Abstract
Atrophic rhinitis (AR) is a chronic debilitating nasal mucosal disease of unknown aetiology and the definitive treatment is still elusive. This often leads to the patient suffering during the entire life span often depleting the individual psychologically. On the contrary, Rhinoscleroma (RS) for which the aetiology is clearly known and is completely curable has atrophic stage which is clinically indistinguishable from AR. Many cases of atrophic stage of RS are undetected and often they end up being treated as AR. This study was conducted to know the role of histopathology and other factors in differentiating AR and Atrophic stage of RS, which can significantly alter the course of treatment and outcome. Forty-five cases of clinically diagnosed AR were included in the study. Punch biopsy of nasal mucosa was obtained from the anterior end of inferior turbinate. Core culture was performed on one sample and histopathological examination for the other sample. Among the 45 patients, 38 (84.44%) of cases were diagnosed to be AR and 7 (15.56%) cases were diagnosed to be RS by histopathology. Core culture of RS subjects showed positive culture for Klebsiella rhinoscleromatis in five subjects (71%). To conclude, AR cases should be confirmed by histopathological examination to rule out RS, for effective management and to prevent complications.
Keywords: Atrophic rhinitis, Rhinoscleroma, Histopathology
Introduction
The aetiology of primary AR is not definitively known. For many years, rhinologists all over the world have attempted to solve the problem of aetiology, yet their opinions are divergent [1]. Though the condition is relatively rare in the developed countries, it continues to be common in the developing countries like India [2]. Many patients of AR get socially dejected which in turn can lead to divorce, suicide, depressive psychosis and stigmas [3]. Many cases of atrophic stage of Rhinoscleroma (RS) are diagnosed and treated to be a case of AR. Such cases progress over a period of time to nodular and subsequently to cicatricle stage. These cases unlike exudative stage and atrophic stage of RS are non-responsive to medical treatment alone and might require recanalization or excision surgeries. These stages are difficult to manage and might need stent placements for as long as 4–6 weeks. Few cases might need revision surgeries to keep the nasal cavity patent. This period is emotionally and psychologically draining to the patients and can be easily avoided by simple easily available investigations.
The present study was conducted with the objective to diagnose the atrophic stage of RS among patients presenting with AR. This study highlights the role of histopathology, core culture and symptom duration in identifying atrophic stage of RS especially in a developing country where the disease is commonly found and facilities for immunological tests to differentiate between the two conditions are not easily available.
Patients and Methods
A cross sectional descriptive study was conducted for a period of one year from January 2009 and December 2009 in a tertiary referral hospital of North Karnataka. We included patients presenting with history suggestive of AR, like foul smell emanating from the nose (Fetor), hyposmia or anosmia, nasal obstruction, nasal discharge, crusting and less commonly headache and epistaxis. In addition, patients with clinical evidence of AR like nasal mucosal atrophy, nasal crusting, and enlargement of nasal space with paradoxical nasal obstruction, viscid secretions and dried crusts were also included. However, we excluded histopathologically confirmed cases of AR (both primary and secondary).
Sample size was estimated by using the diagnostic accuracy of Core culture in diagnosing RS. From the pilot study diagnostic accuracy of 70% was observed. Using this value sample size of 40 was obtained at 95% confidence limit, 10% non-response rate and 15% allowable error. Forty-five patients who met the inclusion criteria were included into the study during the study duration and were evaluated by history, clinical examination, complete blood counts, urine routine, serological tests for Syphilis (VDRL), HIV I and II and HBsAg. Chest X-ray PA view was taken to rule out granulomatous diseases. Informed consent was taken prior to the inclusion and Ethical clearance was obtained from the institutional ethical committee prior to the start of the study.
Under aseptic conditions nasal swab was obtained and sent for culture and sensitivity (surface culture). Cottonoids soaked in 4% lidocaine were placed along the length of the inferior turbinate for 5 min. They were removed and anterior end of the inferior turbinate was infiltrated with 1 ml of 2% lidocaine. Later under endoscopic guidance, two samples of punch biopsy were obtained from the anterior end of inferior turbinate. One specimen in formalin was sent for histopathological examination and the other in normal saline was sent for culture (Core culture).
Data was entered in Microsoft excel data sheet and was analysed by SPSS 22 version statistical software. Qualitative data was represented as frequencies and proportions. Chi square test and Fisher Exact test was the test of significance and p value <0.05 was considered as statistically significant.
Results
Forty-five patients who met the inclusion criteria during the study period were included into the analysis and it was observed that majority of subjects were females, with female to male ratio of 1.8:1. Patients’ ages were within the range of 12–71 years with 73% of patients aged <50 years and 27% were aged >50 years.
