Abstract
Aim: To evaluate the role of CHADS2 and CHA2DS2-VASc scores in predicting the risk of ischemic stroke or transient ischemic attack (TIA) outcomes in patients with interatrial block (IAB) without a history of atrial fibrillation (AF).
Methods: A retrospective study was conducted, including 1,046 non-anticoagulated inpatients (612 males, 434 females; mean age: 63 ± 10 years) with IAB and without AF. IAB was defined as P-wave duration > 120 ms using a 12-lead electrocardiogram. CHADS2 and CHA2DS2-VASc scores were retrospectively calculated. The primary outcomes evaluated were ischemic stroke or TIA.
Results: During the mean follow-up period of 4.9 ± 0.7 years, 55 (5.3%) patients had an ischemic stroke or TIA. Receiver operating characteristic (ROC) curve analysis showed that the CHADS2 score [area under the curve (AUC), 0.638; 95% confidence interval (CI), 0.562–0.715; P = 0.001] and the CHA2DS2-VASc score (AUC, 0.671; 95% CI, 0.599–0.744; P <0.001) were predictive of ischemic strokes or TIA. Cut-off point analysis showed that a CHADS2 score ≥ 3 (sensitivity = 0.455 and specificity = 0.747) and a CHA2DS2-VASc score ≥ 4 (sensitivity = 0.564 and specificity = 0.700) provided the highest predictive value for ischemic stroke or TIA. The multivariate Cox regression analysis showed that CHADS2 [hazard ratio (HR), 1.442; 95% CI, 1.171–1.774; P = 0.001] and CHA2DS2-VASc (HR, 1.420; 95% CI, 1.203–1.677; P <0.001) scores were independently associated with ischemic stroke or TIA following adjustment for smoking, left atrial diameter, antiplatelet agents, angiotensin inhibitors, and statins.
Conclusions: CHADS2 and CHA2DS2-VASc scores may be predictors of risk of ischemic stroke or TIA in patients with IAB without AF.
Keywords: CHADS2, CHA2DS2-VASc, Interatrial block, Ischemic stroke, Transient ischemic attack
Introduction
Interatrial block (IAB) involves a conduction delay between the right and left atria and is manifested on a 12-lead electrocardiogram (ECG) by a P-wave duration of > 120 ms1). A recent review of the prevalence of IAB has described this condition as being an underappreciated clinical “pandemic,” particularly in the aging population2). IAB may be a preceding or causative risk factor for atrial arrhythmias, particularly atrial fibrillation (AF)3–6), and can be associated with left atrial dilation1), left atrial electromechanical dysfunction7), and thromboembolic ischemic stroke8–10). Because of the association between IAB and increased risk of ischemic stroke, it has been suggested that these patients could benefit from anticoagulation therapy. However, since the IAB population may be heterogeneous in terms of their risk of developing ischemic stroke, an accurate, objective approach for evaluating the risk of ischemic stroke in these patients is urgently sought to allow physicians and patients to select and plan the most appropriate antithrombotic therapy.
The CHADS2 and CHA2DS2-VASc scores are currently the recommended clinical risk prediction tools used to evaluate the risk of thromboembolism in patients with non-valvular AF11, 12). Recently, CHADS2 and CHA2DS2-VASc scores have also been reported to have a predictive role for outcomes in patients without known AF, including the risks of adverse clinical events in patients with pacemakers and sinus node dysfunction13), new-onset peripheral arterial occlusive disease14), incident AF15, 16), ischemic stroke or TIA in patients with acute coronary syndrome17), and ischemic stroke in hypertensive patients aged 65 years or older18). However, there is little information available regarding the predictive value of the CHADS2 and CHA2DS2-VASc scores for ischemic stroke or TIA in IAB patients without known AF. The purpose of this retrospective study was to investigate the role of the CHADS2 and CHA2DS2-VASc scores in predicting clinical outcomes including ischemic stroke or TIA in patients with IAB without a history of AF.
Methods
Study Subjects
Our prospectively established database of ECG recordings of patients who were hospitalized in the Zhengzhou University People's Hospital for diagnosis and treatment between 1st March and 31st March 2010 were retrospectively reviewed. If patients underwent more than one ECG during the index hospitalization, only the first ECG recording was analyzed. We excluded from the analysis patients with a history of diagnosis of AF, patients who under anticoagulant therapy, patients with missing data for calculation of the CHADS2 and CHA2DS2-VASc scores, and patients lost to follow-up. The study protocol was approved by the Local Institutional Review Board. The requirement for informed consent was waived because of the anonymous nature of the data.
