Abstract
Neurological disorders are a major cause of chronic disability globally among which stroke is a leading cause of chronic disability. The advances in the medical management of stroke patients over the past decade have significantly reduced mortality, but at the same time increased numbers of disabled survivors. Unfortunately, this reduction in mortality was not paralleled by satisfactory therapeutics and rehabilitation strategies that can improve functional recovery of patients. Motor recovery after brain injury is a complex, dynamic, and multifactorial process in which an interplay among genetic, pathophysiologic, sociodemographic and therapeutic factors determines the overall recovery trajectory. Although stroke recovery is the most well-studied form of post-injury neuronal recovery, a thorough understanding of the pathophysiology and determinants affecting stroke recovery is still lacking. Understanding the different variables affecting brain recovery after stroke will not only provide an opportunity to develop therapeutic interventions but also allow for developing personalized platforms for patient stratification and prognosis. We aim to provide a narrative review of major determinants for post-stroke recovery and their implications in other forms of brain injury.
Keywords: Brain recovery, Stroke recovery, Neurorehabilitation, Brain injury
1. Introduction
Stroke has declined to the fifth most common cause of death in the United States after devoting extensive efforts for controlling stroke risk factors and optimizing acute care of stroke patients [1]. However, stroke remains a leading cause of disability among adults in the United States and globally [1–3]. Of the estimated 800,000 strokes that occur in the US per year, the majority of stroke survivors develop long-term functional deficits [1]. The NINDS sponsored r-tPA trials have reported that the percentage of patients that still had mild to moderate functional deficits (Modified Rankin Scale of 2–5) at 3 and 12 months after a stroke were 44% and 35%, respectively, despite the fact that they received r-tPA in the acute phase [4,5]. Although these functional deficits may include cognitive, speech, visual, sensory and motor deficits, the most commonly recognized deficit after stroke is motor impairment that have negative impact on subject’s mobility and quality of life [5].
Functional deficits after stroke are also associated with huge financial burden on the patient, family, and society. It is estimated the average lifetime cost of caring for one stroke patient (across all stroke sub-types) was about $103,576 in 1990 which included the cost of acute care, long-term ambulatory care, and nursing home care [6]. The overall financial cost of post-stroke management of patients as well as the demand on rehabilitation therapy has also increased with the increase in numbers of stroke survivors reaching an annual total of $3.4 billion in the US [1].
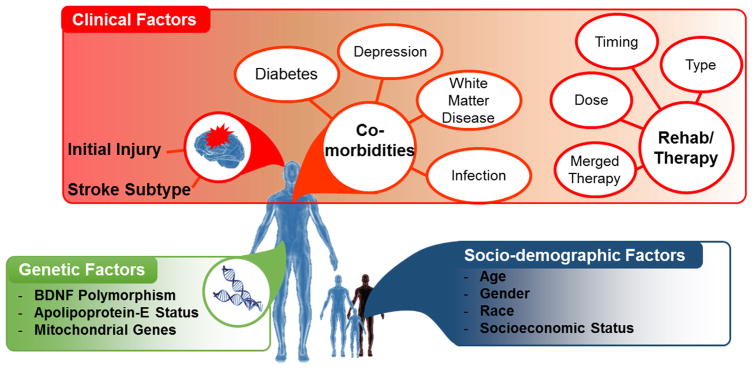
Post-stroke motor recovery is a complex, dynamic, and multifactorial process in which an interplay among genetic, pathophysiologic, sociodemographic and therapeutic factors determines the overall recovery trajectory. Therefore, rehabilitation strategies that aim to improve post-stroke recovery outcomes require a thorough understanding of those major determinants. In this paper, we review the major factors influencing post-stroke motor recovery and its implication for neurotherapy after brain injury. We categorized those factors into three groups socio-demographic factors (age, gender, race, socio-economic status and others), clinical factors (the initial injury, co-morbidities, post-stroke depression and rehabilitation therapeutics), and genetic factors (Fig. 1).
Fig. 1.
Summary of Factors Influencing Post-stroke Motor Recovery.
2. Socio-demographic factors
2.1. Age
Older age is commonly identified as a significant prognostic factor for poorer outcome after ischemic and hemorrhagic stroke where nearly half of older stroke survivors experience mild-to-severe disability [7–11], However, the proposed prognostic value of age have been challenged by studies on long-term recovery [12,13]. Whereas age may be an independent predictor of early outcomes after stroke [14–16], the effect of age on long-term outcome measures is less pronounced and may have minimal clinical relevance after adjustment of other factors in regression analysis [12,17]. Therefore, the influence of age on post-stroke recovery should be distinguished from age-associated factors to assess for the independent prognostic value of age alone. Age-associated confounding factors including co-morbidities and social variables should be taken into consideration before interpretation.
2.2. Gender
Females appear less likely to achieve complete functional independence and/or are more likely to be disabled after stroke than male [18–20]. The underlying causes behind this gender difference are not fully understood, but women are more likely to suffer depressive symptoms [21] and fatigue [22] that can indirectly have negative impact on recovery. A gender-age interaction has been hinted as post-stroke outcomes are actually better in young females as compared to young males [14]. However, this has been challenged by studies reporting lower overall quality of life in female stroke survivors compared to males regardless of age [20,23].
