Abstract
Atherosclerosis is a chronic inflammatory disease. Interventions targeting the inflammatory process could provide new strategies for preventing atherosclerotic cardiovascular diseases (CVD). Previously, we have reported that oral administration of anti-CD3 antibodies, or active vitamin D3, reduced atherosclerosis in mice via recruiting regulatory T cells and tolerogenic dendritic cells to the gut-associated lymphoid tissues. From this, it is reasonable to propose that the intestine could be a novel therapeutic target for prevention of atherosclerotic CVD. Recently, the association between cardio-metabolic diseases and gut microbiota has attracted increased attention. Gut microbiota, reported to be highly associated with intestinal immunity and metabolism, were shown to aggravate CVD by contributing to the production of trimethylamine-N-oxide (TMAO), a pro-atherogenic compound. We have also previously investigated the relationship between patient susceptibility to coronary artery disease (CAD) and gut microbiota. We found that the order Lactobacillales was significantly increased and the phylum Bacteroidetes was decreased in CAD patients compared with control patients. In this review article, we discuss the evidence for the relationship between the gut microbiota and cardio-metabolic diseases, and consider the gut microbiota as new potential diagnostic and therapeutic tool for treating CVD.
Keywords: Intestinal immunity, Regulatory T cell, Tolerogenic dendritic cell, Gut microbiota, TMAO
Introduction
Atherosclerosis and resulting cardiovascular diseases (CVD) are the leading causes of mortality in many developed and developing countries. Clinical studies and animal experiments have demonstrated that elevated plasma cholesterol, mainly transported by low density lipoprotein (LDL), promotes CVD, including coronary artery disease (CAD). On the basis of this finding, subsequent studies have shown that statin-based lipid lowering therapies reduce CV events. However, several clinical trials have revealed that more than 50% of residual cardiovascular risk remains, even after the aggressive reduction of LDL cholesterol1). Atherosclerosis is considered a chronic inflammatory disease in which both innate and acquired immunity are involved2–6). Inflammation of the vessel walls is an important feature of atherosclerosis, and contributes to both instability of plaques and thrombotic occlusion of arteries, resulting in CV events such as acute coronary syndrome and stroke. As a next-generation treatment, many researchers, including us, have been interested in anti-inflammation therapy for atherosclerotic CVD2–12). We subsequently proposed that the intestine could be a novel therapeutic target for prevention of atherosclerosis and treating CVD, and have focused our research on intestinal immunity11, 12).
The gut mucosa is one of the largest immunologically active organs in human body. It protects the host from invading microorganisms, and harbors several hundred trillion bacteria, which are collectively referred to as the “gut microbiota.” Fortunately, majority of these microorganisms are not harmful to the host, and in fact contribute to the maintenance of health. However, if disrupted, they have the potential to drive gastrointestinal and extragastrointestinal disorders13). Over the past decade, the expanded use of a mouse model lacking gut microbiota, known as “germ-free (GF) mice”, as well as the development of various omic technologies, including genomics, transcriptomics, proteomics, and metabolomics, have enriched our understanding of an ecological system of commensal bacteria in the intestine. Recent studies have demonstrated that gut microbe-derived factors may actually lead to many metabolic and digestive tract diseases. In this review, we describe how specific changes in the gut microbiome could affect host metabolism and immune regulation, and how these findings can lead to novel therapeutic targets for CVD and metabolic disorders.
The Intestine as a Therapeutic Target for Preventing Atherosclerosis
The intestinal immune system differentiates potentially harmful foreign antigens from harmless ones. The gut tolerates harmless antigens, but remains able to eliminate harmful pathogens. To accommodate the exposure to harmless antigens, including food components and commensal gut bacteria, the gut has evolved an anti-inflammatory environment. Recent research revealed that tolerogenic dendritic cells (DCs) in the gut present food antigens to T cells as tolerogens and induce antigen-specific immune suppression14). The oral tolerance is shown to involve both anergy/apoptosis of CD4+ effector T cells and induction of regulatory T cells (Tregs) which appear to come in several different forms. The naturally occurring Tregs (nTreg), originally described as CD4+CD25+ cells generated in the thymus, express the transcription factor Foxp3 and are involved in maintaining systemic homeostasis and preventing autoimmunity. Immunosuppressive mediators of Tregs include inhibitory molecules such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), immunoregulatory cytokine interleukin-10 (IL-10), and transforming growth factor-β (TGF-β)14).
