Abstract
Frankfurter sausages were reformulated to produce better lipid compositions by replacing the pork backfat by healthy oils. Sausages in, three different batches were manufactured: control (CO) with 100% of pork backfat, and modified sausages where the pork backfat was replaced with 50% by microencapsulated fish oil (ME) and by unencapsulated olive and fish oil mixture (OM). The ME treatments showed the lowest pH, fat and energy values and the highest protein and carbohydrates levels. The fat replacement by oils significantly (P < 0.05) affected to color parameters, since the ME batches presented the highest L* and b* values, whereas the OM treatments showed the highest values of a* values. As expected, the replacement of backfat by oils also greatly modified the fatty acids profile, since the OM group had the highest MUFA and n-3 PUFA contents. The microencapsulation process significantly (P < 0.001) increased the lipid oxidation. The ME batch presented the highest TBARS values and volatile compounds derivate from lipid oxidation, while the OM treatment showed the same lipid oxidation rate as CO group.
Keywords: Fat replacement, Physicochemical properties, Fatty acids, Volatile compounds, Microencapsulated fish oil, Olive oil
Introduction
Frankfurter-type sausages are non-fermented emulsified sausages with a 20–30% fat content, being chicken skin, beef, and pork, the main fat sources used for this product (Ospina et al. 2015). Pork backfat is the most interesting of these fats due to its technical benefits during meat processing and to the flavor and texture characteristics it provides to the final products (Ospina et al. 2010). But, consumers are becoming more aware of unhealthy effects of high amount of fat and saturated fatty acids in meat products (Josquin et al. 2012). Which together with a trend to health lifestyle did food industry has been focused on reducing the consumption of animal fats in recent years (Ritzoulis et al. 2010).
Frankfurters have a large market, especially among a particular sector of the population (Salcedo-Sandoval et al. 2013), thus, it is very important to keep as much as possible the sensory quality of the final product at the same time the quality of lipids is improved. Taking into account that recommendations about lipid consumption (WHO 2010) encourage to reduce the intake of saturated fat and to increase the unsaturated lipids, one way to enhance fatty acids profile and to obtain functional and healthier frankfurters is through the reformulation of the product, replacing part of pork backfat by vegetable or marine oils. This can be done through direct addition of liquid oil, pre-emulsion of oil and encapsulation of oil in the product formulation (Jiménez-Colmenero 2007).
On the other hand, recently, there has been a growing interest in developing products with a health concept such as a high n-3 fatty acid product (Lee et al. 2007). A way to increase the intake of n-3 polyunsaturated fatty acids (PUFA) is by incorporating them in traditional products (Marchetti et al. 2014; Domínguez et al. 2016a; Lorenzo et al. 2016). That is why fish oil can be another good fat replacer from the health point of view. Fish and other sea-foods are a source of long chain n-3 PUFA like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are recognized as essential constituents for normal animal development and growth (Zhang et al. 2010). In addition, the increase in the consumption of PUFA had been related with potential health effects as keep normal cholesterol blood level. Nevertheless, long chain n-3 PUFA are exceptionally susceptible to oxidation processes, therefore the microencapsulation technique would be a good alternative to control lipid oxidation (Jimenez-Martín et al. 2015a). Other strategy to decrease saturated fatty acids in traditional meat products and improve their nutritional characteristics is increase the proportion of monounsaturated fatty acids (MUFA). In fact, there are many meat products that pork backfat was replaced by olive oil, such as frankfurter sausages (Jimenez-Colmenero et al. 2010; Delgado-Pando et al. 2010; Herrero et al. 2012), chicken sausages (Andrés et al. 2009) or pâté (Domínguez et al. 2016b). Olive oil is also rich in tocopherols which act as antioxidants such as the phenolic substances and several components have beneficial health effects on the atherosclerotic (especially oleic acid and the polyphenols) and thrombotic pathways, which include lipid oxidation, hemostasis, platelet aggregation, coagulation, and fibrinolysis (Huang and Sumpio 2008). With this regards, Reddy et al. (2015) showed the functional components and properties of olive and its importance in meat and meat products in terms of animal fat replacer. Therefore, the objective of this research was to investigate the effect of the partial replacement of pork backfat by healthy oils (microencapsulated fish oil and olive and fish oil mixture) on chemical composition, color parameters, fatty acids and volatile compounds of frankfurter sausages.