2.3. Race
Compared to Whites, Blacks have significantly higher stroke incidence [24], less access to acute therapy [25], higher stroke mortality [26], greater initial stroke severity [27], and higher stroke recurrence [24]. Given the racial variation in co-morbidities, initial stroke severity and rehabilitation service utilization, it is logical to infer the presence of racial variation in post-stroke recovery outcomes. In general, Blacks, along with other minorities, have been shown to have poorer stroke outcomes when compared to Whites [28,29]. Nonetheless, racial disparities with regard to post-stroke recovery and rehabilitation remains poorly characterized [30,31]. It is still unclear whether Blacks and Whites follow different post-stroke recovery patterns regardless of associated socioeconomic racial disparities. Horner et al. has demonstrated a difference in the trajectory of functional recovery between Blacks and Whites stroke survivors even when there were no major disparities between the two groups in terms of access to or utilization of rehabilitation services [29]. Blacks tend to have greater functional impairment acutely and appear to improve slowly; however, within 3–6 months they reach the approximate activity of daily living (ADL) capacity of their Whites counterparts. Some studies have also reported racial variation in the detection of post stroke depression (PSD) showing that Whites are more likely to be diagnosed with PSD [32], even after controlling for socio-demographic and clinical characteristics. In addition, compared to Whites, Blacks are found to be less likely to accept or use antidepressants after stroke [33,34]. Collectively, these studies implicate racial disparities in altering post-stroke motor recovery especially when comparing Blacks and Whites; however, studies in this domain are still scarce, and there is a lack of focused and well-controlled studies comparing the effects of racial factors on stroke recovery.
2.4. Socioeconomic status (SES)
Several indicators are used as surrogate measures of SES (e.g., insurance status, education level, household income). SES is also tied with racial factor as Blacks, and other minority groups, have a relatively lower SES. Being uninsured or underinsured may delay or prevent the process of getting access to rehabilitation services and is likely to be associated with poorer recovery outcomes [35]. Higher educational level was significantly associated with a better motor and functional recovery during the inpatient rehabilitation period while income level was only associated with rehabilitation care after discharge from a rehabilitation facility [36]. From a global perspective, socioeconomically deprived individuals in low, middle- and high-income countries are reported to have higher stroke incidence and poorer short and long-term outcomes after stroke [37]. The most likely underlying reasons for SES-based disparities is the lack of equal access to general healthcare as well as rehabilitation services. It is noteworthy that individuals of lower SES are also more likely to have co-morbidities and cerebrovascular risk factors [37].
2.5. Other factors
While other factors, such as, caregiver support, marital status, disease awareness, mistrust, healthcare system access have been suggested as potential determinants in stroke recovery, additional studies are required to investigate whether these socio-demographic factors independently or collectively contribute to the outcome of stroke survivors [9,30,38].
3. Clinical factors
3.1. Stroke subtype
The two major subtypes of stroke, hemorrhagic and ischemic stroke, result in different patterns of acute and chronic recovery. In general, hemorrhagic stroke patients tend to have greater functional impairment at presentation. However, patients with ICH tend to have a more pronounced and faster recovery than those with ischemic stroke of comparable severity [39–41].
3.2. The initial injury
Although several reparatory and regenerative processes occur following stroke, the extent of initial injury is a major determinant of chronic recovery as it defines the residual neuronal reservoir that is capable to engage in functional recovery. Therefore, a more severe acute motor impairment is a predictor of more severe chronic deficits, and at the same time, successful thrombolytic therapy may limit initial injury and result in less severe chronic deficits. Duncan et al. [42] reported that initial motor impairment, as measured by the Fugl-Meyer Upper Extremity (FM-UE) Scale, accounted for half of the variance in motor impairment (also measured by FM-UE scale) at 6 months post-stroke (R2 = 0.53 from the regression analysis). Across the different stroke severity groups, the most dramatic recovery generally occurs in the first 30 days. Additionally, patients with moderate to severe initial impairment continue to experience additional recovery for 90 days and beyond. Baseline measurements of specific muscle and/or joint movement has been proven to have prognostic value. For instance, patients with active finger extension scores more than 3 had a high probability of achieving good performance (as measured by the Motricity Index) at 30, 90, and 180 days [43]. Confirming this data, Nijland et al. showed that patients who are able to perform shoulder abduction and finger extension on day 2 after stroke have 0.98 probability of regaining some dexterity by 6 months compared to a probability of only 0.25 for those without this voluntary motor capability on day 2 [44].
Assessment of the degree of injury of ipsilesional corticospinal tract (CST), the descending motor pathway that control distal arm and hand muscles, in acute stroke can be used to predict chronic motor deficits. Feng et al. [45] developed an imaging biomarker (weighted CST lesion load, wCST-LL) that indirectly quantifies the extent of involvement of the CST by the stroke lesion. wCST-LL in the acute phase of stroke was found to have a strong predictive value for post-stroke motor outcomes (measured by FM-UE scores) at 3 months, especially in the subgroup of patients with severe motor impairment at baseline. Once the weighted CST lesion load exceeded a threshold of 7.0cc, patients had higher odds to have poor motor outcomes defined as FM-UE score less than 25 at 3 months post-stroke. Interestingly, in subgroup of patient with mild-moderated impairment at baseline, most patients recovered approximately 70% of their maximal recovery potential [46]. Studies performed in heterogeneous cohorts of stroke patients from multiple countries have supported these findings and emphasized the fact that the extent of initial injury is a major prognostic factor of recovery after ischemic stroke [47–49]. In fact, similar to motor recovery, recovery of language deficits after stroke was also found to have similar proportional recovery [1]. Findings in stroke patients also reflects data from preclinical research showing that although stroke is associated with a significant window of neuroplasticity, the ability for brain re-wiring and plasticity is highly dependent on the amount of residual neuronal substrate that can engage in the remodeling process [50].