Electrocardiogram Analysis
In all patients, interatrial conduction was assessed using a resting 12-lead ECG in sinus rhythm (high-pass filter 0.05Hz, low-pass filter 150 Hz, 25 mm/s, 10 mm/mv), which was previously described in detail19). Briefly, P-waves were manually measured using a caliper to identify the longest P-wave duration for all 12 ECG leads. At least three beats were measured on each ECG lead. The onset of the P-wave was the point of initial upward or downward deflection from the ECG baseline, and the P-wave endpoint was identified as the point where the waveform returned to the baseline. IAB was defined as a P-wave duration > 120 ms on the 12-lead ECG1). The ECG analysis was independently performed by two observers who were blinded to the patient details, and any differences between the observers were resolved by consensus. The clinical records of all patients with IAB were then reviewed.
Calculation of the CHADS2 and CHA2DS2-VASc Scores
The CHADS2 score was calculated by assigning 1 point each for age > 75 years, hypertension, diabetes mellitus, and congestive heart failure and 2 points for a previous stroke or TIA11). The CHA2DS2-VASc score was calculated for each patient by assigning 1 point each for age 65–74 years, hypertension, diabetes mellitus, congestive heart failure, vascular disease, and female sex and 2 points for a previous stroke or TIA and age ≥75 years12). Hypertension was defined as a systolic blood pressure of ≥ 140 mm Hg, a diastolic blood pressure of ≥ 90 mm Hg or treatment with antihypertensive drugs. Congestive heart failure was considered present for patients with a history of heart failure or a measured left ventricular ejection fraction < 0.35. Diabetes was defined as a fasting blood glucose level of > 126 mg/dl or treatment with hypoglycemic agents.
Patient Follow-up
All patients included in the study were followed until March 1, 2015 or until the occurrence of an ischemic stroke or TIA. All study subjects were contacted by telephone every 6 months, and follow-up interviews were conducted with participants or their relatives or carers to identify any deaths or hospitalizations that occurred during the previous interval. For any reported event, medical records were retrieved and reviewed. Hemorrhagic stroke was defined as rapidly developing clinical signs of neurological dysfunction attributable to a focal collection of blood within the brain parenchyma or ventricular system that was not caused by trauma20). Ischemic stroke was defined as central nervous system infarction of the brain due to ischemia, based on neuroimaging and/or clinical evidence of permanent injury20). Embolic stroke was identified based on the patient's clinical history and presentation (sudden onset, initial maximal symptoms, and/or subsequent rapid recovery among others), as well as the newly-evidenced lesion size, number, and anatomical cerebrovascular location10, 21). The diagnosis of TIA was made according to the World Health Organization (WHO) criteria22), which include rapidly developing clinical signs of focal or global disturbance of cerebral function lasting less than 24 h, with no apparent non-vascular cause.
Statistical Analysis
Data analysis was performed using SPSS software version 17.0. Continuous data are presented as the mean ± standard deviation. Univariate analysis to assess the predictive value of clinical variables on ischemic stroke or TIA was performed using the unpaired independent-samples t-test for continuous variables and the χ2 test and Fisher's exact test, if necessary, for categorical variables. The cumulative event incidence rates were calculated for the CHADS2 and CHA2DS2-VASc scores. A Kaplan–Meier estimation with a log-rank test was performed for unadjusted analysis of the association between increased CHADS2 and CHA2DS2-VASc scores and the risk of ischemic stroke or TIA. Receiver operating characteristic (ROC) curves were constructed to test the ability of the CHADS2 and CHA2DS2-VASc scores to predict ischemic stroke or TIA and to identify optimal cut-off values. A Cox regression analysis was performed to assess the ability of the CHADS2 and CHA2DS2-VASc scores to predict the incidence of ischemic stroke or TIA, following multivariate adjustment. Based on clinical validity and the review of the previous literature, an adjustment was added for five covariates: current smoking23), left atrial (LA) diameter24–27), use of antiplatelet agents28), use of angiotensin inhibitors29, 30), and used of statins31). All probability values were two-sided, and a P-value of <0.05 was considered to be significant.
Results
We analyzed the ECG recordings during sinus rhythm of a total of 3,487 patients between March 1, 2010 and March 31, 2010. IAB was detected in 38.2% (1,332/3,487) of these patients. On the basis of review of the clinical records of the patients with IAB, 1,084 patients were initially enrolled, and 248 patients were excluded. Of the 1,084 patients, 1,046 (612 males, 434 females; mean age: 63 ± 10 years) completed follow-up (96.5%) and were included in the final study sample.