Based on the histopathological features diagnosis of AR was made in 38 (84.44%) cases and RS was made in 7 (15.56%) cases. Diagnosis of AR was made based on epithelial features and stromal features. Total squamous metaplasia (Fig. 1) was the commonest form of epithelial changes observed in 20 (44.4%) cases followed by squamous metaplasia with ciliated epithelium in 13 (28.9%) cases and squamous metaplasia with hyperkeratosis in 12 (26.7%) cases. The stroma showed chronic cellular infiltrate (Fig. 2), glandular changes and fibrosis. The cellular infiltrate included dense lymphocytic infiltrate in majority of the cases. The blood vessels were predominantly found to be small and congested with 37 (86%) cases showing endarteritis obliterans (Fig. 3) while the dilatation of vessels were noted in 6 (14%) cases. Eosinophils were found in few cases which showed dense infiltrates whenever the maggots were associated. The glands were reduced in 19 (42.22%) cases and absent in 22 (48.89%) cases. Fibrosis was noticed in 42 (93.33%) cases.
Fig. 1.

Squamous metaplasia of epithelium
Fig. 2.

Chronic cellular infiltrate in stroma
Fig. 3.

Stromal fibrosis and endarteritis obliterans (right upper quadrant vessel)
RS was diagnosed based on the presence of Russell bodies and Mikulicz cells. Russell bodies (Fig. 4) are homogenous eosinophilic inclusion bodies found in the plasma cells. They occur due to the accumulation of immunoglobulin’s secreted by plasma cells. On the other hand, Mikulicz cells (Fig. 5) are large foamy macrophages with a central nucleus and vacuolated cytoplasm containing the causative organism.
Fig. 4.
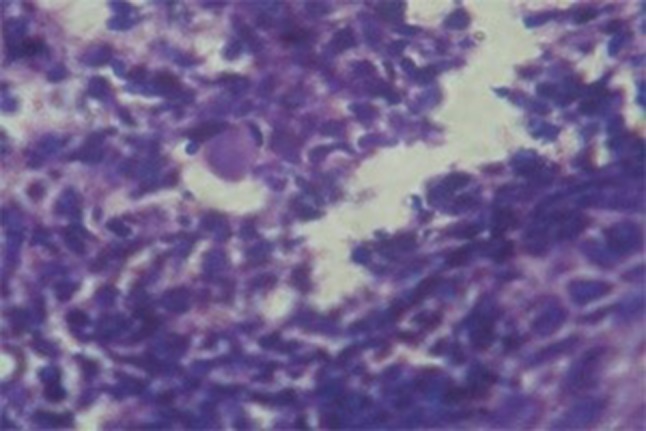
Russel body
Fig. 5.
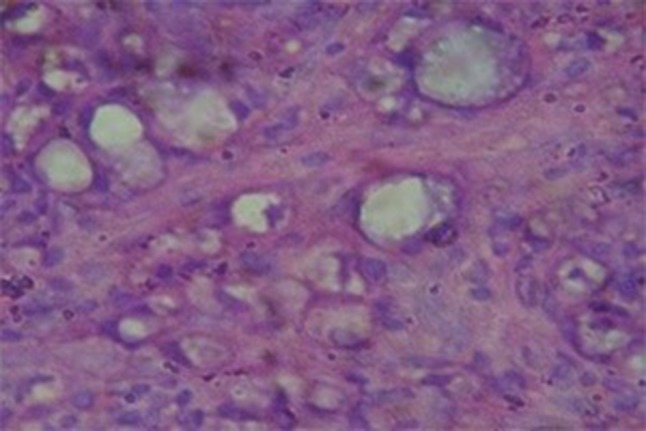
Mikulicz cell
Core culture was better in detecting both K. Ozenae and K. Rhinoscleromatis compared to surface culture. Most strikingly K. Rhinoscleromatis was not isolated in any of the surface cultures and was isolated in 5 cases out of 7 histopathologically proven RS cases in core culture (Table 1). Surface culture for K. Ozaenae had a diagnostic accuracy of 64.44% and for K. Rhinoscleromatis was 28.57% (Table 2). Hence it is important in AR that core culture is done at the earliest to identify K. Rhinoscleromatis and treat appropriately.
Table 1.
Comparison of organisms isolated by surface and core culture
| Organism isolated | Surface culture | Core culture | Both by surface culture and core culture |
|---|---|---|---|
| K. Ozaenae | 23 | 39 | 23 |
| K. Rhinoscleromatis | 0 | 5 | 0 |
| S. aureus | 10 | 4 | 0 |
| P. aeruginosa | 3 | 0 | 0 |
| P. mirabilis | 3 | 0 | 0 |
| Cirtrobacter | 0 | 1 | 0 |
Table 2.