3.3. Post-stroke depression (PSD)
PSD is the most frequent neuropsychiatric consequence of stroke affecting as high as one third of stroke survivors [51,52]. About 12% of males and 16% of females stroke survivors reported that they “always” or “often” felt depressed at 3 months after stroke [21]. The proposed hypothesis on the pathophysiological mechanism behind PSD is the amine hypothesis that suggests a stroke-induced decrease in bioavailability of the biogenic amines (i.e. serotonin, dopamine and norepinephrine) in the brain [53]. Lack of family or social support, stressful events prior to stroke, female gender, major physical disability and prior history of depression have been identified as risk factors for PSD [54]. The interaction between PSD and post-stroke recovery is rather complex, several studies have demonstrated that PSD likely impedes the post-stroke rehabilitation and recovery process and jeopardizes quality of life [51,55]. Antidepressant use immediately after stroke can enhance motor as well as cognition recovery [56]. In the FLAME study [57], patients who received fluoxetine 20 mg per day (5–7 days after stroke) gained an average of 34.0 points on FM-UE motor scale at 3 months after stroke compared to 24.3 points in patients taking placebo (p = 0.003) and also had reduced rate of depression. However, the mechanism of the antidepressant may go beyond motivating stroke patients and elevating their mood to be more engaged in rehabilitative therapy. Anti-depressants can also modulate activity in the primary motor cortex through modulating serotonergic signaling, which subsequently induces an extended window of neural plasticity [58,59].
3.4. Co-morbidities
Patients with diabetes, especially with uncontrolled diabetes, are associated with poorer outcome after stroke [60]. Similarly, severe peri-ventricular white matter disease can adversely affect outcome after stroke [61]. Charlson Index [62], a measure of number of comorbidities at discharge, is negatively correlated with post-stroke global outcomes (measured by modified Rankin scale) [63]. Careful identification of the contribution of different stroke co-morbidities is key for accurate evaluation of the prognostic value of other factors affecting stroke recovery. In addition to co-morbidities, complications of stroke, such as urinary tract infections, may negatively impact stroke recovery during the acute phase, prolong hospital stay and increase medical costs; however, it is still unknown whether urinary tract infections affect long-term outcomes [64].
3.5. Rehabilitation therapeutics
When discussing the effect of different rehabilitation therapies on chronic motor recovery, it is key to emphasize that in addition to the type of treatment, the timing and the dosage of the treatment also contribute to influencing motor outcome [5]. The effect of the dose of rehabilitation therapy on motor recovery is challenging due to the lack of evidence supporting the hypothesis that a high dose of rehabilitation therapy results in a better motor recovery as systemically reviewed by Cooke et al. [65] These findings are specifically problematic given evidence from independent preclinical labs using rodent models showing that high intensity rehabilitation therapy delivered too early after stroke may impede rather than promote recovery. For instance, high intensity training of the impaired limb can enlarge lesion volume, exacerbate injury, and worsen limb outcome in rodents [66–69]. Similar findings have been seen in human stroke rehabilitation trials. For instance, Constraint-induced Movement Therapy (CIMT) is a neurobehavioral intervention whose efficacy for upper extremity motor recovery in chronic stroke patients was demonstrated by a multi-center randomized clinical trial [70]. CIMT has two main components: Constraint – restraint of the unaffected upper extremity, and Inducement – forced training of the hemiparetic upper extremity using a shaping paradigm [71]. Benefit from CIMT over traditional therapy was documented in patients who suffered stroke 3–9 months prior to therapy with some residual wrist and finger movement. However, if CIMT is given to patients immediately after stroke, it is found to be only equally as effective as, but not superior to, an equal dose of traditional occupational therapy. Additionally, a higher dose of CIMT failed to provide a similar benefit compared to low dose of CIMT [72]. Lack of dose-response effect in rehabilitation therapy after stroke was also supported by the AVERT-3 trial using a very early mobilization protocol for patients within 24 h of stroke onset. A higher dose (intensity) of mobilization was associated with a reduction in the odds of a favorable outcome at 3 months [73]. Slope or speed of improvement (vs. the degree of improvement) is also important and may have implication for healthcare cost-saving. For example, The Locomotor Experience Applied Post-Stroke (LEAPS) study [74] failed to support the hypothesis that body weight-supported treadmill training (both early and late after stroke) is superior to home-based therapy. However, the study actually showed that the early treatment group appears to reach recovery plateau quicker than the delayed treatment group although the final endpoint is not different between the two groups.
Currently there is no consensus on the optimal neurorehabilitative intervention for enhancing motor recovery after stroke. Several studies to date have investigated a wide variety of single and combinatory rehabilitation strategies to enhance motor recovery after stroke; however, a significant superiority over traditional occupational and physical therapy has not been established yet [5]. Regardless, several innovative neuromodulatory therapies have utilized combinatory paradigms, such as combining brain stimulation with physical rehabilitation, to facilitate the utilization or extension of the window of brain plasticity after stroke. These emerging therapeutics include transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), robotic-assisted devices (RAD), brain-computer interfaces (BCI), and cell-based therapy that may change the recovery trajectory. (summarized in Table 1).
Table 1.
Emerging Neuromodulatory Therapies for Enhancing Post-stroke Motor Recovery.