The mean follow-up period was 4.9 ± 0.7 years, during which 8 (0.8%) patients without baseline AF were subsequently diagnosed with hemorrhagic stroke and 55 (5.3%) were diagnosed with ischemic stroke or TIA, including 29 (52.7%) patients with a probable embolic stroke, 20 (36.4%) with a probable thrombotic stroke, and 6 (10.9%) with TIA. The baseline clinical characteristics of IAB patients with and without ischemic stroke or TIA are shown in Table 1. Ischemic stroke or TIA during follow-up was reported significantly more often in patients above the age of 75 (P = 0.023), patients with diabetes mellitus (P = 0.015), and patients with a previous recorded stroke or TIA (P = 0.037). More patients with ischemic stroke or TIA were receiving antiplatelet agents compared with those without ischemic stroke or TIA. Patients with ischemic stroke or TIA had larger LA diameters and higher CHADS2 (P <0.001) and CHA2DS2-VASc (P <0.001) scores than those without ischemic stroke or TIA.
Table 1. Baseline characteristics of IAB patients with and without ischemic stroke or TIA.
| Parameters | Stroke or TIA | No-Stroke or TIA | P-value |
|---|---|---|---|
| (n = 55) | (n = 991) | ||
| Age, years | 66 ± 10 | 63 ± 10 | 0.054 |
| Age ≥ 75 | 18 (32.7%) | 198 (20.0%) | 0.023 |
| Age 65–74 | 8 (14.5%) | 104 (10.5%) | 0.344 |
| Female, n (%) | 27 (49.1%) | 407 (41.1%) | 0.240 |
| Congestive heart failure, n (%) | 8 (14.5%) | 118 (11.9%) | 0.558 |
| Hypertension, n (%) | 36 (65.5%) | 526 (53.1%) | 0.073 |
| Diabetes mellitus, n (%) | 33 (60.0%) | 429 (43.3%) | 0.015 |
| Previous stroke or TIA, n (%) | 20 (36.4%) | 237 (23.9%) | 0.037 |
| Coronary artery disease, n (%) | 21 (38.2%) | 278 (28.1%) | 0.106 |
| PCI during index admission, n (%) | 9 (16.4%) | 110 (11.1%) | 0.767 |
| CABG during index admission, n (%) | 3 (5.6%) | 39 (3.9%) | 0.576 |
| Current Smoker (%) | 17 (30.9%) | 227 (22.9%) | 0.172 |
| LVEF, % | 58.3 ± 13.6 | 60.2 ± 11.3 | 0.064 |
| LA diameter, mm | 38.4 ± 4.6 | 36.9 ± 4.4 | 0.013 |
| Medication Use | |||
| Antiplatelet Agent, n (%) | 26 (47.3%) | 320 (32.3%) | 0.022 |
| β-blockers, n (%) | 29 (52.7%) | 415 (41.9%) | 0.113 |
| Angiotensin inhibitors, n (%) | 18 (32.7%) | 285 (28.8%) | 0.528 |
| Statins, n (%) | 28 (50.9%) | 445 (44.9%) | 0.384 |
| Antiarrhythmics, n (%) | 7 (12.7%) | 90 (9.1%) | 0.364 |
| CHADS2 score | 2.5 ± 1.4 | 1.8 ± 1.3 | <0.001 |
| 0 | 3 (5.5%) | 158 (15.9%) | |
| 1 | 13 (23.6%) | 299 (30.2%) | |
| 2 | 14 (25.5%) | 283 (28.6%) | |
| ≥ 3 | 25 (45.5%) | 252 (25.4%) | |
| CHA2DS2-VASc score | 3.8 ± 1.8 | 2.7 ± 1.7 | <0.001 |
| 0 | 1 (1.8%) | 102 (10.3%) | |
| 1 | 5 (9.1%) | 165 (16.6%) | |
| 2 | 8 (14.5%) | 206 (20.8%) | |
| 3 | 10 (18.2%) | 221 (22.3%) | |
| ≥ 4 | 31 (56.4%) | 297 (30.0%) |
TIA, transient ischemic attack; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; LA, left atrial.
The incidence of ischemic stroke or TIA increased with an increased CHADS2 score. For CHADS2 scores of 0, 1, 2, and ≥ 3, the event incidences were 0.37, 0.85, 0.96, and 1.92 per 100 person-years, respectively (Fig. 1A). ROC curve analysis showed that the CHADS2 score [area under the curve (AUC), 0.638; 95% confidence interval (CI), 0.562–0.715; P = 0.001] was a significant predictor of ischemic stroke or TIA (Fig. 2A). Cut-off point analysis showed that a CHADS2 score ≥ 3 gave the highest predictive value for ischemic stroke or TIA (sensitivity = 0.455 and specificity = 0.747). The incidence of ischemic stroke or TIA was significantly higher in patients with CHADS2 score ≥ 3 compared with those with a CHADS2 score < 3 (the log-rank test, P = 0.001) (Fig. 3A).