CTLA-4 is a co-inhibitory molecule mainly expressed in CD4+Foxp3+ Tregs. It binds to CD80/CD86 on the DCs to suppress their function. IL-10-producing type 1 regulatory T (Tr1) cells, which do not express Foxp3, have been observed in Peyer's patches of mice fed a low dose of β-lactogloblin, and produce high amounts of IL-1014). T helper 3 (Th3) cells are TGF-β-producing LAP (latency-associated peptide) +CD4+ T cells originally isolated from mesenteric lymph nodes of orally tolerant mice. TGF-β induces expression of Foxp3 in naive CD4+ T cells, and Th3 cells can influence Treg development in neighboring cells. Importantly, retinoic acid promotes the conversion of naive CD4+ T cells into Foxp3+ peripherally inducible Treg (pTreg) with the help of TGF-β in the gut, suggesting significant roles for intestinal DCs that produce retinoic acid (Fig. 1)14).
Fig. 1.
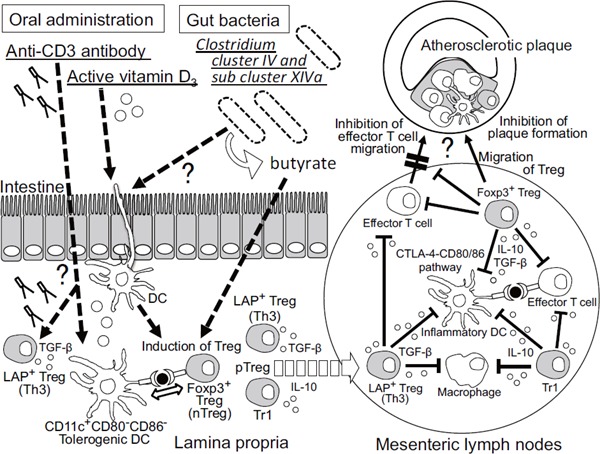
Intestinal immunity as a possible therapeutic target for controlling inflammatory diseases including atherosclerosis
Oral administration of anti-CD3 antibody and active vitamin D3 induces Tregs and tolerogenic DCs in mesenteric lymph nodes. Clostridium cluster IV and subcluster XIV a was shown to induce Foxp3+ Tregs in the colon of mice through butyrate-dependent manner. Some DCs in the intestine have a crucial role in determining tolerogenic immune responses by prompting the generation of Tregs. Peripherally inducible Treg (pTreg) is reported to differentiate mainly in the intestine. Foxp3+ Tregs inhibit antigen presentation through a cell-contact dependent manner or production of anti-inflammatory cytokines (IL-10 or TGF-β). These phenomena are similar to oral tolerance and may result in inhibiting atherosclerosis. Treg; regulatory T cell, LAP+ Treg; latency-associated peptide+ Tregs, pTreg; peripherally inducible Treg, DC; dendritic cell, IL; interleukin, IFN-γ; interferon-γ, TGF-β; transforming growth factor-β, CTLA-4; cytotoxic T-lymphocyte-associated protein 4
Oral tolerance is locally induced in an antigen-specific manner, but its effects are not constrained to the local immune response within the gut. Anti-inflammatory immune responses could be seen in other tissues and organs including distal non-lymphoid organs. From this, we hypothesized that modulation of intestinal immunity or induction of oral tolerance must affect the systemic immune responses, including vessel walls, and might prevent atherosclerosis.