| Therapeutic Approach | Potential Mechanism | Clinical Status | Effect Size | Target Stroke Population | Other Application in Motor Recovery after Neuronal Injury |
|---|---|---|---|---|---|
| tDCS | Modulates cortical excitability [104]. Anodal stimulation increase while cathodal stimulation decreases cortical excitability. | Proof-of-concept studies showed inconsistent data. Meta-analysis showed a dose-response relationship [105]. Up to 2 mA is well tolerated by patient, but higher doses have not been tested. [105] |
Variable 0.43–2.87 | Chronic patients with mild-to-moderate deficits. | Multiple sclerosis [106]. Traumatic brain injury [107]. Spinal cord injury [108]. |
| rTMS | TMS uses a high frequency stimulation to stimulate cortical firing or low frequency stimulation to inhibit cortical firing [109]. | Meta-analysis demonstrated positive impact of rTMS on motor recovery [110,111]. Low-frequency stimulation of unaffected hemisphere provided better results than high-frequency stimulation of affected hemisphere. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Functions in Patients With Stroke [110,111] |
Varies depending on lesion location and stimulation strategy. 0.55–0.73 |
Sub-acute and chronic patients with mild-to-moderate deficits. | Multiple sclerosis [112]. Traumatic brain injury [113]. |
| Epidural Cortical Stimulation | Invasive stimulation of the peri-infarct cortex using epidural electrodes [114,115]. | Phase II multicenter clinical trials demonstrated a positive effect on motor recovery upon combination with basic rehabilitation [114]. Phase III trial failed to replicate a positive additive effect of epidural stimulation on rehabilitation therapy [115]. |
Variable | Sub-acute and chronic patients. | Traumatic brain injury [116]. |
| Robotic-Assisted Devices | Mechanical assisstance in supporting and extending the strength of muscles during movement. Restrictive devices inhibit un-intended movements. | Several randomized controlled studies have reported variable results. Metanalaysis revealed a positive effect on upper limb motor function [5]. Benefit is maximal when combined with other neurorehabilitative therapies [5]. |
0.12–0.81 | Sub-acute and chronic patients. | Spinal cord injury [117]. Multiple Sclerosis [118]. Cererbellar Ataxia [115]. |
| Brain Computer Interfaces | Using bio-feedback to facilitate motor cortical re-organization and modulate neuronal networks after stroke. | Small trials have demonstrated positive effect on outcome; however, brain computer interfaces were not superior to robotic assisted devices [119]. | Variable | Stroke patients with variable disease severity. | Spinal cord injury [126]. |
| Stem Cell Therapy | Contributes to self-repair and neurogenesis after stroke by providing trophic factors and/or replacing missing neurons. | Several single arm studies have suggested a positive effect of stem cell therapy on stroke therapy [120]. Phase 1/2a trial showed safety and clinical improvement at 12 months [121]. |
Variable | Investigated in both acute and chronic stroke. | Traumatic brain injury [122]. Multiple Sclerosis [123]. Spinal cord injury [124]. |
4. Genetic factors
Several studies on genetic variations in stroke and cardiovascular risk have associated multiple single nucleotide polymorphisms (SNPs) with increased risk [75,76] or severity of ischemic stroke [77]. Yet, investigating genetic variants that may influence chronic recovery and response to rehabilitation therapy is still in infancy. Such genetic polymorphism may account for some of the inter-individual variability seen in stroke recovery, and may have implication for individualized rehabilitation programs. A number of gene products, such as Brain Derived Neurotrophic Factor (BDNF) polymorphism [78–83], Apolipoprotein E (Apo-E) [84], Neurotrophic tyrosine kinase receptor [85,86], and mitochondrial DNA gene variation may have potential influence on stroke recovery [87]. BDNF is one of the most studied neurotrophic factors in the brain and is implicated in synaptic plasticity [80], learning and memory [79]. This warrants the hypothesis that variations in BDNF levels post-stroke is implicated in chronic recovery; a hypothesis strongly backed by preclinical studies where exogenous administration of BDNF by either intranasal [81] or intravenous routes [82] enhanced chronic stroke recovery after experimental stroke. In human studies, a functional SNP (rs6252) has been identified in the BDNF gene, in which a G to A substitution at nucleotide 19 results in an amino acid switch from Valine to Methionine at codon 66 (Val66Met). An ethnic variation in this polymorphism exists where 18% of the Finnish [83], 66% of the Japanese [88], 52% of the Italian [89] and 32% of the American population [79] has Val66Met polymorphism. This polymorphism has been shown to partially affect activity-dependent BDNF secretion by reducing BDNF secretion by 30% in Met/Met neurons in-vitro [90] and to impair motor skill acquisition in-vivo [78,79]. In patients with ischemic stroke, the Val66Met allele was associated with poor outcomes and physical disability after stroke, and with slower motor recovery [83,91–93]. Analysis of pathophysiological difference between Val and Met patients suggests that both can retain the ability to recovery after stroke; however, the rate and trajectory of recovery appears to be difference [94]. In addition, the presence of Met allele was also found to negatively impact the response to rehabilitative treatment including brain stimulation by rTMS [95–97]. Work by Fritsch et al. and others has also shown that motor learning is enhanced by anodal tDCS in a BDNF-dependent manner where tDCS can promote BDNF-dependent synaptic plasticity [78].
Apolipoprotein E (Apo-E), another genetic candidate for influencing post-stroke motor recovery [84], plays an important role in the growth and regeneration of central nervous system tissues and in modulating neuronal repair, remodeling, and protection. Although not fully confirmed in large trials, polymorphism in Apo-E has been associated with variability in recovery after ischemic stroke [98–100].
Other less commonly studied genetic factors that may have potential influence on motor recovery after stroke are a polymorphism of Neurotrophic tyrosine kinase receptor [85,87], the enzyme catechol-O-methyl transferase (COMT) [101,102]. Overall, large collaborative studies are needed to better investigate genetic determinants in stroke recovery, and to provide a personalized approach of rehabilitation therapy.
5. Conclusion
The increased number of stroke survivors creates a high demand for effective and accessible neuro-rehabilitation therapies. A thorough understanding of the pathophysiology and pattern of stroke recovery will boost the existing therapeutics and develop new rehabilitational interventions in the pipeline. Equally important is the understanding of non-pathophysiological and modifiable factors that may negatively or positively impact recovery process. Therefore, significant areas for further research in stroke recovery would be to optimize post-stroke outcome prediction, to identify more sensitive and specific biomarkers for recovery, to individualize recovery therapy depending on the severity and pattern of injury, to investigate efficacy of combined several therapeutic modalities, and to understand and ameliorate socioeconomic hurdles to the recovery process. Due to the similarity in neurophysiological and neuropathological response to stroke and other forms of acquired neural injury [103], identifying determinants of motor recovery after stroke may provide insight into potential factors influencing recovery after other forms of neuronal injury including traumatic brain injury, multiple sclerosis, and spinal cord injury. Finally, it is important to note that understanding the factors influencing post-stroke recovery can be incorporated into individualized recovery plan for stroke patients. For instance, for patients with BDNF polymorphism, a higher intensity of rehabilitation therapy may overcome the genetic impact on stroke recovery [125].