Materials and methods
Raw material and ingredients used in frankfurter sausages formulation
Frankfurter sausages were prepared using fresh pork (shoulder lean, heart, jowl and backfat) purchased from a local processor at 48 h post-mortem. All subcutaneous and visible connective tissue were removed from fresh shoulder muscle. The fatty acids profile and the cholesterol content of backfat, olive oil and fish oil were analysed according to the procedures described below. Fatty acid and cholesterol composition of the pork backfat was as follows: MUFA, 47.25% [oleic acid, 40.53%]; SFA, 34.59%; PUFA, 18.15%; n-3 PUFA, 1.30% [eicosapentaenoic acid (EPA), 0.01% and docosahexaenoic acid (DHA), 0.04%]; cholesterol, 44.04 mg/100 g. Fish oil (provided by Biomega Natural Nutrients S.L., Galicia, Spain) was used as source of n-3 PUFA. Fatty acid and cholesterol composition of the fish oil was as follows: MUFA, 37.90% [oleic acid, 20.32%]; SFA, 28.90%; PUFA, 33.20%; n-3 PUFA, 28.78% [eicosapentaenoic acid (EPA), 4.78% and docosahexaenoic acid (DHA), 20.55%]; cholesterol, 265.38 mg/100 g. The fish oil contains 0.02% of BHT as antioxidant. Some of this fish oil was also microencapsulated by Biomega Natural Nutrients S.L. (Galicia, Spain). The monolayered microcapsules were prepared with maltodextrin, gum arabic and caseinate as follow: In the first step, a solution of 120 kg of water (80%), 19.5 kg of maltodextrin (13%), 9 kg of caseinate (6%) and 1.5 kg of gum arabic (1%) was mixed at 75 °C until complete dissolution of the components. Then the solution was cooled to 40 °C, and 10 kg of the fish oil was added and mixed. In the second step, the previous mix was homogenized at 80 °C during 2 h using a homogenizer Ariete Mod. NS3006H (Niro Soavi S.p.A, Parma, Italy). In the final step, the homogenate was dried using a spray-dryer Galaxy Mod. 2520 (Galaxy Secado Spray, Buenos Aires, Argentina). The homogenate was maintained at room temperature during the spray-drying process. The aspirator feed rate was 75 L/h, inlet temperature was 180 °C and outlet temperature 80 °C. The drying process lasts 3 h. The collected dried powders were stored in containers at room temperature until frankfurter sausages elaboration. The microencapsulated fish oil powder containing 35% of fish oil. The fish oil amount of the microencapsulated powder was quantified according to the AOCS Official Procedure Am 5-04 (AOCS 2005).
On the other hand, the extra-virgin olive oil (provided by Aceites Abril S.L., Galicia, Spain) was used as source of MUFA. Fatty acid composition of the olive oil was as follows: MUFA, 77.68% [oleic acid, 74.39%]; SFA, 14.92%; PUFA, 7.39%; n-3 PUFA, 0.73% [eicosapentaenoic acid (EPA), 0.05% and docosahexaenoic acid (DHA), not detected]. The commercial mix “073 Salchichas frankfurter” was provided by Laboratorios Ceylamix (Valencia, Spain) and it was composed, in unknown proportions, of potato starch, salt, dextrose, spices, soy and milk protein, monosodium glutamate (E621), phosphates (E450i and E452i), sodium ascorbate (E301), sodium nitrite (E250) and paprika extract (E160c).
Sausages preparation and processing
For this study, three different batches were manufactured: a control batch (CO), prepared only with pre-emulsified pork backfat; a batch enriched with microencapsulated fish oil (ME) since the 50% of pork backfat were replaced by microencapsulated fish oil powder (containing 35% of fish oil); a batch enriched with a mixture of olive and fish oil (OM) since the 50% of pork backfat were replaced by a mixture of extra-virgin olive and fish oil (in 1:1 proportion). The fat replacement by oils was performed weight by weight. The ingredients of the three formulations are listed in Table 1. The backfat (CO batch) or oils (ME and OM treatments) were pre-emulsified with sodium caseinate. Water (approximately 60 °C) and sodium caseinate were mixed with an Ultraturrax T25 basic (IKA-Werke, Staufen, Germany) for 2 min at a ratio of 5:1. Next, five parts of oil or pork backfat were added and emulsified for 3 min. After emulsifying, the mixture was cooled to room temperature (approximately 25 °C).
Table 1.