Recent studies have shown that the oral anti-CD3 antibody is biologically active and induces TGF-β-producing CD4+LAP+ Tregs, namely Th3, that suppress experimental autoimmune encephalitis15) and autoimmune diabetes in a TGF-β-dependent fashion16). Autoimmune diseases are suppressed even by low doses of oral anti-CD3 antibody, associated with an increase in LAP+ Tregs. However, there is no evidence of antigen specificity with oral anti-CD3 antibodies and the cellular and molecular mechanisms underlying induction of Tregs remain unclear15). In one study, we applied this method to the treatment of atherosclerosis in apolipoprotein E-deficient (apoE-/-) mice. Here we demonstrated that oral anti-CD3 antibody treatment induced Th3 and Foxp3+ Tregs, which suppressed pathogenic immune processes crucial for atherogenesis through a TGF-β-dependent mechanism, consequently inhibiting atherosclerotic plaque formation11). In another study, we examined the effect of oral anti-CD3 antibody treatment on the phenotypes of DCs in the mesenteric lymph nodes in mice, and confirmed that expression of CD80 and CD86 in DCs were reduced in anti-CD3 antibody-treated mice compared to controls (Fig. 1). We found that active vitamin D3 (calcitriol) was induced immature DCs and Tregs. Further, we tried to examine the effects of orally administrated calcitriol on atherosclerosis in animal models, and demonstrated that it decreases atherosclerosis in apoE-/- mice, by promoting induction of tolerogenic DCs and Foxp3+ Tregs 12). Although further studies are needed to clarify the precise role of several types of vascular DCs in atherogenesis, effective methods to induce atheroprotective DCs could be novel therapies for prevention of atherosclerosis11, 12). Taken together, modulation of the intestinal immunity, including the function and quantity of Tregs and tolerogenic DCs, could be a novel strategy for preventing atherosclerotic CVD.
Gut Microbiota and their Regulatory Effects on the Intestinal Immunity
During birth, we are colonized with many microorganisms that will play a crucial role in defining our future metabolism and immunity. The profile of the predominant phylum in the gut changes during childhood and youth, and nearly stabilizes by adulthood17). Most bacterial species in the adult human and mouse gut belong to the phyla Firmicutes and Bacteroidetes, with less abundant bacterial phyla, such as Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia also present (Fig. 2). A lot of gut bacteria cannot be cultivated in vitro, making categorization and identification of gut bacteria complicated. Although there is no clear definition of a “healthy” gut microbiome in humans, a recent metagenome study has allowed three major clusters, or “Enterotypes,” of gut bacteria to be distinguished in humans, based on the predominant bacterial genera in fecal specimens: type I is characterized by high levels of Bacteroides; type II has few Bacteroides but Prevotella are common; and type III has high levels of Ruminococcus (Fig. 2)18). The composition of our gut microbiota is remarkably diverse. Because dietary exposures significantly affect our microbial community, it is very dynamic and can change rapidly in a short period of time19). However, its composition appears to remain remarkably stable over longer periods, and to be conserved between individuals and their family members.
Fig. 2.
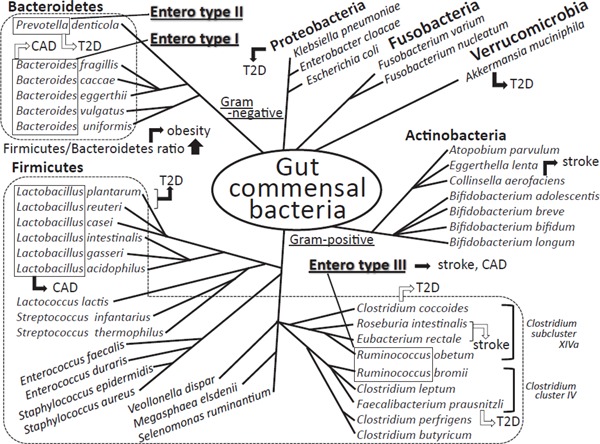
Human gut commensal microbiota, their classification, and their relation to cardio-metabolic diseases
The phylum Firmicutes and Bacteroidetes is the most dominant gram-positive and gramnegative bacteria phylum in human gut microbiota, respectively. The major 4 phyla of Firmicutes, Bacteroides, Actinobacteria, and Proteobacteria occupy more than 98% of all human gut microbiota. The total number of bacteria in human intestine is more than one hundred trillions and are classified in several hundreds of species. The gut bacteria phenotype named “Enterotype” was reported in humans, based on the predominant bacterial genera in fecal specimens; type I is characterized by high levels of Bacteroides, type II predominates Prevotella, and type III has high levels of Ruminococcus. The Firmicutes/Bacteroidetes ratio was reported to be associated with obesity and lean. Black and white arrows show the positive and negative correlations with the incidence of the indicated diseases, respectively. T2D; type 2 diabetes mellitus, CAD; coronary artery disease
It has recently been demonstrated that specific bacterial species are associated with differentiation of specific subsets of T cells in the intestine19). Both human and mouse Clostridium cluster IV and subcluster XIVa, spore-forming components of indigenous intestinal microbiota, have been implicated in the induction of Foxp3+ Tregs in the colon of mice (Fig. 1)20, 21). Furthermore, butyrate, a short chain fatty acid (SCFAs) produced by Clostridium species, promotes Foxp3+ Treg induction22). It can therefore be speculated that the propagation or sterilization of some specific bacterial species, resulting in augmented generation of Tregs or reduced differentiation of pathogenic T cells, may prevent inflammatory diseases, including atherosclerosis. Further studies are needed to prove this hypothesis and may contribute to the development of novel strategies for preventing atherosclerosis by modulating intestinal immunity.