HIGHLIGHTS.
Motor recovery after stroke is a multifactorial and dynamic process.
Advanced age, African American race, and female gender are major socioeconomic factors affecting stroke recovery.
Extent of initial injury after stroke is a major independent predictor of recovery.
Neurorehabilitation strategies provide a unique opportunity for enhancing recovery.
Genetic polymorphisms especially in BDNF may influence post-stroke recovery process.
Acknowledgments
Dr. Feng acknowledges grant support from National Institute of Health (P20GM109040 and HD086844), from American Heart Association (14SDG1829003 and 15SFDRN26030003) and South Carolina Clinical & Translational Research Insitute (UL1 TR001450). Mr. Alawieh acknowledges grant support from the American Heart Association (15PRE25250009). Dr. Zhao acknowledge grant support from National Natural Science Foundation(81572232) and Shanghai Natural Science Foundation(13ZR1436600).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ. Executive summary: heart disease and stroke statistics—2016 update a report from the American Heart Association. Circulation. 2016;133(4):447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42(8):2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Tissue plasminogen activator for acute ischemic stroke. the national institute of neurological disorders and stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 5.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 6.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25(4):808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 8.Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17(4):765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen HS, Reith J, Nakayama H, Kammersgaard LP, Raaschou HO, Olsen TS. What determines good recovery in patients with the most severe strokes? The Copenhagen Stroke Study. Stroke. 1999;30(10):2008–2012. doi: 10.1161/01.str.30.10.2008. [DOI] [PubMed] [Google Scholar]
- 10.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 11.D’Amore C, Paciaroni M, Silvestrelli G, Agnelli G, Santucci P, Lanari A, Alberti A, Venti M, Acciarresi M, Caso V. Severity of acute intracerebral haemorrhage, elderly age and atrial fibrillation: independent predictors of poor outcome at three months. Eur J Intern Med. 2013;24(4):310–313. doi: 10.1016/j.ejim.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002;33(1):179–185. doi: 10.1161/hs0102.101224. [DOI] [PubMed] [Google Scholar]
- 13.Luk JK, Cheung RT, Ho SL, Li L. Does age predict outcome in stroke rehabilitation? A study of 878 Chinese subjects. Cerebrovasc Dis. 2006;21(4):229–234. doi: 10.1159/000091219. [DOI] [PubMed] [Google Scholar]
- 14.Umeano O, Phillips-Bute B, Hailey CE, Sun W, Gray MC, Roulhac-Wilson B, McDonagh DL, Kranz PG, Laskowitz DT, James ML. Gender and age interact to affect early outcome after intracerebral hemorrhage. PLoS One. 2013;8(11):e81664. doi: 10.1371/journal.pone.0081664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weimar C, König I, Kraywinkel K, Ziegler A, Diener H, Collaboration GSS. Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia development and external validation of prognostic models. Stroke. 2004;35(1):158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 16.Kwah LK, Harvey LA, Diong J, Herbert RD. Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: an observational study. J Physiother. 2013;59(3):189–197. doi: 10.1016/S1836-9553(13)70183-8. [DOI] [PubMed] [Google Scholar]
- 17.Denti L, Agosti M, Franceschini M. Outcome predictors of rehabilitation for first stroke in the elderly. Eur J Phys Rehabil Med. 2008;44(1):3–11. [PubMed] [Google Scholar]
- 18.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34(5):1114–1149. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 19.Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2(3):A13. [PMC free article] [PubMed] [Google Scholar]
- 20.Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007;38(9):2541–2548. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson M, Asplund K, Glader EL, Norrving B, Stegmayr B, Terent A, Asberg KH, Wester PO. Self-reported depression and use of antidepressants after stroke: a national survey. Stroke. 2004;35(4):936–941. doi: 10.1161/01.STR.0000121643.86762.9a. [DOI] [PubMed] [Google Scholar]
- 22.Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33(5):1327–1333. doi: 10.1161/01.str.0000014248.28711.d6. [DOI] [PubMed] [Google Scholar]
- 23.Gray LJ, Sprigg N, Bath PM, Boysen G, De Deyn PP, Leys D, O’Neill D, Ringelstein EB Investigators T. Sex differences in quality of life in stroke survivors: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST) Stroke. 2007;38(11):2960–2964. doi: 10.1161/STROKEAHA.107.488304. [DOI] [PubMed] [Google Scholar]
- 24.Feng W, Nietert PJ, Adams RJ. Influence of age on racial disparities in stroke admission rates, hospital charges, and outcomes in South Carolina. Stroke. 2009;40(9):3096–3101. doi: 10.1161/STROKEAHA.109.554535. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SC, Fung LH, Gillum LA, Smith WS, Brass LM, Lichtman JH, Brown AN. Utilization of intravenous tissue-type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke. 2001;32(5):1061–1068. doi: 10.1161/01.str.32.5.1061. [DOI] [PubMed] [Google Scholar]
- 26.Feng W, Hendry RM, Adams RJ. Risk of recurrent stroke, myocardial infarction, or death in hospitalized stroke patients. Neurology. 2010;74(7):588–593. doi: 10.1212/WNL.0b013e3181cff776. [DOI] [PubMed] [Google Scholar]
- 27.Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith-Hammond CA, Matchar DB, Oddone EZ. Racial variation in initial stroke severity. Stroke. 2000;31(3):563–567. doi: 10.1161/01.str.31.3.563. [DOI] [PubMed] [Google Scholar]
- 28.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 29.Horner RD, Matchar DB, Divine GW, Feussner JR. Racial variations in ischemic stroke-related physical and functional impairments. Stroke. 1991;22(12):1497–1501. doi: 10.1161/01.str.22.12.1497. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, Peterson E, Rosamond W, Trimble B, Valderrama AL. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 31.McNaughton H, Feigin V, Kerse N, Barber PA, Weatherall M, Bennett D, Carter K, Hackett M, Anderson C. Ethnicity and functional outcome after stroke. Stroke. 2011;42(4):960–964. doi: 10.1161/STROKEAHA.110.605139. [DOI] [PubMed] [Google Scholar]