Recipe used for the preparation of Frankfurt sausages elaborated with backfat replacement by healthy oils (expressed as g/100 g of raw batter)
| Ingredients | Batch | ||
|---|---|---|---|
| CO | ME | OM | |
| Fat/oil emulsion | |||
| Pork backfat | 9.2 | 4.6 | 4.6 |
| Olive oil | 2.3 | ||
| Fish oil | 2.3 | ||
| Microencapsulated fish oil | 4.6 | ||
| Sodium caseinate | 1.8 | 1.8 | 1.8 |
| Water | 9.2 | 9.2 | 9.2 |
| Pork jowl | 23.4 | 23.4 | 23.4 |
| Pork lean | 10.6 | 10.6 | 10.6 |
| Pork heart | 12.8 | 12.8 | 12.8 |
| Ice | 22.3 | 22.3 | 22.3 |
| Sodium caseinate | 0.6 | 0.6 | 0.6 |
| Commercial Mix for Frankfurt sausages | 10.1 | 10.1 | 10.1 |
CO Control (100% of backfat); ME microencapsulated fish oil (50% backfat; 50% microencapsulated fish oil); OM Oil mixture (50% backfat; 25% fish oil; 25% olive oil)
The CO group was manufactured using 23.4% of pork jowl, 10.6% of pork lean, 12.8 of pork heart and 9.2% of pork backfat. The ME batch was manufactured with 50% of backfat substitution by microencapsulated fish oil powder (containing 35% of fish oil), whereas OM treatment was manufactured with 50% of backfat substitution by a mixture of extra-virgin olive and fish oil (1:1). The pork meat, jowl and heart were cut in a cutter (Cutter K30, Talsa, Talsabell S.A., Valencia, Spain) for 1 min. The pork backfat was added and the meat mixture was chopped for 1 min. Then, the pre-emulsified oils (ME and OM batches), ice (22.3 g/100 g), sodium caseinate (0.6 g/100 g) and commercial mix for frankfurter sausages (10.1 g/100 g) (Ceylamix®: 073 Salchichas frankfurter, Manufacturas Ceylan S.L., Valencia, Spain) was added to meat mixture and mixed for 5 min. Final temperature of batter was maintained below 8 °C throughout preparation. After emulsification, meat batter was stuffed into 25 mm collagen casings and hand-linked to form links approximately 8 cm in length. The raw sausages were then cooked in a temperature-controlled bath-water (Marmite Mera 120*70, Talsa, Talsabell S.A., Valencia, Spain) at 90 °C for 20 min. The cooked meat sausages were cooled in an ice water-bath, placed in polyethylene bags, vacuum packaged and pasteurized at 90 °C for 30 min. Finally, frankfurters were maintained at 2 °C during 24 h until the laboratory analysis. The three batches mentioned before (CO, ME and OM) were manufactured with the same ingredients, formulation and technology in two different weeks (two replicates). After pH and color analysis, the four frankfurter sausages (about 10 g per sausage) of each package were minced and were considered as one sample. All analyses were carried out in triplicate for each formulation.
Physicochemical properties
The pH of the samples was measured using a digital portable pH-meter (Hanna Instruments, Eibar, Spain) equipped with a penetration probe. Color parameters were measured using a portable colorimeter (Konica Minolta CM-600d, Osaka, Japan) with pulsed xenon arc lamp filtered to illuminant D65 lighting conditions, 0° viewing angle geometry and 8 mm aperture size, to estimate sausages color in the CIELAB space: lightness, (L*); redness, (a*); yellowness, (b*). The sausages were sliced and the color was measured in cut surface in three different points of each sample. Before each series of measurements, the instrument was adjusted using a white ceramic tile.
Proximate composition analysis
Moisture, protein and ash were quantified according to the ISO recommended standards (ISO: 1442 1997; ISO 937:1978 and ISO 936:1998, respectively). Total fat was extracted according to the AOCS Official Procedure Am 5-04 (AOCS 2005), while carbohydrate contents were calculated by the difference. For determination of total cholesterol, saponification, extraction and identification was performed in normal phase following the procedure described by Domínguez et al. (2015). Total calorie estimates (kcal) for frankfurter sausages were calculated on the basis of a 100 g portion using Atwater values for fat (9 kcal/g), protein (4.02 kcal/g), and carbohydrate (3.87 kcal/g) (Mansour and Khalil 1999).
Fatty acids
Total fat was extracted from 12.5 g of ground sausage sample, according to Bligh and Dyer (1959) method. Fifty milligrams of fat was used to determine the fatty acid profile. Total fatty acids were transesterified according to Domínguez et al. (2015) procedure. Separation and quantification of the fatty acids methyl esters (FAMEs) was carried out using a gas chromatograph (GC-Agilent 6890 N; Agilent Technologies Spain, S.L., Madrid, Spain) equipped with a flame ionization detector following the chromatographic conditions described by Domínguez et al. (2015). Data regarding FAME composition were expressed in g/100 g of fat.