Gut Microbial Alternations Associated with Obesity and Type 2 Diabetes
Recent studies in both mice and humans have suggested that gut microbiota may function as an environmental factor contributing to obesity and type 2 diabetes (T2D). This was first demonstrated by showing the increase of the phylum Firmicutes and the decrease of the phylum Bacteroidetes (increase of the Firmicutes/Bacteroidetes (F/B) ratio) in obese patients (Fig. 2), attracting attention from researchers in all over the world23, 24). Although these findings suggest that gut microbiota are altered in obesity, it is unclear whether the change of microbial composition is a cause or a result of obesity. If the altered microbiota contribute to the pathogenesis of obesity, modulating microbial composition could be a new therapeutic option for extreme obesity. In this regard, it was demonstrated that the obesity phenotype, an increase in body fat and weight, was transmissible by fecal microbiota transplantation into lean GF mice, resulting in increased capacity for energy harvest25). Another paper demonstrated that metabolic disorders, such as insulin resistance, were also transmissible by fecal transplantation26). Accordingly, the obese phenotype can be transplanted between individuals through the microbiota.
One potential mechanism by which gut microbiota contribute to the pathophysiology of obesity has been intensively examined. The microbiome of the obesity subjects was found to have increased fermentation capabilities, resulting in increased levels of fecal SCFAs that could be used as energy by the host. It was recently revealed that SCFAs such as acetate and propionate regulate appetite via gut endocrine hormone and central homeostatic mechanisms27). Taken together, these findings suggest that gut microbiota directly contribute to obesity via increasing appetite and energy harvest from the diet.
Two recent papers demonstrated the diagnostic and clinical value of fecal microbiota composition in T2D28, 29). In both studies, diabetic subjects were characterized by a reduction of Clostridiales including butyrate-producing Roseburia species and Feacalibacterium prausnitzii. Whereas Karlsson et al. reported an enrichment of Lactobacillus gasseri and Streptococcus mutans in diabetic patients29), Qin et al. found that an increased number of Proteobacteria could be a predictor of T2D28). Using a sensitive quantitative reverse transcription PCR method, in fecal samples of Japanese patients with T2D, it became obvious that the Clostridium coccoides group, Atopobium cluster, and Prevotella were significantly lower, while the counts of total Lactobacillus were higher than in those of control subjects30). Specifically, the counts of Lactobacillus reuteri and Lactobacillus plantarum subgroups were significantly higher in Japanese T2D patients (Fig. 2). Other papers demonstrated that Akkermansia muciniphila (A. muciniphila), a mucin-degrading bacterium, was associated with glucose metabolism28, 31). In human studies, the relationship between the abundance of A. muciniphila and type 2 diabetes is controversial. Oral administration of A. muciniphila led to decreased metabolic endotoxemia, improved glucose tolerance, and reduced atherosclerosis in mice. These findings indicate the need for further investigation to ascertain the therapeutic applicability of A. muciniphila.