- 32.Jia H, Chumbler NR, Wang X, Chuang HC, Damush TM, Cameon R, Williams LS. Racial and ethnic disparities in post-stroke depression detection. Int J Geriatr Psychiatry. 2010;25(3):298–304. doi: 10.1002/gps.2339. [DOI] [PubMed] [Google Scholar]
- 33.Kirby JB, Hudson J, Miller GE. Explaining racial and ethnic differences in antidepressant use among adolescents. Med Care Res Rev. 2010;67(3):342–363. doi: 10.1177/1077558709350884. [DOI] [PubMed] [Google Scholar]
- 34.Cooper LA, Gonzales JJ, Gallo JJ, Rost KM, Meredith LS, Rubenstein LV, Wang NY, Ford DE. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care. 2003;41(4):479–489. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 35.Horner RD, Swanson JW, Bosworth HB, Matchar DB. Effects of race and poverty on the process and outcome of inpatient rehabilitation services among stroke patients. Stroke. 2003;34(4):1027–1031. doi: 10.1161/01.STR.0000060028.60365.5D. [DOI] [PubMed] [Google Scholar]
- 36.Putman K, De Wit L, Schoonacker M, Baert I, Beyens H, Brinkmann N, Dejaeger E, De Meyer AM, De Weerdt W, Feys H, Jenni W, Kaske C, Leys M, Lincoln N, Schuback B, Schupp W, Smith B, Louckx F. Effect of socioeconomic status on functional and motor recovery after stroke: a European multicentre study. J Neurol Neurosurg Psychiatry. 2007;78(6):593–599. doi: 10.1136/jnnp.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14(12):1206–1218. doi: 10.1016/S1474-4422(15)00200-8. [DOI] [PubMed] [Google Scholar]
- 38.Hartman-Maeir N, Soroker N, Oman SD, Katz N. Awareness of disabilities in stroke rehabilitation—a clinical trial. Disabil Rehabil. 2003;25(1):35–44. [PubMed] [Google Scholar]
- 39.Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, De Angelis D, Fusco FR, Morelli D, Venturiero V, Troisi E, Pratesi L. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: a matched comparison. Stroke. 2003;34(12):2861–2865. doi: 10.1161/01.STR.0000102902.39759.D3. [DOI] [PubMed] [Google Scholar]
- 40.Kelly PJ, Furie KL, Shafqat S, Rallis N, Chang Y, Stein J. Functional recovery following rehabilitation after hemorrhagic and ischemic stroke. Arch Phys Med Rehabil. 2003;84(7):968–972. doi: 10.1016/s0003-9993(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 41.Bhalla A, Wang Y, Rudd A, Wolfe CD. Differences in outcome and predictors between ischemic and intracerebral hemorrhage The South London Stroke Register. Stroke. 2013;44(8):2174–2181. doi: 10.1161/STROKEAHA.113.001263. [DOI] [PubMed] [Google Scholar]
- 42.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23(8):1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 43.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, Moretto G, Bovi P, Gambarin M. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 2007;38(3):1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 44.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 2010;41(4):745–750. doi: 10.1161/STROKEAHA.109.572065. [DOI] [PubMed] [Google Scholar]
- 45.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, Kautz SA, Schlaug G. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78(6):860–870. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krakauer JW, Marshall RS. The proportional recovery rule for stroke revisited. Ann Neurol. 2015;78(6):845–847. doi: 10.1002/ana.24537. [DOI] [PubMed] [Google Scholar]
- 47.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015;78(6):848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- 48.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, Marshall RS, Krakauer JW. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 49.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2015;29(7):614–622. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- 50.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 51.Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, Sharpe M. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356(9224):122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 52.Gaete JM, Bogousslavsky J. Post-stroke depression. Expert Rev Neurother. 2008;8(1):75–92. doi: 10.1586/14737175.8.1.75. [DOI] [PubMed] [Google Scholar]
- 53.Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med. 2012;16(9):1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guiraud V, Gallarda T, Calvet D, Turc G, Oppenheim C, Rouillon F, Mas J-L. Depression predictors within six months of ischemic stroke: the DEPRESS study. Int J Stroke. 2016;11(5):519–525. doi: 10.1177/1747493016632257. http://dx.doi.org/10.1177/1747493016632257. [DOI] [PubMed] [Google Scholar]
- 55.Robinson RG, Jorge RE. Post-Stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 56.Mead GE, Hsieh CF, Lee R, Kutlubaev M, Claxton A, Hankey GJ, Hackett M. Selective serotonin reuptake inhibitors for stroke recovery: a systematic review and meta-analysis. Stroke. 2013;44(3):844–850. doi: 10.1161/STROKEAHA.112.673947. [DOI] [PubMed] [Google Scholar]
- 57.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, Guillon B, Moulin T, Marque P, Pariente J, Arnaud C, Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 58.Dam M, Tonin P, De Boni A, Pizzolato G, Casson S, Ermani M, Freo U, Piron L, Battistin L. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27(7):1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 59.Pariente J, Loubinoux I, Carel C, Albucher JF, Leger A, Manelfe C, Rascol O, Chollet F. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001;50(6):718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 60.Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, Lavallee P, Cabrejo L, Guidoux C, Klein I, Amarenco P, Mazighi M. Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke. 2013;44(7):1915–1923. doi: 10.1161/STROKEAHA.111.000813. [DOI] [PubMed] [Google Scholar]