Diagnostic accuracy of surface culture compared to core culture in diagnosing organisms in addition to the other parameters
| Parameter | K. Ozaenae (%) | K. Rhinoscleromatis (%) |
|---|---|---|
| Sensitivity | 58.97 | 0.0 |
| Specificity | 100 | 100 |
| Positive predictive value | 100 | – |
| Negative predictive value | 27.27 | 28.57 |
| Diagnostic accuracy | 64.44 | 28.57 |
Majority of subjects with AR i.e. 81.58% had duration of symptoms for more than 3 years and in 18.42% duration was less than 3 years, where as in RS cases majority i.e. 85.71% cases had duration of symptoms ranging from 1 to 3 years and 14.29% had duration more than 3 years. This observation was associated significantly i.e. when the duration was less than 3 years, AR cases should be evaluated for RS (Table 3).
Table 3.
Duration of symptoms and histopathological diagnosis
| Duration of symptoms | Atrophic rhinitis | Rhinoscleroma | p value (Fisher Exact test) |
|---|---|---|---|
| <3 years | 7 (18.42%) | 6 (85.71%) | 0.0012 |
| >3 years | 31 (81.58%) | 1 (14.29%) | |
| Total | 38 | 7 |
Discussion
A cross sectional study on 45 cases of AR showed that 38 (84.44%) cases were AR and 7 (15.56%) cases were rhinoscleromatis. In a study by Datti et al. [4], 10% of nasal diseases in Hubli was AR. The diagnosis of AR is essentially clinical as it poses little diagnostic challenge. Histopathology is sought only if secondary causes are suspected and similar observation was made by Barbary AS were in 12% cases were rhinoscleromatis [5]. AR secondary to granulomatous conditions like Tuberculosis, Leprosy, and Sarcoidosis; which are relatively easily diagnosed due to the systemic manifestations they present, in addition to the characteristic histopathology in many conditions. The presence of K. Ozaenae in the purulent, foul smelling nasal discharge forms the basis for diagnosis [6]. In developing countries both RS and AR are relatively common and especially the atrophic stage of RS poses diagnostic challenge because of clinical similarities.
RS is a chronic granulomatous disease caused by K. Rhinoscleromatis which primarily affects the nose, but may extend into nasopharynx, Para nasal sinuses, oropharynx, occasionally the larynx and tracheobronchial tree [7].
There are four recognised clinical stages of RS [8].
Exudative stage (Catarrhal phase)
Atrophic stage
Granular or nodular stage (Proliferative granulomatous growth)
Cicatricle stage (Pronounced scarring and retraction of the tissues)
The diagnosis of RS in the nodular and cicatricle stages poses little challenge as they can be identified very easily, even on clinical examination. However, it is the atrophic stage of the disease which is difficult to diagnose clinically and hence histopathological examination becomes imperative. Further, the diagnosis has a bearing on the treatment and outcome of the condition as the atrophic stage is amenable to conservative treatment.
RS commonly occurs in regions like central and north Africa, central and south America, central and Eastern Europe, India and Indonesia. It is predominantly found in rural conditions especially among people with poor socio economic conditions [7, 9, 10]. Histologically the presence of Mikulicz cells with entrapped, rod like gram negative bacilli and Russell bodies suggests RS [11, 12]. RS poses diagnostic challenge and if not detected early can go for significant complications which can involve lower airways. Early diagnosis and appropriate treatment helps to reduce morbidity [13]. Gaffer HA studied 56 patients of RS over a period of 10 years and reported high incidence of recurrence reaching up to 25%. The modalities of treatment are often unsatisfactory and tendency for recurrence is the rule [14].
Electron microscopy demonstrates numerous phagosomes in the cytoplasm of Mikulicz cells where many K. Rhinoscleromatis are present. A small number of organelles like endoplasmic reticulum and lysozymes are often squeezed to the side of cells. Many granular substances were observed on the surface of intracellular bacteria which are not found on the extracellular KR. KR is a facultative intracellular bacteria which can resist the digestion of macrophages and proliferate in them. The major cause of tissue injury is the formation of granulomas and fibrosis in KR infiltrated regions. In the plasma cells bag like dilatation of ergastoplasm (Granular endoplasmic reticulum) and Russell bodies are observed [15, 16].
In the study, Surface culture for K. Ozaenae had a diagnostic accuracy of 64.44% and for K. Rhinoscleromatis was 28.57%. Core culture involves deeper tissue for culture which depicts the true representation of organism for growth compared to the surface culture. The chances of contaminants could be higher with surface culture as evident in the present study where P. aurigenosa and P. mirabilis have been isolated from surface but not core culture. More importantly none of the samples from RS have grown on surface culture where as 5 out of the 7 histopathologically proven cases of RS have yielded growth form core culture highlighting the value of core culture. None of the previous studies have stated this fact. In addition, surface culture has missed K. Ozaenae in a significant number of cases.