CVD and the Chemical TMAO Connection
Recently, Hazen SL et al. have splendidly reported that gut microbial-derived metabolites, trimethylamine (TMA) and trimethylamine N-oxide (TMAO), are pro-atherogenic in both mice and humans (Fig. 3)32, 33). First, they used a metabolomics approach to generate small-molecule metabolomics profiles in human plasma that can predict for CVD32). Three metabolites of the dietary lipid phosphatidylcholine; choline, TMAO, and betaine, were identified and these metabolites were associated with atherosclerotic CV risks in humans and the promotion of atherosclerosis in mice. Oral feeding of choline, rather than parenteral delivery, was necessary to generate TMAO, suggesting that a crucial phase in this biochemical pathway was performed within the intestine. Generation of TMA, a precursor of TMAO, was shown to be dependent on the gut microbiota in both humans and mice. That is, they found that dietary choline is metabolized by gut bacteria to TMA, which is subsequently absorbed into the host and metabolized to TMAO in the liver by the FMO (flavin-containing monooxygenase) family of enzymes (Fig. 3)34). Oral administration of phosphatidylcholine promoted up-regulation of multiple macrophage receptors and enhanced atherosclerosis in apoE-/- mice32). Deletion of gut microbiota using broad spectrum antibiotics canceled the pro-atherosclerotic effect of dietary choline, which was associated with the reduction of plasma TMAO levels in antibiotic-treated mice. Furthermore, inhibition of microbial TMA lyases, enzymes that produce TMA, by 3,3-dimethyl-1-butanol (DMB) could reduce atherosclerotic lesion formation in apoE-/- mice35).
Fig. 3.
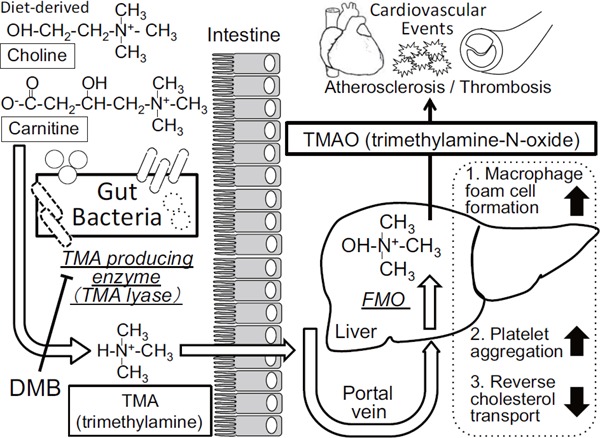
Gut microbiota metabolic pathways related with the incident adverse cardiovascular events
Gut microbial-derived metabolites, trimethylamine (TMA) and trimethylamine N-oxide (TMAO), are shown to be associated with the incidence of cardiovascular events in humans and increase atherosclerosis in mice. TMAO is shown to increase the foam cell formation in macrophages, activate platelet aggregation, and decrease the reverse cholesterol transport from the atherosclerotic lesions to the liver. DMB; 3,3-dimethyl-1-butanol, FMO; flavin-containing monooxygenase
They also investigated the relationship between fasting plasma levels of TMAO and incidents of major adverse cardiovascular events (death, myocardial infarction, or stroke) during three years of follow-up in 4007 patients undergoing elective diagnostic cardiac catheterization33). Increased plasma TMAO levels were associated with an increased risk of a major adverse CV event. Even after adjustment for traditional risk factors, elevated TMAO levels could predict an increased risk of major adverse CV events. Collectively, their findings suggesed that pathways dependent on gut microbiota may contribute to the pathophysiology of atherosclerotic CVD and are potential therapeutic targets.
Microbial processing L-carnitine, which is abundant in red meat and contains a trimethylamine structure similar to that of choline, was found to elevate plasma TMAO concentrations and enhance atherosclerosis in a microbial-dependent manner36). It was also recently found that γ-butyrobetaine is the major proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMA and TMAO37). One of the proatherosclerotic mechanisms of TMAO might be that it increases macrophage foam cell formation, suppresses reverse cholesterol transport, and enhances platelet hyperreactivity (Fig. 3) 34). Bacterial taxa belonging to the families Clostridiaceae and Peptostreptococcaceae were positively associated with TMAO production in humans, suggesting that L-carnitine-metabolizing bacteria may belong to these families. Although the molecular mechanisms through which gut microbial formation of TMAO leads to increasing atherosclerosis and CV events are not entirely clear, this study may in part explain why excessive red meat consumption has been associated with increased CVD and mortality risks. In any case, these studies suggest that targeting the gut microbial TMAO pathway as a treatment strategy, whether it is via dietary manipulation, alteration in microbial community with a probiotic, or pharmacological inhibition of microbial enzymes (e.g., DMB) involved in TMA production, has the potential to inhibit atherosclerotic CVD associated with elevated TMAO.