Fig. 1.
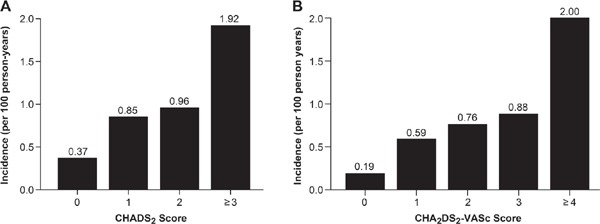
Incidence of ischemic stroke or TIA based on the CHADS2 (A) and CHA2DS2-VASc (B) scores
Fig. 2.
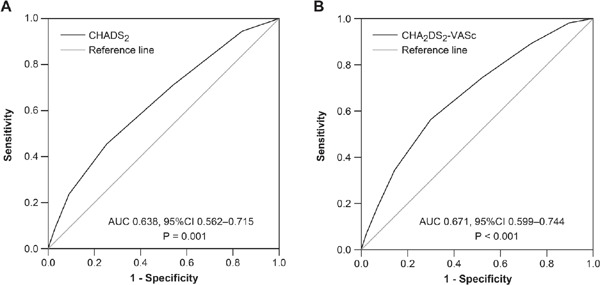
Receiver operating characteristic (ROC) curves for the CHADS2 (A) and CHA2DS2-VASc (B) scores for prediction of ischemic stroke or TIA
Fig. 3.
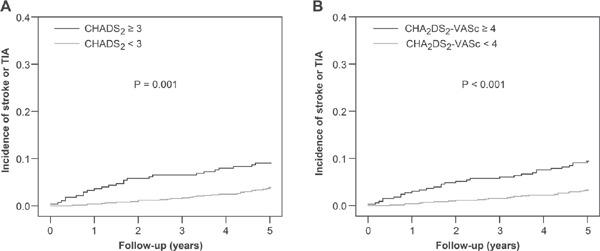
Kaplan–Meier curves showing the incidence of ischemic stroke or TIA stratified by CHADS2 and CHA2DS2-VASc scores; A: Patients with a CHADS2 score ≥ 3 had a higher incidence of ischemic stroke or TIA than those with a CHADS2 score <3 (P = 0.001). B: Patients with a CHA2DS2-VASc score ≥ 4 had a higher incidence of ischemic stroke or TIA than those with a CHA2DS2-VASc score <4 (P <0.001).
Similarly, there was an ascending pattern of the incidence of ischemic stroke or TIA with increasing CHA2DS2-VASc scores: for a CHA2DS2-VASc score of 0, 1, 2, 3, and ≥ 4, the event incidences were 0.19, 0.59, 0.76, 0.88, and 2.0 per 100 person-years, respectively (Fig. 1B). ROC curve analysis showed that the CHA2DS2-VASc score (AUC, 0.671; 95% CI, 0.599–0.744; P <0.001) (Fig. 2B) was also a significant predictor of ischemic stroke or TIA. The optimal cut-off point for a CHA2DS2-VASc score displaying the best predictive value was ≥ 4 (sensitivity = 0.564 and specificity = 0.700). The incidence of ischemic stroke or TIA was significantly higher in patients with a CHA2DS2-VASc score ≥ 4 than in those with a CHA2DS2-VASc score < 4 (the log-rank test, P <0.001) (Fig. 3B).
The multivariate Cox regression analysis showed that the CHADS2 score [hazard ratio (HR), 1.442; 95% CI, 1.171–1.774; P = 0.001] was independently associated with ischemic stroke or TIA following adjustment for smoking, LA diameter, antiplatelet agents, angiotensin inhibitors, and statins in model 1 (Table 2). The CHA2DS2-VASc score (HR, 1.420; 95% CI, 1.203–1.677; P <0.001) was also an independent predictor of ischemic stroke or TIA following adjustment for the same potential clinical confounders in model 2 (Table 2). In addition, the multivariate analysis showed that the LA diameter was also independently associated with ischemic stroke or TIA in model 1 (HR, 1.067; 95% CI, 1.006–1.133; P = 0.032) and model 2 (HR, 1.065; 95% CI, 1.004–1.131; P = 0.037) (Table 2).