Lipid oxidation (TBARS values and volatile compounds)
The 2-thiobarbituric acid (TBARS) assay was carried out according to the extraction method described by Vyncke (1975). The TBARS values were calculated from a standard curve (between 0.2 and 1 µg MDA) performed with 1,1,3,3-tetraethoxypropane and the final results were expressed as mg MDA/kg sample. The analysis of the volatile compounds was performed using HS-SPME-GC/MS method. One gram of each sample was minced and weighed into a 24 mL headspace vial and sealed with a PTFE-faced silicone septum (Supelco, Bellefonte, PA, USA). A SPME device (Supelco, Bellefonte, PA, USA) containing a fused silica fibre (10 mm length) coated with a 50/30 μm layer of DVD/CAR/PDMS was used. Headspace SPME extraction and chromatographic conditions were carried out as described by Domínguez et al. (2014). The results are expressed as area units (AU) × 106/g of dry matter.
Statistical analysis
A total of 36 samples (six samples for each batch x three batches x two replicates) were analyzed for different parameters. The effect of different formulation on chemical composition, color parameters, fatty acids and volatile compounds was examined using a mixed-model ANOVA, where these parameters were set as dependent variables, fat source as fixed effect, and replicate as random effect. The pairwise differences between least-square means were evaluated by Duncan’s method. Differences were considered significant if P < 0.05. Correlations between variables were determined by Pearson’s linear correlation coefficient (P < 0.05). The values were given in terms of mean values and standard deviation. All statistical analysis was performed using IBM SPSS Statistics 21 software.
Results and discussion
Physicochemical properties
Physicochemical properties and chemical composition of frankfurter sausages are shown in Table 2. The values of pH were significantly (P < 0.001) affected by the replacement of pork backfat by oils, being in the range of 6.33–6.41. The pH values were slightly higher in OM samples compared to CO batch. This increase, which has been described by other authors (Caceres et al. 2008; Delgado-Pando et al. 2010), was possibly related to the protein component and to the lipid material of the oil-in-water emulsion. However, this was in disagreement with those previously reported by Muguerza et al. (2002) who did not observe any difference in pH values in samples where the backfat was replaced by oil. On the other hand, the sausages manufactured with microencapsulated fish oil (ME batch) presented the lowest pH values. This fact could be related with the low pH value (4.98) that presented the microencapsulated. Contrary, Pelser et al. (2007) did not find difference between the control and the modified products (pre-emulsified oil and encapsulated oil) on pH values (ranged from 4.61 to 4.69).
Table 2.
Proximate composition, calorific content and color properties of frankfurter type sausages formulated with pork backfat replacement by healthy oils
| Parameters | Batch | Sig. | ||
|---|---|---|---|---|
| CO | ME | OM | ||
| pH | 6.39 ± 0.03b | 6.33 ± 0.01a | 6.41 ± 0.01b | *** |
| Composition (g/100 g) | ||||
| Moisture | 62.14 ± 0.41 | 61.69 ± 0.91 | 61.35 ± 0.84 | ns |
| Fat | 15.80 ± 0.46b | 14.41 ± 0.64a | 17.40 ± 0.97c | *** |
| Protein | 12.49 ± 0.39ab | 12.76 ± 0.21b | 12.23 ± 0.26a | * |
| Ash | 2.62 ± 0.08 | 2.59 ± 0.07 | 2.54 ± 0.08 | ns |
| Carbohydrates | 6.87 ± 0.34a | 8.55 ± 0.42b | 6.48 ± 0.13a | *** |
| Cholesterol (mg/100 g) | 12.39 ± 2.23a | 19.45 ± 1.98b | 24.55 ± 2.08c | *** |
| Energy (Kcal/100 g) | 219.94 ± 3.43a | 214.91 ± 6.59a | 231.46 ± 8.27b | ** |
| Color parameters | ||||
| Lightness (L*) | 59.53 ± 3.42a | 62.64 ± 0.41b | 57.71 ± 1.45a | ** |
| Redness (a*) | 12.71 ± 1.35a | 11.91 ± 0.24a | 13.80 ± 0.62b | ** |
| Yellowness (b*) | 17.95 ± 0.67a | 20.80 ± 0.39b | 18.23 ± 0.28a | *** |
Batches: CO Control (100% of backfat); ME microencapsulated fish oil (50% backfat; 50% microencapsulated fish oil); OM Oil mixture (50% backfat; 25% fish oil; 25% olive oil)
a–c Mean values in the same row (corresponding to the same parameter) not followed by a common letter differ significantly (P < 0.05)
Sig significance: * (P < 0.05), ** (P < 0.01), *** (P < 0.001), ns (not significant); SEM Standard error of the mean