Gut Microbial Alternations Associated with Atherosclerosis
The studies using GF mice of how the intestinal bacteria make an impact on the development of atherosclerosis are limited38, 39). One study showed that, although plasma cholesterol levels were significantly higher in GF/apoE-/- mice than in conventional apoE-/- mice, the effect of gut microbiota on atherosclerosis was ambiguous38). Lipidomics analysis on GF and conventionally raised mice proposed that gut microbiota affect host lipid metabolism40). A detailed mechanism for how the commensal bacteria may contribute to host lipid and cholesterol metabolism could potentially be explained by microbial regulation of bile acid synthesis and metabolism, but further studies are required.
A sequencing study comparing the gut microbiome from patients who had stenotic and symptomatic atherosclerotic plaques in the carotid artery and from healthy controls showed that the microbiome was more pro-inflammatory in the people with atherosclerotic plaques41). The shotgun sequencing of the gut metagenome demonstrated that the genus Collinsella was increased in patients with symptomatic stroke, whereas Roseburia and Eubacterium were enriched in healthy controls (Fig. 2). They also demonstrated that patients with symptomatic atherosclerosis were underrepresented in enterotype I, and overrepresented in enterotype III18).
We tried to clarify the specific profile of gut microbiota in CAD patients to investigate a diagnostic and therapeutical potential of gut microbiota42). We used terminal restriction fragment length polymorphism (T-RFLP) analysis to detect the profile of gut microbiota in CAD and control patients who had T2D, hypertension, and/or dyslipidemia. T-RFLP analysis is one of the most well-established and reliable 16S ribosomal RNA-based methods, especially when considering its high throughput and reproducibility. T-RFLP using BslI could classify gut microbiota into ten groups: Prevotella, Bacteroides, Lactobacillales, Bifidobacterium, Clostridium cluster IV, Clostridium subcluster XIVa, Clostridium cluster IX, Clostridium cluster XI, Clostridium cluster XVIII, as well as others, combining the operational taxonomic units that belonged to the same group. Arumugam et al. suggested that the human gut microbiota could be stratified into three enterotypes18). According to the report and our data, we defined enterotype I as Bacteroides >30%, enterotype II as Prevotella >15%, and the remaining as enterotype “others” (III), using this T-RFLP analysis of gut microbiota. Our data revealed that CAD patients were overrepresented in the enterotype “others” (III) compared with the control group (p <0.001, chi-squared test; Fig. 4A)42).
Fig. 4.
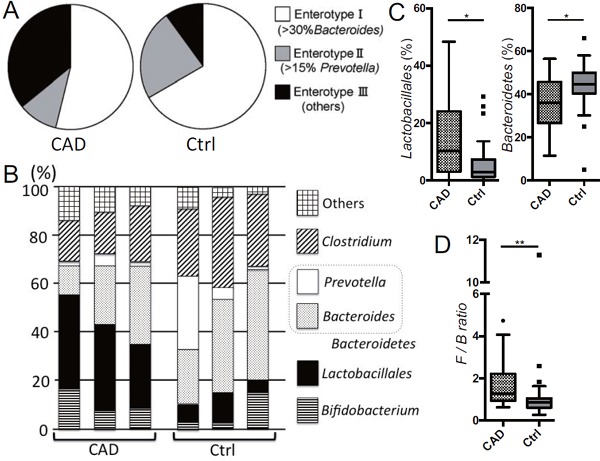
Dysbiosis in coronary artery disease patients determined by terminal restriction fragment length polymorphism (T-RFLP)
(A) Entero type III (high levels of Ruminococcus type) is predominant in coronary artery disease (CAD) patients. (B) Representative proportions of gut micobiota in CAD and contol (Ctrl) patients were shown. (C) The percentage of the order Lactobacillales was increased; while the percentage of the phylum Bacteroidetes (Prevotella+Bacteroides) was decreased in CAD patients compared with Ctrl patients. (D) The F/B (Fimicutes / Bacteroidetes) ratio was increased in CAD patients. Kruskal-Wallis test followed by Dunn's post-hoc analysis was used to calculate p-values (*p <0.05 and **p <0.01). Ctrl = ageand sex-matched controls with hypertension, type 2 diabetes and/or dyslipidemia who did not have coronary or other vascular diseases.