Table 2. Risk of ischemic stroke or TIA using multivariate analysis.
| HR (95%CI) | P-values | |
|---|---|---|
| Model 1 | ||
| Smoking | 1.719 (0.967–3.056) | 0.065 |
| LA diameter | 1.067 (1.006–1.133) | 0.032 |
| Antiplatelet Agent | 1.696 (0.618–4.654) | 0.305 |
| Angiotensin inhibitors | 0.886 (0.503–1.560) | 0.675 |
| Statins | 0.703 (0.256–1.930) | 0.494 |
| CHADS2 score | 1.416 (1.147–1.749) | 0.001 |
| Model 2 | ||
| Smoking | 1.792 (1.008–3.185) | 0.047 |
| LA diameter | 1.065 (1.004–1.131) | 0.037 |
| Antiplatelet Agent | 1.381 (0.507–3.762) | 0.528 |
| Angiotensin inhibitors | 0.887 (0.504–1.562) | 0.679 |
| Statins | 0.676 (0.253–1.808) | 0.436 |
| CHA2DS2-VASc score | 1.402 (1.184–1.661) | <0.001 |
HR, hazard ratio; CI, confidence interval. For other abbreviations, see Table 1.
In 18.6% (195/1046) of patients, there was an incidental finding of AF at the hospital discharge diagnosis. To determine whether the predictive abilities of the CHADS2 and CHA2DS2-VASc scores for ischemic stroke or TIA were mediated by incidental AF, the ROC curve analysis was repeated after excluding patients who developed AF during follow-up. However, excluding these patients from the analysis had no impact on the predictive ability of these risk-scoring methods for ischemic stroke or TIA (CHADS2: AUC, 0.643; 95% CI, 0.555–0.730; P = 0.002, and CHA2DS2-VASc: AUC, 0.673; 95% CI, 0.589–0.757; P <0.001). The ROC curve analysis was also performed to evaluate the utility of these scores for prediction of new-onset AF. The analysis showed that the CHADS2 score (AUC, 0.578; 95% CI, 0.533–0.622; P = 0.001) and the CHA2DS2-VASc score (AUC, 0.623; 95% CI, 0.583–0.662; P <0.001) were both predictive of new-onset AF.
Discussion
The main findings presented in this study are as follows: (1) CHADS2 and CHA2DS2-VASc scores may be used to predict ischemic stroke or TIA in patients with IAB without a history of AF and (2) CHADS2 scores ≥ 3 and CHA2DS2-VASc scores ≥ 4 displayed the highest predictive value for ischemic stroke or TIA.
It was previously reported that IAB is a common but poorly recognized condition in the hospital inpatient population2, 32–34) and that patients with IAB have an increased risk of ischemic stroke8–10). Ischemic stroke is a major cause of premature death and disability and can occur without any warning symptoms20, 22, 35, 36). Hence, it is crucial to identify IAB patients who are at high risk of ischemic stroke.
In the present study, we sought to investigate the role of the CHADS2 and CHA2DS2-VASc scores in predicting ischemic stroke or TIA in patients with IAB without a history of AF. The ROC curve analysis showed that CHADS2 and CHA2DS2-VASc scores were both predictive of ischemic strokes or TIA, and multivariate analysis showed that CHADS2 and CHA2DS2-VASc were independently associated with ischemic stroke or TIA. Several potential mechanisms may explain these findings. First, the various components of the CHADS2 and CHA2DS2-VASc scoring systems may include independent risk factors for ischemic stroke. In 1997, Dries and colleagues reported that patients with heart failure are at a risk of embolism due to ventricular thrombi, even in sinus rhythm37). In 2007, Ariyarajah and colleagues found that there was direct and significant correlation between hypertension and embolic stroke10). Furthermore, a recent study found that diabetes mellitus and dyslipidemia, along with hypertension and smoking, are the leading risk factors for the occurrence of ischemic stroke38). In addition, patients with high CHADS2 and CHA2DS2-VASc scores may have a higher risk of developing AF. In a study by Mitchell and colleagues17), the CHADS2 and CHA2DS2-VASc scores were predictive for subsequent hospital discharge with a diagnosis of AF in acute coronary syndrome. In 2013, Chao and colleagues39) also reported that the incidence of AF progressively increased when patients had more complicated systemic diseases, as indicated by a high CHADS2 score; this is in agreement with the finding of Tischer and colleagues that the prevalence of AF increases with increasing CHADS2 and CHA2DS2-VASc scores40). These studies support the finding that higher CHADS2 and CHA2DS2-VASc scores can identify patients who are more likely to develop asymptomatic AF. Finally, the various components of the CHADS2 and CHA2DS2-VASc scores may directly contribute to LA remodeling, a process characterized by atrial dilatation and mechanical dysfunction41); this may lead to blood stasis and an increased thromboembolic risk, regardless of the cardiac rhythm.