- 61.Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Flaherty ML, Air E, Broderick J, Tsevat J. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40(2):530–536. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35(8):1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 63.Bushnell CD, Lee J, Duncan PW, Newby LK, Goldstein LB. Impact of comorbidities on ischemic stroke outcomes in women. Stroke. 2008;39(7):2138–2140. doi: 10.1161/STROKEAHA.107.509281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poisson SN, Johnston SC, Josephson SA. Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke. 2010;41(4):e180–4. doi: 10.1161/STROKEAHA.109.576413. [DOI] [PubMed] [Google Scholar]
- 65.Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: a systematic review and meta-analysis. BMC Med. 2010;8:60. doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16(15):4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Risedal A, Zeng J, Johansson BB. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999;19(9):997–1003. doi: 10.1097/00004647-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke. 2000;31(5):1144–1152. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- 69.Jones TA, Allred RP, Adkins DL, Hsu JE, O’Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40(3 Suppl):S136–S138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D Investigators E. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 71.Taub E, Uswatte G. Constraint-induced movement therapy: bridging from the primate laboratory to the stroke rehabilitation laboratory. J Rehabil Med. 2003;(41 Suppl):34–40. doi: 10.1080/16501960310010124. [DOI] [PubMed] [Google Scholar]
- 72.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73(3):195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lele A. Efficacy and safety of very early mobilisation within 24 hours of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386(9988):46–55. doi: 10.1016/S0140-6736(15)60690-0. [DOI] [PubMed] [Google Scholar]
- 74.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL, Rosamond WD, Rivadeneira F, Kelly-Hayes M, Lopez OL, Coresh J, Hofman A, DeCarli C, Heckbert SR, Koudstaal PJ, Yang Q, Smith NL, Kase CS, Rice K, Haritunians T, Roks G, de Kort PL, Taylor KD, de Lau LM, Oostra BA, Uitterlinden AG, Rotter JI, Boerwinkle E, Psaty BM, Mosley TH, van Duijn CM, Breteler MM, Longstreth WT, Jr, Wolf PA. Genomewide association studies of stroke. N Engl J Med. 2009;360(17):1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NINDS Stroke Genetics Network and International Stroke Genetics Consortium. Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 2016;15(2):174–184. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greisenegger S, Endler G, Haering D, Schillinger M, Lang W, Lalouschek W, Mannhalter C. The (− 174) G/C polymorphism in the interleukin-6 gene is associated with the severity of acute cerebrovascular events. Thromb Res. 2003;110(4):181–186. doi: 10.1016/s0049-3848(03)00376-1. [DOI] [PubMed] [Google Scholar]
- 78.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 80.Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 81.Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38(7):2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 83.Siironen J, Juvela S, Kanarek K, Vilkki J, Hernesniemi J, Lappalainen J. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(10):2858–2860. doi: 10.1161/STROKEAHA.107.485441. [DOI] [PubMed] [Google Scholar]
- 84.Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil. 2009;16(4):282–299. doi: 10.1310/tsr1604-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, Simmons MS, Perry RT, Wiener HW, Harrell LE, Go RC. Genetic association of neurotrophic tyrosine kinase receptor type 2 (NTRK2) With Alzheimer’s disease. Am J Med Genet Part B: Neuropsychiatr Genet. 2008;147(3):363–369. doi: 10.1002/ajmg.b.30607. [DOI] [PubMed] [Google Scholar]
- 86.Hayashi A, Nakatani K, Nishioka J, Sakamoto Y, Jinda S, Wada H, Nobori T. Neurotrophic receptor tyrosine kinase B induces c-fos-associated cell survival. Int J Mol Med. 2009;24(6):807–811. doi: 10.3892/ijmm_00000296. [DOI] [PubMed] [Google Scholar]
- 87.Chinnery PF, Elliott HR, Syed A, Rothwell PM. Mitochondrial DNA haplogroups and risk of transient ischaemic attack and ischaemic stroke: a genetic association study. Lancet Neurol. 2010;9(5):498–503. doi: 10.1016/S1474-4422(10)70083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Itoh K, Hashimoto K, Kumakiri C, Shimizu E, Iyo M. Association between brain-derived neurotrophic factor 196 G/A polymorphism and personality traits in healthy subjects. Am J Med Genet Part B: Neuropsychiatr Genet. 2004;124B(1):61–63. doi: 10.1002/ajmg.b.20078. [DOI] [PubMed] [Google Scholar]
- 89.Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M. Association between the BDNF 196A/G polymorphism and sporadic Alzheimer’s disease. Mol Psychiatry. 2002;7(2):136–137. doi: 10.1038/sj.mp.4000952. [DOI] [PubMed] [Google Scholar]
- 90.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Helm EE, Tyrell CM, Pohlig RT, Brady LD, Reisman DS. The presence of a single-nucleotide polymorphism in the BDNF gene affects the rate of locomotor adaptation after stroke. Exp Brain Res. 2015:1–11. doi: 10.1007/s00221-015-4465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim EJ, Park CH, Chang WH, Lee A, Kim ST, Shin YI, Kim YH. The brain-derived neurotrophic factor Val66Met polymorphism and degeneration of the corticospinal tract after stroke: a diffusion tensor imaging study. Eur J Neurol. 2016;23(1):76–84. doi: 10.1111/ene.12791. [DOI] [PubMed] [Google Scholar]
- 93.Kim JM, Stewart R, Park MS, Kang HJ, Kim SW, Shin IS, Kim HR, Shin MG, Cho KH, Yoon JS. Associations of BDNF genotype and promoter methylation with acute and long-term stroke outcomes in an East Asian cohort. PLoS One. 2012;7(12):e51280. doi: 10.1371/journal.pone.0051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Pino G, Pellegrino G, Capone F, Assenza G, Florio L, Falato E, Lotti F, Di Lazzaro V. Val66Met BDNF polymorphism implies a different way to recover from stroke rather than a worse overall recoverability. Neurorehabil Neural Repair. 2016;30(1):3–8. doi: 10.1177/1545968315583721. http://dx.doi.org/10.1177/1545968315583721. [DOI] [PubMed] [Google Scholar]