We found that the order Lactobacillales was significantly increased and the phylum Bacteroidetes (Bacteroides + Prevotella) was significantly decreased in the CAD group compared with the control group (Fig. 4B and C). The F/B ratio, reported to increase in obesity24), was also increased in the CAD group compared with the control group (Fig. 4D). The order Lactobacillales is one of the main components of the human gut microbiome and belongs to the phylum Firmicutes. The order Lactobacillales is divided into several genera, including Lactobacillus, Streptococcus, and Enterococcus. Because of the study protocol, we could not deny that medication could affect the composition of gut microbiota. The biological significance of the increase of the order of Lactobacillaes and the decrease of the phylum of Bacteroidetes in CAD patients remains, as of yet, unknown. Although there are differences between carotid and coronary atherosclerosis, we found consistent results of low levels of the phylum Bacteroidetes. Bacteroides fragilis, which belongs to the phylum Bacteroidetes, affects mucosal T cell homeostasis by promoting regulatory T cell function. Other Bacteroides species can also establish mutualistic relationships with the host, by being able to flourish in the plant polysaccharide-enriched gut environment and by providing the biological byproducts necessary for the well-being of the host. Our work, and previous reports support the hypothesis that the phylum Bacteroidetes might help to prevent coronary atherosclerosis. Therefore, the role of bacteria in the phylum Bacteroidetes in CAD should be assessed.
Conclusion and Perspectives
Intestinal immunity has been attracting much attention as a novel therapeutic target to treat atherosclerotic CVD. Since the discovery of Tregs and DCs, knowledge about the biology and pathophysiology of these regulatory immune cells has accumulated in the field of atherosclerosis as well as autoimmune diseases. It is now clear that several types of Tregs and tolerogenic DCs are essential for the regulation of pathogenic T cell immune responses in atherogenesis. Gutassociated immune tolerance induction by oral administration of drugs or therapeutic agents possessing immunoregulatory activities is a hopeful way to regulate inflammation. However, the details of mechanisms by which the intestinal immunity affect the systemic immunity remain to be clarified.
In association with intestinal immunity in atherogenesis, we have been interested in the gut bacteria that may be involved in the pathogenesis of atherosclerosis. We had already identified the types of gut microbiota susceptible to CAD. Further studies are needed to understand the functional level of some specific microbial pathways and their products, which contribute to maintaining our physiological homeostasis and drive disease progression. The next important step might be the application of this new knowledge to diagnostic and therapeutic approaches. The present status of the association between the gut microbiota and the incidence of T2D and atherosclerotic CVD was demonstrated in this review article. We hope that novel therapeutic strategies targeting the gut microbiome to prevent atherosclerotic CVD will be developed in the near future.
Disclosures
The author received some research funding described below and declared no other conflict of interest.
Sources of Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant No. 24591114, Suzuken Memorial Foundation, Takeda Scientific Foundation, Mochida Memorial Foundation, Senshin Medical Research Foundation, Yakult Bioscience Research Foundation, and Hyogo Science and Technology Association.