One of the findings of the present study was that there were patients without baseline AF who were subsequently diagnosed with AF. Exclusion of these patients from the analysis did not change the predictive ability of the risk-scoring methods to predict ischemic stroke or TIA. This suggests that CHADS2 and CHA2DS2-VASc scores can predict ischemic stroke or TIA in patients with IAB but no history of AF, regardless of the incidental finding of AF during follow-up. In agreement with these findings, IAB has previously been reported to be associated with the subsequent development of AF3–6). Also, our study found that CHADS2 and CHA2DS2-VASc scores were both predictive of new-onset AF, which is in accordance with the findings of a recent study by Mitchell and colleagues17).
In the present study, patients in the stroke or TIA group had larger LA diameters than those in the no-stroke or TIA group. This may be because patients in the stroke or TIA group had higher CHADS2 or CHA2DS2-VASc scores, which are associated with LA enlargement42, 43). In addition to the CHADS2 and CHA2DS2-VASc scores, the LA diameter was also found to be independently associated with ischemic stroke or TIA in the multivariate analysis. This is supported by the findings of previous studies24–27), which showed that LA enlargement may be a risk factor for ischemic stroke. In addition, more patients in the stroke or TIA group were receiving antiplatelet agents compared with the no-stroke or TIA group. There are two potential explanations for this finding. First, patients in the stroke or TIA group had a higher prevalence of previous stroke or TIA, for which antiplatelet therapy is recommended by the stroke prevention guidelines44, 45), than those in the no-stroke or TIA group. Second, patients in the stroke or TIA group had higher CHADS2 and CHA2DS2-VASc scores, indicating more atherosclerotic risk factors; thus, these patients may have had a higher prevalence of antiplatelet agent use.
There were several limitations to this study. Ischemic stroke and TIA were analyzed in a global manner, and no distinction was made between the various causes of these conditions (embolic, atherothrombotic, etc.). As we excluded patients previously diagnosed with AF at baseline ECG from this study, it is possible that individuals with asymptomatic paroxysmal AF who were in sinus rhythm at the time of the baseline ECG could have been misclassified as non-AF participants and included in the analysis. It is also possible that AF was under-diagnosed, as it was identified using hospital discharge data. However, since AF is asymptomatic in some patients, only continuous ECG monitoring would have correctly classified all patients. Additionally, because this study did not evaluate the prognostic performance of the CHADS2 and CHA2DS2-VASc scores for predicting the risk of ischemic stroke in patients without IAB, it was difficult to determine whether the utility of these scores for predicting the risk of ischemic stroke was specific for patients with IAB; thus, further studies are needed. Finally, prescription data obtained during follow-up was unavailable, which may have affected the accuracy of the predictive models.
In conclusion, the CHADS2 and CHA2DS2-VASc scores may be predictors of the risk of ischemic stroke or TIA in patients with IAB without AF.
Acknowledgements
This research was supported by the Medical Science and Technology Project of the Henan Province (No. 201403163).
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1). Bayés de Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R, Bayés-Genis A, Guindo J, Viñolas X, Garcia-Niebla J, Barbosa R, Stern S, Spodick D: Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol, 2012; 45: 445-451 [DOI] [PubMed] [Google Scholar]
- 2). Chhabra L, Devadoss R, Chaubey VK, Spodick DH: Interatrial Block in the Modern Era. Curr Cardiol Rev, 2014; 10: 181-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Bayés de Luna A, Cladellas M, Oter R, Torner P, Guindo J, Martí V, Rivera I, Iturralde P: Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J, 1988; 9: 1112-1118 [DOI] [PubMed] [Google Scholar]
- 4). Agarwal YK, Aronow WS, Levy JA, Spodick DH: Association of interatrial block with development of atrial fibrillation. Am J Cardiol, 2003; 91: 882. [DOI] [PubMed] [Google Scholar]