- 95.Chang WH, Bang OY, Shin YI, Lee A, Pascual-Leone A, Kim YH. BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain Stimul. 2014;7(4):553–558. doi: 10.1016/j.brs.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Hwang JM, Kim YH, Yoon KJ, Uhm KE, Chang WH. Different responses to facilitatory rTMS according to BDNF genotype. Clin Neurophysiol. 2015;126(7):1348–1353. doi: 10.1016/j.clinph.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 97.Uhm KE, Kim Y-H, Yoon KJ, Hwang JM, Chang WH. BDNF genotype influence the efficacy of rTMS in stroke patients. Neurosci Lett. 2015;594:117–121. doi: 10.1016/j.neulet.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 98.Cramer S, Procaccio V. Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012;19(5):718–724. doi: 10.1111/j.1468-1331.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 99.Martinez-Gonzalez NA, Sudlow CL. Effects of apolipoprotein E genotype on outcome after ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77(12):1329–1335. doi: 10.1136/jnnp.2006.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarron MO, Muir KW, Nicoll JA, Stewart J, Currie Y, Brown K, Bone I. Prospective study of apolipoprotein E genotype and functional outcome following ischemic stroke. Arch Neurol. 2000;57(10):1480–1484. doi: 10.1001/archneur.57.10.1480. [DOI] [PubMed] [Google Scholar]
- 101.Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36(2):184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 102.Warburton DM, Rusted JM, Muller C. Patterns of facilitation of memory by nicotine. Behav Pharmacol. 1992;3(4):375–378. [PubMed] [Google Scholar]
- 103.Albert-Weissenberger C, Siren AL, Kleinschnitz C. Ischemic stroke and traumatic brain injury: the role of the kallikrein-kinin system. Prog Neurobiol. 2013;101–102:65–82. doi: 10.1016/j.pneurobio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation-technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- 105.Feng W, Enabore Ja, Kautz S, Adams RJ, Chhatbar P. Dose response relationship in transcranial direct current stimulation stroke motor recovery studies. Stroke. 2015;46(Suppl 1):A7–A7. doi: 10.1016/j.brs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cuypers K, Leenus DJ, Van Wijmeersch B, Thijs H, Levin O, Swinnen SP, Meesen RL. Anodal tDCS increases corticospinal output and projection strength in multiple sclerosis. Neurosci Lett. 2013;554:151–155. doi: 10.1016/j.neulet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Clayton E, Kinley-Cooper S, Weber R, Adkins D. Brain stimulation: neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res. 2016;1640:30–138. doi: 10.1016/j.brainres.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat® gait training. Neurosci Lett. 2016;620:143–147. doi: 10.1016/j.neulet.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 109.Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 110.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43(7):1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 111.Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil Assoc Acad Physiatr. 2014;93(5):422–430. doi: 10.1097/PHM.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 112.Centonze D, Koch G, Versace V, Mori F, Rossi S, Brusa L, Grossi K, Torelli F, Prosperetti C, Cervellino A. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. 2007;68(13):1045–1050. doi: 10.1212/01.wnl.0000257818.16952.62. [DOI] [PubMed] [Google Scholar]
- 113.Nielson DM, McKnight CA, Patel RN, Kalnin AJ, Mysiw WJ. Preliminary guidelines for safe and effective use of repetitive transcranial magnetic stimulation in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2015;96(4):S138–S144. doi: 10.1016/j.apmr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 114.Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108(4):707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- 115.Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, Sobue G, Nishizawa M. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012;26(5):515–522. doi: 10.1177/1545968311425918. [DOI] [PubMed] [Google Scholar]
- 116.Shin SS, Dixon CE, Okonkwo DO, Richardson RM. Neurostimulation for traumatic brain injury: a review. J Neurosurg. 2014;121(5):1219–1231. doi: 10.3171/2014.7.JNS131826. [DOI] [PubMed] [Google Scholar]
- 117.Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85(1):52–66. [PubMed] [Google Scholar]
- 118.Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008;22(6):661–671. doi: 10.1177/1545968308318473. [DOI] [PubMed] [Google Scholar]
- 119.Ang KK, Chua KS, Phua KS, Wang C, Chin ZY, Kuah CW, Low W, Guan C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci. 2015;46(4):310–320. doi: 10.1177/1550059414522229. [DOI] [PubMed] [Google Scholar]
- 120.Borlongan CV, Jolkkonen J, Detante O. The future of stem cell therapy for stroke rehabilitation. Future Neurol. 2015;10(4):313–319. doi: 10.2217/FNL.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang J, Phelan M, Cummings BJ. A meta-analysis of efficacy in pre-clinical human stem cell therapies for traumatic brain injury. Exp Neurol. 2015;273:225–233. doi: 10.1016/j.expneurol.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 123.Rice CM, Marks DI, Ben-Shlomo Y, Evangelou N, Morgan PS, Metcalfe C, Walsh P, Kane NM, Guttridge MG, Miflin G. Assessment of bone marrow-derived cellular therapy in progressive multiple sclerosis (ACTiMuS): study protocol for a randomised controlled trial. Trials. 2015;16(1):1–8. doi: 10.1186/s13063-015-0953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2015;78(3):436–447. doi: 10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 125.McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Exp Brain Res. 2011;213(4):415–422. doi: 10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- 126.Enzinger C, Ropele S, Fazekas F, Loitfelder M, Gorani F, Seifert T, Reiter G, Neuper C, Pfurtscheller G, Müller-Putz G. Brain motor system function in a patient with complete spinal cord injury following extensive brain–computer interface training. Exp Brain Res. 2008;190(2):215–223. doi: 10.1007/s00221-008-1465-y. [DOI] [PubMed] [Google Scholar]