Color parameters of frankfurter sausages were significantly (P < 0.01) affected by the replacement of pork fat with oils (Table 2). These parameters were closely related to color properties of raw material used in the formulation, and therefore, the proportion of the ingredients used might led to different colors in the final product (Jiménez-Colmenero et al. 2010). These findings were in agreement with those observed by Domínguez et al. (2016) who found significant difference in color parameters in pâté samples where the backfat was replaced by oil in the formulation. According with Marquez et al. (1989), lightness (L*) values were significantly (P < 0.01) affected by fat content, since the highest L* values were found in ME group (62.6 vs. 59.5 vs. 57.7 for ME, CO and OM batches, respectively). Regarding redness (a*) values, the use of oil mixture in the manufacture of the frankfurter sausages produced a significantly (P < 0.01) increase of this color parameter respect to CO group. These outcomes were in agreement with those reported by Morales-Irigoyen et al. (2012) as to lightness and redness, since they found significant (P < 0.05) changes in these parameters when backfat was substituted by emulsified canola oil. However, Pelser et al. (2007) did not show difference between the control and the modified products (pre-emulsified oil and encapsulated oil) on lightness and redness values.
On the other hand, the highest yellowness (b*) values were observed in the sausages manufactured with microencapsulated fish oil (20.8 vs. 17.9 vs. 18.2, for ME, CO and OM treatments, respectively). This fact could be linked to the yellow color of fish oil. This was in agreement with data reported by Pelser et al. (2007) who observed that the control sausages were less yellow than the modified products (pre-emulsified oil and encapsulated oil). In addition, Rodríguez-Carpena et al. (2012) and Domínguez et al. (2016) also observed an increase in yellowness in meat products where pork backfat was replaced with vegetable oil.
Chemical composition
Statistical analysis showed significant (P < 0.05) differences in proximate composition among batches, except for moisture and ash content (Table 1). The addition of the same amount of water in all formulations could be related to the fact that there was no significant difference in moisture content between three formulations. This outcome was in agreement with those reported by Pelser et al. (2007) who did not find difference in moisture content for sausages with encapsulated flaxseed oil and fish oil. As expected, the partial replacement of pork backfat by healthy oils significantly (P < 0.001) affected the fat content of the frankfurter sausages. Unlike as reported in the literature (Muguerza et al. 2002; Josquin et al. 2012), the substitution of pork backfat by microencapsulated oil reduced significantly (P < 0.001) fat content from 15.80 to 14.41%, for CO and ME batches, respectively. This behaviour might be due to microencapsulated fish oil containing only 35% of fish oil. However, the addition of oil mixture significantly (P < 0.001) increased the fat level of the frankfurter sausages till 17.40%. This fact could be related to the lean meat and membranes inside of pork backfat which would decrease the amount of fat.
The type of fat used in the formulation of frankfurter sausages had a significant (P < 0.05) effect on the protein content. With this regards, Josquin et al. (2012) observed that protein content seemed to be influenced significantly (P < 0.05) by adding encapsulated fish oil and the percentage substitution, probably due to addition of extra dry matter that was in the encapsulated oil. Our protein values were similar to those found by other authors (Delgado-Pando et al. 2010; Salcedo-Sandoval et al. 2013) in frankfurter sausages. Although all batches were manufactured with the same meat content, these differences could be due to the lower contribution of protein from the pork fat as a consequence of reformulation. As opposed to happen with fat, the sausages manufactured with oil mixture displayed the highest protein contents (12.76 vs. 12.49 vs. 12.23% for OM, CO and ME batches, respectively). This was in disagreement with data reported by Pelser et al. (2007) who did not show difference in protein content between the control and the modified products (pre-emulsified oil and encapsulated oil). The replacement of pork backfat with oils did not show significant (P > 0.05) difference in ash content of frankfurter sausages, ranged from 2.62 to 2.54%, for CO and OM treatments, respectively.