References
- 1). Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group : Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med, 2008; 359: 2195-2207 [DOI] [PubMed] [Google Scholar]
- 2). Koga J, Matoba T, Egashira K: Anti-inflammatory Nanoparticle for Prevention of Atherosclerotic Vascular Diseases. J Atheroscler Thromb, 2016; 23: 757-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Usman A, Ribatti D, Sadat U, Gillard JH: From Lipid Retention to Immune-Mediate Inflammation and Associated Angiogenesis in the Pathogenesis of Atherosclerosis. J Atheroscler Thromb, 2015; 22: 739-749 [DOI] [PubMed] [Google Scholar]
- 4). Eguchi K, Manabe I: Toll-Like Receptor, Lipotoxicity and Chronic inflammation: The Pathological Link Between Obesity and Cardiometabolic Disease. J Atheroscler Thromb, 2014; 21: 629-639 [DOI] [PubMed] [Google Scholar]
- 5). Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 6). Libby P, Lichtman AH, Hansson GK: Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity, 2013; 38: 1092-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Yodoi K, Yamashita T, Sasaki N, Kasahara K, Emoto T, Matsumoto T, Kita T, Sasaki Y, Mizoguchi T, Sparwasser T, Hirata K: Foxp3+ regulatory T cells play a protective role in angiotensin II-induced aortic aneurysm formation in mice. Hypertension, 2015; 65: 889-895 [DOI] [PubMed] [Google Scholar]
- 8). Kita T, Yamashita T, Sasaki N, Kasahara K, Sasaki Y, Yodoi K, Takeda M, Nakajima K, Hirata K: Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice. Cardiovsac Res, 2014; 102: 107-117 [DOI] [PubMed] [Google Scholar]
- 9). Kasahara K, Sasaki N, Yamashita T, Kita T, Yodoi K, Sasaki Y, Takeda M, Hirata K: CD3 antibody and IL-2 complex combination therapy inhibits atherosclerosis by augmenting a regulatory immune response. J Am Heart Assoc, 2014; 3: e000719. 10.1161/JAHA.113.000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, Hirata K: Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol, 2011; 31: 1963-1972 [DOI] [PubMed] [Google Scholar]
- 11). Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K: Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation, 2009; 120: 1996-2005 [DOI] [PubMed] [Google Scholar]
- 12). Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Ishida T, Hirata K: Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol, 2010; 30: 2495-2503 [DOI] [PubMed] [Google Scholar]
- 13). Round JL, Mazmanian SK: The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol, 2009; 9: 313-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Weiner HL, da Cunha AP, Quintana F, Wu H: Oral tolerance. Immunol Rev, 2011; 241: 241-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, Maron R, Weiner HL: Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med, 2006; 12: 627-635 [DOI] [PubMed] [Google Scholar]
- 16). Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL: Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes, 2007; 56: 2103-2109 [DOI] [PubMed] [Google Scholar]
- 17). Human Microbiome Project Consortium : Structure, function and diversity of the healthy human microbiome. Nature, 2012; 486: 207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, MetaHIT Consortium. Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P: Enterotypes of the human gut microbiome. Nature, 2011; 473: 174-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R: Diversity, stability and resilience of the human gut microbiota. Nature, 2012; 489: 220-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K: Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 2013; 500: 232-236 [DOI] [PubMed] [Google Scholar]
- 21). Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K: Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 2011; 331: 337-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H: Commensal microbe derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 2013; 504: 446-450 [DOI] [PubMed] [Google Scholar]
- 23). Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI: A core gut microbiome in obese and lean twins. Nature, 2009; 457: 480-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: human gut microbes associated with obesity. Nature, 2006; 444: 1022-1023 [DOI] [PubMed] [Google Scholar]
- 25). Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 2006; 444: 1027-1031 [DOI] [PubMed] [Google Scholar]
- 26). Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR: Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab, 2015; 22: 516-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD: The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun, 2014; 5: 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J: A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 2012; 490: 55-60 [DOI] [PubMed] [Google Scholar]
- 29). Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F: Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature, 2013; 498: 99-103 [DOI] [PubMed] [Google Scholar]
- 30). Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T, Tamura Y, Sakurai Y, Yamamoto R, Mita T, Fujitani Y, Fukuda H, Nomoto K, Takahashi T, Asahara T, Hirose T, Nagata S, Yamashiro Y, Watada H: Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care, 2014; 37: 2343-2350 [DOI] [PubMed] [Google Scholar]
- 31). Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, MICRO-ObesConsortium. Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K: Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut, 2016; 65: 426-436 [DOI] [PubMed] [Google Scholar]
- 32). Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011; 472: 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med, 2013; 368: 1575-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Tang WH, Hazen SL: The contributory role of gut microbiota in cardiovascular disease. J Clin Invest, 2016; 124: 4204-4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL: Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell, 2015; 163: 1585-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL: Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med, 2013; 19: 576-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab, 2014; 20: 799-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, Sparrow CP, Detmers PA, Chao YS: Infectious agents are not necessary for murine atherogenesis. J Exp Med, 2000; 191: 1437-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Stepankova R, Tonar Z, Bartova J, Nedorost L, Rossman P, Poledne R, Schwarzer M, Tlaskalova-Hogenova H: Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atheroscler Thromb, 2010; 17: 796-804 [DOI] [PubMed] [Google Scholar]
- 40). Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Oresic M, Bäckhed F: The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res, 2010; 51: 1101-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J: Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun, 2012; 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K: Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb, 2016; 23: 908-921 [DOI] [PMC free article] [PubMed] [Google Scholar]