Conclusion
From the study it can be inferred that the probability of clinically diagnosed cases of AR, being atrophic stage of RS is high when the symptoms are present for a short duration. This highlights the need for histopathological examination in every clinically diagnosed case of AR especially when the symptoms are present for a short duration. The high incidence among the younger age group substantiates the fact that in a developing country like India, both AR and RS are still highly prevalent and there is a need for early diagnosis and intervention. Hence, a combination of histopathology, core culture and duration of symptoms can effectively distinguish cases of primary AR from atrophic stage of RS. As this does not involve too much of a cost, it can be performed easily in any basic set up.
Funding
This study was self-funded.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflict of interest
Human and Animal Rights Statement
This article does not contain any studies with animals performed by any of the authors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Umesh S. Nagalotimath, Email: umesh_nagalotimath@yahoo.com
Krishnamurthy Naveen, Email: naveenk1381@gmail.com, Email: naveenkrishnamurthy8113@gmail.com.
Rekha B. Puranik, Email: rekhakp2007@rediffmail.com
Dandinarasaiah Manjunath, Email: drmanjud@gmail.com.
Mahesh Venkatesha, Email: maheshpsm1984@gmail.com.
References
- 1.Anand CSAS. A histopathological study in Atrophic rhinitis. J Indian Med Assoc. 1972;59(7):278–281. [PubMed] [Google Scholar]
- 2.Mehrotra RSJ, Kawatra M, Gupta SC, Mangal S. Pre and post-treatment histopathological changes in Atrophic rhinitis. Indian J Pathol Microbiol. 2005;48(3):310–313. [PubMed] [Google Scholar]
- 3.Pattanaik S. Interesting observations in primary Atrophic rhinitis. Indian J Otolaryngol Head Neck Surg. 2006;58(3):264–267. doi: 10.1007/BF03050835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datti PV. Closure of nostril in Atrophic rhinitis. Indian J Otolaryngol. 1974;24(4):187–192. [Google Scholar]
- 5.Barbary AS, Yassin A, Fouad H. Histopathological and histochemical studies on Atrophic rhinitis. J Laryngol Otol. 1970;84:1103–1112. doi: 10.1017/S0022215100156506. [DOI] [PubMed] [Google Scholar]
- 6.Deshazo RD, Stringer SP. Atrophic rhinosinusitis: progress toward explanation of an unsolved medical mystery. Curr Opin Allergy Clin Immunol. 2011;11(1):1–7. doi: 10.1097/ACI.0b013e328342333e. [DOI] [PubMed] [Google Scholar]
- 7.Nayak P, Pramod RC, Suresh KV, Desai D, Pandit S, Ingaleshwar PS. Rhinoscleroma of nose extruding into oral cavity. J Coll Phys Surg Pak. 2015;25(11):S27–S29. [PubMed] [Google Scholar]
- 8.DiBartolomio JR. Scleroma of the nose and pharynx. West J Med. 1976;124(1):13–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Bonacina E, Chianura L, Sberna M, Ortisi G, Gelosa G, Citterio A, et al. Rhinoscleroma in an immigrant from Egypt: a case report. J Travel Med. 2012;19(6):387–390. doi: 10.1111/j.1708-8305.2012.00659.x. [DOI] [PubMed] [Google Scholar]
- 10.Eguia AI, Vicario GF. Rhinoscleroma. Acta Otorrinolaringol Esp. 2010;61(2):160–162. doi: 10.1016/j.otorri.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 11.August C, Hustert B. Nasal scleroma (rhinoscleroma): pathological and clinical results. Pathologe. 1998;19(5):384–387. doi: 10.1007/s002920050302. [DOI] [PubMed] [Google Scholar]
- 12.Kumari JO. Coexistence of rhinoscleroma with Rosai-Dorfman disease: is Rhinoscleroma a cause of this disease? J Laryngol Otol. 2012;126(6):630–632. doi: 10.1017/S0022215112000552. [DOI] [PubMed] [Google Scholar]
- 13.Tan SL, Neoh CY, Tan HH. Rhinoscleroma: a case series. Singapore Med J. 2012;53(2):e24–e27. [PubMed] [Google Scholar]
- 14.Gaafar HA, Gaafar AH, Nour YA. Rhinoscleroma: an updated experience through the last 10 years. Acta Otolaryngol. 2011;131(4):440–446. doi: 10.3109/00016489.2010.539264. [DOI] [PubMed] [Google Scholar]
- 15.Balazs M, Elo J, Juhasz J. Light and electron microscopy of Rhinoscleroma (author’s transl) HNO. 1975;23(2):35–42. [PubMed] [Google Scholar]
- 16.Zhang S, Lu Z, Ni X, Zhang Y, Hong M. An etiological and pathologic study of Rhinoscleroma. Zhonghua Bing Li Xue Za Zhi. 2000;29(6):421–423. [PubMed] [Google Scholar]