Statistical analysis showed that the replacement of pork backfat significantly (P < 0.001) affected the carbohydrate content, since the highest value was observed for frankfurter sausages manufactured with microencapsulated fish oil (8.55 vs. 6.87 vs. 6.48% for ME, CO and OM batches, respectively). This could be related to the ingredients used (maltodextrin, gum arabic and caseinate) to prepare the microcapsules in the ME batch. On the other hand, the cholesterol content vary significantly (P < 0.001) among the three batches, since frankfurter sausages manufactured with oil mixture in its formulation had the highest cholesterol content (Table 2). This could be due to fish oil, extracted from fish liver (where the cholesterol is synthesized and accumulated) and therefore the cholesterol content was inherent to the liver itself. In addition, this result was reflected in cholesterol contents of the ingredients, as reported in materials and methods section, fish oil had content of 265.38 mg/100 g vs. 44.04 mg/100 g that contained pork backfat.
The energy values of the frankfurter sausages showed significant (P < 0.01) difference among batches (Table 2). The substitution of pork backfat with microencapsulated fish oil reduced the energy content compared to CO treatment. However, the frankfurter sausages manufactured with oil mixture in its formulation exhibited the highest energy levels (231.46 vs. 219.94 vs. 214.91 kcal/100 g for OM, CO and ME batches, respectively). As mentioned above, this increase may be associated with fat intake; the formulation with oil mixture involved the addition of oil almost totally. This showed that fat accounted for almost 65% of the total energy content (67.64 vs. 64.63 vs. 60.31% for OM, CO and ME treatments, respectively). In fact, energy content was related with fat content and as reflected in the significant correlation was observed between them (r = 0.96, P < 0.01). Similar result reported by Delgado-Pando et al. (2010) for low-fat frankfurter sausages. However, Choi et al. (2010) noticed that the replacement of animal fat with vegetable oils reduced energy contents.
Fatty acid composition
The effect of the partial replacement of pork backfat by healthy oils on fatty acid profile (g/100 g of fat) of frankfurter sausages is summarised in Table 3. As expected, the pork fat replacement with healthier oils had a significant (P < 0.05) effect on the fatty acid composition of the frankfurter sausages. In fact, 24 out of 25 fatty acids showed significant differences among batches. In all samples the most abundant fatty acids were monounsaturated fatty acids (MUFA), followed by saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA). Fatty acid composition showed that the substitution of pork backfat with microencapsulated fish oil (ME batch) and with fish/olive oil mixture (OM batch) reduced the SFA content from 33.14 g/100 g for CO group to 31.65 and 29.22 g/100 g for ME and OM batches, respectively. Thus, the total amount of SFA in the modified sausages decreased by 4.5-11.8% compared to the CO treatment. Similar results were reported by Choi et al. (2010) who observed that the pork backfat replacement by fish and/or vegetable oils decreased the SFA content by 3–25% in frankfurter type sausages. In addition, Josquin et al. (2012) observed that the total amount of SFA in the encapsulated fish oil sausages decreased by approximately 3–8% compared to the controls. The most abundant SFA were C16:0 and C18:0. It was known that all SFA did not have the same hypercholesterolaemic effect. In the present study, the C16:0 content decreased from 21.04 g/100 g for CO group to 20.21 and 18.81 g/100 g for ME and OM batches, respectively; while the C18:0 level decreased from 10.04 g/100 g for CO treatment to 9.20 and 8.25 g/100 g for ME and OM groups, respectively. This fact is caused by the low SFA amount in fish (28.9%) and olive oil (14.9%) compared to the pork backfat (34.6%). These outcomes are consistent with those reported by Delgado-Pando et al. (2010) and Salcedo-Sandoval et al. (2013), who described a large decreased of these fatty acids content since the pork fat was replaced by vegetable and/or fish oils in frankfurter type sausages.
Table 3.