- 5). Conde D, Baranchuk A: Interatrial block as anatomical–electrical substrate for supraventricular arrhythmias: Bayes' syndrome. Arch Mex Cardiol, 2014; 84: 32-40 [DOI] [PubMed] [Google Scholar]
- 6). Conde D, Seoane L, Gysel M, Mitrione S, Bayés de Luna A, Baranchuk A: Bayés' syndrome: the association between interatrial block and supraventricular arrhythmias. Expert Rev Cardiovasc Ther, 2015; 13: 541-550 [DOI] [PubMed] [Google Scholar]
- 7). Goyal SB, Spodick DH: Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J, 2001; 142: 823-827 [DOI] [PubMed] [Google Scholar]
- 8). Lorbar M, Levrault R, Phadke JG, Spodick DH: Interatrial block as a predictor of embolic stroke. Am J Cardiol, 2005; 95: 667-668 [DOI] [PubMed] [Google Scholar]
- 9). Ariyarajah V, Apiyasawat S, Najjar H, Mercado K, Puri P, Spodick DH: Frequency of interatrial block in patients with sinus rhythm hospitalized for stroke and comparison to those without interatrial block. Am J Cardiol, 2007; 99: 49-52 [DOI] [PubMed] [Google Scholar]
- 10). Ariyarajah V, Puri P, Apiyasawat S, Spodick DH: Interatrial block: a novel risk factor for embolic stroke? Ann Noninvasive Electrocardiol, 2007; 12: 15-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ: Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA, 2001; 285: 2864-2870 [DOI] [PubMed] [Google Scholar]
- 12). Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ: Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest, 2010; 137: 263-272 [DOI] [PubMed] [Google Scholar]
- 13). Glotzer TV, Hellkamp AS, Lee KL, Lamas GA: CHA2DS2-VAS(C) and CHADS2 scores predict adverse clinical events in patients with pacemakers and sinus node dysfunction independent of atrial fibrillation. Can J Cardiol, 2015; 31: 1004-1011 [DOI] [PubMed] [Google Scholar]
- 14). Hsu PC, Chiu CA, Chu CY, Lee WH, Su HM, Lin TH, Voon WC, Lai WT, Sheu SH: CHADS2 score and risk of new-onset peripheral arterial occlusive disease in patients without atrial fibrillation: a nationwide cohort study in Taiwan. J Atheroscler Thromb, 2015; 22: 490-498 [DOI] [PubMed] [Google Scholar]
- 15). Sciacqua A, Perticone M, Tripepi G, Tassone EJ, Cimellaro A, Mazzaferro D, Sesti G, Perticone F: CHADS2 and CHA2DS2-VASc scores are independently associated with incident atrial fibrillation: the Catanzaro Atrial Fibrillation Project. Intern Emerg Med, 2015; 10: 815-821 [DOI] [PubMed] [Google Scholar]
- 16). Yin L, Ling X, Zhang Y, Shen H, Min J, Xi W, Wang J, Wang Z: CHADS2 and CHA2DS2-VASc scoring systems for predicting atrial fibrillation following cardiac valve surgery. PLoS One, 2015; 10: e0123858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB, APPROACH investigators : Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart, 2014; 100: 1524-1530 [DOI] [PubMed] [Google Scholar]
- 18). Morillas P, Pallarés V, Fácila L, Llisterri JL, Sebastián ME, Gómez M, Castilla E, Camarasa R, Sandin M, García-Honrubia A, FAPRES registry investigators : The CHADS2 Score to Predict Stroke Risk in the Absence of Atrial Fibrillation in Hypertensive Patients Aged 65 Years or Older. Rev Esp Cardiol (Engl Ed), 2015; 68: 485-491 [DOI] [PubMed] [Google Scholar]
- 19). Wu JT, Long DY, Dong JZ, Wang SL, Fan XW, Yang HT, Duan HY, Yan LJ, Qian P, Yang CK: Advanced interatrial block predicts clinical recurrence of atrial fibrillation after catheter ablation. J Cardiol, 2015. November 20. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20). Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism : An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2013; 44: 2064-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Tei H, Uchiyama S, Koshimizu K, Kobayashi M, Ohara K: Correlation between symptomatic, radiological and etiological diagnosis in acute ischemic stroke. Acta Neurol Scand, 1999; 99: 192-195 [DOI] [PubMed] [Google Scholar]
- 22). WHO MONICA Project Principal Investigators : The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol, 1988; 41: 105-114 [DOI] [PubMed] [Google Scholar]
- 23). Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ, Cigarette smoking as a risk factor for stroke : The Framingham Study. JAMA, 1988; 259: 1025-1029 [PubMed] [Google Scholar]
- 24). Di Tullio MR, Sacco RL, Sciacca RR, Homma S: Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke, 1999; 30: 2019-2024 [DOI] [PubMed] [Google Scholar]
- 25). Piotrowski G, Banach M, Gerdts E, Mikhailidis DP, Hannam S, Gawor R, Stasiak A, Rysz J, Gawor Z: Left atrial size in hypertension and stroke. J Hypertens, 2011; 29: 1988-1993 [DOI] [PubMed] [Google Scholar]
