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Grade | 1.29 ± 0.55 | 0.53 ± 0 .79 | 0.002 |
| Roughness | 1.16 ± 0.75 | 0.71 ± 0.69 | 0.052 |
| Asthenia | 1.13 ± 1.08 | 0.47 ± 0.79 | 0.040 |
| Breathiness | 0.83 ± 0.87 | 0.41 ± 0.61 | 0.094 |
| Strain | 1.38 ± 1.01 | 0.53 ± 0.87 | 0.008 |
Statistically significant values are in bold
Fig. 1.
Mean score of perceptual evaluation parameters in patients vs. controls
There was no significant difference in the mean score of VHI-10 in patients vs. controls (p value 0.149).
Acoustic Analysis
There was no significant difference in the means of any of the acoustic parameters between patients and controls except for shimmer which was significantly higher in the asthmatic group (4.81 ± 2.37 vs. 3.47 ± 1.52; p value: 0.037). Even when stratified by gender, still there was no significant difference in the average fundamental frequency and habitual pitch between the two groups (p value: 0.09 and 0.732 respectively).
Aerodyanmic Measurements
The values of VC, PQ and FEVI/FVC were as follows: Vital Capacity VC (L) = 4.41 ± 1.13, Forced Expiry Volume FEV1 (L) = 99.73 ± 23.61, Ratio of Forced Expiry Volume to Forced Vital Capacity FEV1/FVC = 74.39 ± 14.87 and the Phonatory quotient VC/MPT (L/s) = 0.33 ± 0.12. There was no significant difference in the Maximum phonation time between the two groups (p value 0.75).
Discussion
Asthma is a chronic disease of the airway. It is characterized by airway inflammation, bronchial hyper-responsiveness, and episodic airflow obstruction [16]. Inflammation leads to airway thickening and remodeling with progression of the disease to a chronic state [17]. The clinical manifestation includes recurrent cough, wheezing, dyspnea, and chest tightness, with a nocturnal predilection of these symptoms [10]. Asthma is known to be associated with a number of comorbidities, among which are allergic rhinitis, obesity, depression, diabetes mellitus, cardiovascular disease and gastro-esophageal reflux disease. Co-morbidity refers to either co-existing condition or interacting condition that carries a significant influence on management. As such, control of asthma requires proper diagnosis and effective treatment of these co-existing conditions. A review by Ledford and Lockey [18] highlights the importance of optimizing the management of these co-morbidities, with achievable outcome especially regarding rhino sinusitis.
Few studies have investigated the prevalence of phonatory symptoms, acoustic variables and aerodynamic measures in patients with asthma vs. a controlled group [3, 5–9]. Is dysphonia a co-morbid condition to asthma that mandates medical attention? Ihre et al. [3] in his study on 350 patients reported dysphonia in 80% of the cases. Govindaiah et al. [9] reported a prevalence of 46.4% in patients with asthma and allergic rhinitis. The results of our investigation are in partial agreement with many reports in the literature with 32.3% of patients having dypshonia compared to only 5.3% of controls. More so, though not significant, there was higher prevalence of vocal effort and vocal fatigue between the two groups indicating that asthmatic patients had to put more effort to talk and had had more vocal fatigue compared to non asthmatic.
With respect to the reports on acoustic variables, the findings in the literature are inconsistent and conflicting. Lavy et al. [5] reported an increase in the perturbation parameters in 39% of the cases, whereas Dogan et al. [7] found higher jitter percent only in women compared to men. On the other hand Asnaashari et al. [8] reported no significant difference in any of the F0, jitter and shimmer values in a group of 34 asthmatic patients compared to controls. The results of this investigation showed no significant difference in any of the acoustic variables between the two groups, even when stratified by gender except for Shimmer, which was higher in the asthmatic group.