Fatty acid content (expressed as g/100 g of fat) of frankfurter type sausages formulated with pork backfat replacement by healthy oils
| Fatty acids | Batch | Sig. | ||
|---|---|---|---|---|
| CO | ME | OM | ||
| C12:0 | 0.08 ± 0.01b | 0.08 ± 0.00b | 0.07 ± 0.00a | *** |
| C14:0 | 1.36 ± 0.02a | 1.49 ± 0.02b | 1.37 ± 0.02a | *** |
| C14:1n5 | 0.04 ± 0.00a | 0.05 ± 0.00c | 0.04 ± 0.00b | *** |
| C15:0 | 0.06 ± 0.00a | 0.09 ± 0.00b | 0.10 ± 0.00c | *** |
| C16:0 | 21.04 ± 0.42c | 20.21 ± 0.21b | 18.81 ± 0.17a | *** |
| C16:1n7 | 2.40 ± 0.05a | 2.59 ± 0.03c | 2.46 ± 0.01b | *** |
| C17:0 | 0.30 ± 0.01c | 0.29 ± 0.00b | 0.28 ± 0.01a | *** |
| C17:1n7 | 0.29 ± 0.01b | 0.29 ± 0.01b | 0.27 ± 0.00a | *** |
| C18:0 | 10.04 ± 0.19c | 9.20 ± 0.10b | 8.25 ± 0.14a | *** |
| C18:1n9t | 0.32 ± 0.01c | 0.30 ± 0.01b | 0.28 ± 0.01a | *** |
| C18:1n9c | 37.18 ± 0.72b | 35.41 ± 0.27a | 38.58 ± 0.46c | *** |
| C18:1n7c | 2.50 ± 0.06b | 2.54 ± 0.02b | 2.45 ± 0.01a | ** |
| C18:2n6c | 15.79 ± 0.29c | 14.25 ± 0.15b | 12.76 ± 0.10a | *** |
| C20:0 | 0.15 ± 0.00a | 0.16 ± 0.00b | 0.21 ± 0.01c | *** |
| C20:1n9 | 0.74 ± 0.02a | 1.14 ± 0.02b | 1.24 ± 0.03c | *** |
| C18:3n3 | 0.88 ± 0.01b | 0.88 ± 0.01b | 0.85 ± 0.01a | *** |
| c9,t11-CLA | 0.13 ± 0.00 | 0.12 ± 0.03 | 0.12 ± 0.00 | ns |
| C20:2n6 | 0.65 ± 0.02c | 0.62 ± 0.00b | 0.55 ± 0.01a | *** |
| C20:3n6 | 0.14 ± 0.00b | 0.15 ± 0.01c | 0.13 ± 0.00a | *** |
| C22:1n9 | 0.01 ± 0.00a | 0.11 ± 0.01b | 0.14 ± 0.00c | *** |
| C20:3n3 | 0.12 ± 0.00a | 0.13 ± 0.00b | 0.12 ± 0.00a | *** |
| C20:4n6 | 0.56 ± 0.02a | 0.71 ± 0.05b | 0.72 ± 0.03b | *** |
| C20:5n3 | 0.02 ± 0.01a | 0.35 ± 0.02b | 0.62 ± 0.02c | *** |
| C22:5n3 | 0.12 ± 0.00a | 0.26 ± 0.01b | 0.37 ± 0.01c | *** |
| C22:6n3 | 0.05 ± 0.01a | 1.17 ± 0.06b | 2.30 ± 0.08c | *** |
| SFA | 33.14 ± 0.64c | 31.65 ± 0.33b | 29.22 ± 0.34a | *** |
| MUFA | 43.51 ± 0.85b | 42.52 ± 0.35a | 45.59 ± 0.45c | *** |
| PUFA | 18.50 ± 0.32 | 18.68 ± 0.29 | 18.58 ± 0.16 | ns |
| ∑n3 | 1.18 ± 0.02a | 2.77 ± 0.11b | 4.25 ± 0.11c | *** |
| ∑n6 | 17.18 ± 0.32c | 15.78 ± 0.20b | 14.21 ± 0.10a | *** |
| ∑n6/∑n3 | 14.52 ± 0.32c | 5.70 ± 0.18b | 3.35 ± 0.08a | *** |
| AI | 0.43 ± 0.00b | 0.43 ± 0.00b | 0.38 ± 0.00a | *** |
| TI | 0.95 ± 0.00c | 0.82 ± 0.01b | 0.66 ± 0.00a | *** |
| h/H | 2.56 ± 0.01a | 2.57 ± 0.01a | 2.91 ± 0.00b | *** |
Batches: CO Control (100% of backfat); ME microencapsulated fish oil (50% backfat; 50% microencapsulated fish oil); OM Oil mixture (50% backfat; 25% fish oil; 25% olive oil)
a–c Mean values in the same row (corresponding to the same parameter) not followed by a common letter differ significantly (P < 0.05)
Sig. significance: * (P < 0.05), ** (P < 0.01), *** (P < 0.001), ns (not significant); SEM Standard error of the mean; SFA saturated fatty acids; MUFA monounsaturated fatty acids; PUFA polyunsaturated fatty acids; AI atherogenic index; TI Thrombogenic index; h/H hypocholesterolemic/Hypercholesterolemic ratio
The MUFA content was also affected by the pork backfat replace. In this case, OM batch presented the highest MUFA values (45.59 g/100 g), followed by CO (43.51 g/100 g) and ME (42.52 g/100 g) treatments. These differences were due to the C18:1n9c content (38.58 g/100 g in OM batch vs. 37.18 and 35.41 g/100 g in CO and ME groups, respectively). The incorporation of olive oil (with 74.39% of C18:1n9c) in the OM batch showed the highest MUFA content in sausages. These findings were in agreement with those reported by Choi et al. (2010) and Delgado-Pando et al. (2010) who also observed an increase of MUFA content when the pork backfat was replaced by oils (pure olive oil or mixtures with other oils) in frankfurter sausage formulation (pure or encapsulated fish oil). In contrast, the lower C18:1n9c amounts in the fish oil (20.32%) compared to the pork backfat (40.53%) and the olive oil (74.39%) explained the lower MUFA and C18:1n9c levels in the ME batch.