- 26). Pierdomenico SD, Pierdomenico AM, Di Carlo S, Di Tommaso R, Cuccurullo F: Left atrial enlargement and risk of ischemic stroke in elderly treated hypertensive patients. Am J Hypertens, 2014; 27: 1179-1184 [DOI] [PubMed] [Google Scholar]
- 27). Shin HY, Jeong IH, Kang CK, Shin DJ, Park HM, Park KH, Sung YH, Shin DH, Noh Y, Lee YB: Relation between left atrial enlargement and stroke subtypes in acute ischemic stroke patients. J Cerebrovasc Endovasc Neurosurg, 2013; 15: 131-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Hart RG, Benavente O, McBride R, Pearce LA: Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: A meta-analysis. Ann Intern Med. 1999; 131: 492-501 [DOI] [PubMed] [Google Scholar]
- 29). Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K: Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation, 2000; 101: 2612-2617 [DOI] [PubMed] [Google Scholar]
- 30). Tsang TS, Barnes ME, Abhayaratna WP, Cha SS, Gersh BJ, Langins AP, Green TD, Bailey KR, Miyasaka Y, Seward JB: Effects of quinapril on left atrial structural remodeling and arterial stiffness. Am J Cardiol, 2006; 97: 916-920 [DOI] [PubMed] [Google Scholar]
- 31). Bucher HC, Griffith LE, Guyatt GH: Effect of HMG-CoA reductase inhibitors on stroke. Ann Intern Med, 1998; 128: 89-95 [DOI] [PubMed] [Google Scholar]
- 32). Spodick D, Ariyarajah V: Interatrial Block: The pandemic remains poorly perceived. Pacing Clin Electrophysiol, 2009; 32: 667-672 [DOI] [PubMed] [Google Scholar]
- 33). Asad N, Spodick DH: Prevalence of interatrial block in a general hospital population. Am J Cardiol, 2003; 91: 609-610 [DOI] [PubMed] [Google Scholar]
- 34). Proietti R, Russo V, Sagone A, Viecca M, Spodick DH: Interatrial block: an under-recognized electrocardiographic diagnosis with important clinical-therapeutic implications. G Ital Cardiol (Rome), 2014; 15: 561-568 [DOI] [PubMed] [Google Scholar]
- 35). Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation, 2015. December 16. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36). Sorensen AG, Ay H: Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am, 2011; 21: 303-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Dries DL, Rosenberg YD, Waclawiw MA, Domanski MJ: Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials. J Am Coll Cardiol, 1997; 29: 1074-1080 [DOI] [PubMed] [Google Scholar]
- 38). Djelilovic-Vranic J, Alajbegovic A, Zelija-Asimi V, Niksic M, Tiric-Campara M, Salcic S, Celo A: Predilection role diabetes mellitus and dyslipidemia in the onset of ischemic stroke. Med Arch, 2013; 67: 120-123 [DOI] [PubMed] [Google Scholar]
- 39). Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Chen TJ, Chen SA: CHADS2 score and risk of new-onset atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol, 2013; 168: 1360-1363 [DOI] [PubMed] [Google Scholar]
- 40). Tischer Ts, Schneider R, Lauschke J, Nesselmann C, Klemm A, Diedrich D, Kundt G, Bänsch D: Prevalence of atrial fibrillation in patients with high CHADS2- and CHA2DS2VASc-scores: anticoagulate or monitor high-risk patients? Pacing Clin Electrophysiol, 2014; 37: 1651-1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Casaclang-Verzosa G, Gersh BJ, Tsang TSM: Structural and functional remodeling of the left atrium clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol, 2008; 51: 1-11 [DOI] [PubMed] [Google Scholar]
- 42). Hrynkiewicz-Szymanska A, Dluzniewski M, Platek AE, Szymanski FM, Syska-Suminska J, Klos-Szadryn A, Glinka M, Strojek M, Kuciej A, Tomaszewska-Kiecana M: Association of the CHADS2 and CHA 2DS 2-VASc scores with left atrial enlargement: a prospective cohort study of unselected atrial fibrillation patients. J Thromb Thrombolysis, 2015; 40: 240-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Park JH, Joung B, Son NH, Shim JM, Lee MH, Hwang C, Pak HN: The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace, 2011; 13: 1541-1549 [DOI] [PubMed] [Google Scholar]
- 44). Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease : Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2014; 45: 2160-2236 [DOI] [PubMed] [Google Scholar]
- 45). Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA, American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension : Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2014; 45: 3754-3832 [DOI] [PMC free article] [PubMed] [Google Scholar]