The significantly higher prevalence of dysphonia, and the non-significantly higher prevalence of phonatory effort and vocal fatigue in the asthmatic group compared to controls can be explained on several bases: One is the impaired expiration in patients with asthma. The restricted breathing adversely affects phonation and can result in phonatory disturbances. Several studies have documented the importance of breathing as a power supply to voice production with subjects using on average 90% of their vital capacity to sustain phonation [19, 20]. Lung function influences voice and lung volume can markedly affect phonation [21]. Iwarsson et al. [21] demonstrated that with decreased lung volume, the closed quotient increases, while the subglottic pressure, peak to peak flow amplitude and leakage across the glottis decrease. There are also numerous reports on the role of expiratory effort and breathing in the control of vocal intensity and the position of the larynx [22, 23]. Together with the restricted expiration in asthmatics, there is an increase in abdominal wall muscle contraction to compensate for breathing, lung hyperinflation with lowering of the diaphragm, together with fluctuations in intra-thoracic and intra-abdominal pressures. All the aforementioned might hypothetically explain the significantly higher prevalence of dysphonia, phonatory effort and vocal fatigue in the asthmatic group [24].
A second basis for the significant difference is the use of steroid inhalers. There are numerous reports in the literature on dysphonia in patients using steroid inhalers. Dysphonia has been explained on the presence of thyroarytenoid myopathy, laryngeal candidiasis and or steroid induced laryngitis. Common laryngeal stroboscopic findings include bowing of the vocal folds, presence of glottal gap, vocal fold irregularities, and presence of vascular lesions, mucosal thickening and leukoplakia [25]. Other findings include abnormal closure patterns, phase and amplitude asymmetry, and or the presence of exudates like material on the vocal folds. In our study, fifty eight percent of asthmatic patients were using steroid inhalers, the intake of which can explain the higher prevalence of phonatory symptoms.
This study provides a comprehensive evaluation of the prevalence and severity of phonatory symptoms in patients with asthma compared to controls taking into consideration allergic rhinitis as a confounding factor. When present, allergy can skew the true prevalence of dysphonia in the analysis. For that reason, the authors of this manuscript were keen on having both allergic rhinitis and smoking equally present in asthmatic patients and controls. Nevertheless, this study has two limitations: one is the small number of cases, and two: is the lack of laryngeal examination. Patients enrolled in this investigation were assessed in the pulmonary clinic where the acoustic and aerodynamic measurements were taken. Laryngeal video-stroboscopy was not available for further assessment.
Conclusion
Dysphonia is significantly more prevalent in patients with asthma compared to controls. Asthmatic patients have to put more effort to talk and experience vocal fatigue more often than non asthmatic. The primary care physician should be alert to the presence of phonatory symptoms as a co-morbid condition to asthma that is often masked by the respiratory symptoms and the presence of other co-morbidities.
Compliance with Ethical Standards
Conflict of interest
All authors of this manuscript declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants involved in the study.
References
- 1.Toelle BG, Peat JK, Salome CM, Mellis CM, Woolcock AJ. Toward a definition of asthma for epidemiology. Am Rev Respir Dis. 1992;146(3):633–637. doi: 10.1164/ajrccm/146.3.633. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel JR, Sataloff RT, Chon JR, Hawkshaw M. Respiratory dysfunction. In: Sataloff RT, editor. The professional voice: the science and art of clinical care. 2. San Diego: Singular Publishing Group; 1997. pp. 375–386. [Google Scholar]
- 3.Ihre E, Zetterström O, Ihre E, Hammarberg B. Voice problems as side effects of inhaled corticosteroids in asthma patients—a prevalence study. J Voice. 2004;18(3):403–414. doi: 10.1016/j.jvoice.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Watkin KL, Ewanowski SJ. The effects of triamcinolone acetonide on the voice. J Speech Hear Res. 1979;22(3):446–455. doi: 10.1044/jshr.2203.446. [DOI] [PubMed] [Google Scholar]
- 5.Lavy JA, Wood G, Rubin JS, Harries M. Dysphonia associated with inhaled steroids. J Voice. 2000;14(4):581–588. doi: 10.1016/S0892-1997(00)80014-4. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla RK, Watson G, Taylor W, Jones AS, Roland NJ. Acoustic analysis in asthmatics and the influence of inhaled corticosteroid therapy. J Voice. 2009;23(4):505–511. doi: 10.1016/j.jvoice.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Dogan M, Eryuksel E, Kocak I, Celikel T, Sehitoglu MA. Subjective and objective evaluation of voice quality in patients with asthma. J Voice. 2007;21(2):224–230. doi: 10.1016/j.jvoice.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Asnaashari AM, Rezaei S, Babaeian M, et al. The effect of asthma on phonation: a controlled study of 34 patients. Ear Nose Throat J. 2012;91(4):168–171. doi: 10.1177/014556131209100409. [DOI] [PubMed] [Google Scholar]
- 9.Govindaiah RC, Baldwin JL, Sanders GM. Incidence and potential etiologies of dysphonia in patients with allergies and/or asthma. J Allergy Clin Immunol. 2004;113(2 Suppl):S275. doi: 10.1016/j.jaci.2004.01.464. [DOI] [Google Scholar]
- 10.GINA (2012) Global strategy for asthma management and prevention, global initiative for asthma (Cited September 2, 2013). Available from www.ginasthma.org
- 11.Bauchau V, Philippart D, Durham S (2004) A simple and efficient screening tool for allergic rhinitis. Poster presented at 23rd Congress of the European Academy of Allergology and Clinical Immunology, June 12–16, Amsterdam
- 12.Koichi Omari (2014) Diagnosis of voice disorders. Jpn Med Assoc J 54(4):248–253. Retrieved from: https://www.med.or.jp/english/journal/pdf/2011_04/248_253.pdf. Accessed on 22 Oct 2011
- 13.Arffa RE, Krishna P, Gartner-Schmidt J, Rosen CA. Normative values for the Voice Handicap Index-10. J Voice. 2012;26(4):462–465. doi: 10.1016/j.jvoice.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the Voice Handicap Index-10. Laryngoscope. 2004;114(9):1549–1556. doi: 10.1097/00005537-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Kay Elemetrics Corporation . Visi-Pitch II Model 3300 Instruction Manual. New Jersey: Lincolin Park; 1996. p. 120. [Google Scholar]
- 16.Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol. 2013;31(1):3–10. [PubMed] [Google Scholar]
- 17.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledford DK, Lockey RF. Asthma and comorbidities. Curr Opin Allergy Clin Immunol. 2013;13(1):78–86. doi: 10.1097/ACI.0b013e32835c16b6. [DOI] [PubMed] [Google Scholar]
- 19.Sataloff RT, Heman-Ackah YD, Hawkshaw MJ. Clinical anatomy and physiology of the voice. Otolaryngol Clin North Am. 2007;40(5):909–929. doi: 10.1016/j.otc.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Solomon NP, Garlitz SJ, Milbrath RL. Respiratory and laryngeal contributions to maximum phonation duration. J Voice. 2000;14(3):331–340. doi: 10.1016/S0892-1997(00)80079-X. [DOI] [PubMed] [Google Scholar]
- 21.Iwarsson J, Thomasson M, Sundberg J. Effects of lung volume on the glottal voice source. J Voice. 1998;12(4):424–433. doi: 10.1016/S0892-1997(98)80051-9. [DOI] [PubMed] [Google Scholar]
- 22.Iwarsson J, Sundberg J. Effects of lung volume on vertical larynx position during phonation. J Voice. 1998;12(2):159–165. doi: 10.1016/S0892-1997(98)80035-0. [DOI] [PubMed] [Google Scholar]
- 23.Makiyama K, Yoshihashi H, Mogitate M, Kida A. The role of adjustment of expiratory effort in the control of vocal intensity: clinical assessment of phonatory function. Otolaryngol Head Neck Surg. 2005;132(4):641–646. doi: 10.1016/j.otohns.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Ayazi S, DeMeester SR, Hsieh CC, et al. Thoraco-abdominal pressure gradients during the phases of respiration contribute to gastroesophageal reflux disease. Dig Dis Sci. 2011;56(6):1718–1722. doi: 10.1007/s10620-011-1694-y. [DOI] [PubMed] [Google Scholar]
- 25.Mirza N, Kasper Schwartz S, Antin-Ozerkis D. Laryngeal findings in users of combination corticosteroid and bronchodilator therapy. Laryngoscope. 2004;114(9):1566–1569. doi: 10.1097/00005537-200409000-00012. [DOI] [PubMed] [Google Scholar]