The PUFA content did not display significant (P > 0.05) difference among the three batches of sausages. Although, total PUFA did not show difference, great changes were observed in the individual PUFA content between control and modified frankfurter sausages. The CO batch had the highest n-6 PUFA value (17.18 g/100 g) and the lowest n-3 PUFA content (1.18 g/100 g). In contrast, the sausages from OM group had the lowest n-6 PUFA content (14.21 g/100 g) and the highest n-3 PUFA levels (4.25 g/100 g), while the ME treatment presented intermediate values (15.78 and 2.77 g/100 g for n-6 and n-3 PUFA amounts, respectively). These differences were related to the fact that sausages from CO batch had the highest C18:2n6c (15.79 vs. 14.25 vs. 12.76 g/100 g for CO, ME and OM groups respectively) values and the lowest C20:5n3 (EPA; 0.02 vs. 0.35 vs. 0.62 g/100 g for CO, ME and OM batches, respectively), C22:5n3 (DPA; 0.12 vs. 0.26 vs. 0.37 g/100 g for CO, ME and OM groups, respectively) and C22:6n3(DHA; 0.05 vs. 1.17 vs. 2.30 g/100 g for CO, ME and OM treatments, respectively) amounts. The higher n-3 PUFA content in modified frankfurter sausages than in the CO batches were caused by the high long-chain n-3 PUFA content in fish oil (4.78% of EPA and 20.55% of DHA). Similar results were obtained in previous studies when pork backfat were substituted by fish oil in sausages formulation (Delgado-Pando et al. 2010; Salcedo-Sandoval et al. 2013).
It is well known that the high PUFA proportion in itself was not necessarily healthy if it is not balanced in relation to the n-6/n-3 ratio, which should not exceed 4 (Simopoulos, 2004). Excessive n-6 PUFA content and high n-6/n-3 PUFA ratios promote several kinds of pathogenesis, including cardiovascular disease, cancer and inflammatory and autoimmune diseases, whereas the increase n-3 PUFA levels (and low n-6/n-3 PUFA ratios) exert suppressive effects (Simopoulos 2004). In our study, the n-6/n-3 ratios decreased from 14.52 for CO batch to 5.70 and 3.35 for ME and OM treatments, respectively. These results agreed with those obtained by Keenan et al. (2015), who also observed that the inclusion of fish oil in beef burgers decreased this ratio. Regarding nutritional indices, the inclusion of olive and fish oil mixture significantly (P < 0.001) decreased the atherogenic (AI) (0.43 vs. 0.38 for CO and OM batches, respectively) and the thrombogenic (TI) (0.95 vs. 0.66 CO and OM treatments, respectively) indexes. On the other hand, while the hypocholesterolemic/hypercholesterolemic (h/H) ratio was significantly (P < 0.001) higher in the OM group (2.91) than in the CO batch (2.56).
Nutritional improvements with the replacement of animal fat by healthier oils were also observed by Delgado-Pando et al. (2010) and Salcedo-Sandoval et al. (2013) who noticed lower AI and TI values and higher h/H ratio in the reformulated meat products than in products manufactured with pork backfat. According to a European Commission Regulation (European Commission 2010) regarding nutritional claims, a “high omega 3 fatty acids” claim may only be made where the product contains a minimum of 80 mg of the sum of EPA and DHA per 100 g of product. In the present study, the ME batch contained 50.4 mg of EPA and 168.6 mg of DHA per 100 g of product, while OM treatment contained 107.9 mg of EPA and 400.2 mg of DHA per 100 g of product. Therefore, reformulated frankfurter sausages may be claimed to be high in omega 3 fatty acids. Therefore, in the present study, the frankfurter sausages formulated with addition of olive and fish oil mixture (OM batch) presented the best nutritional value and balanced fatty acid profile.
Lipid oxidation (TBARS values and volatile compounds)
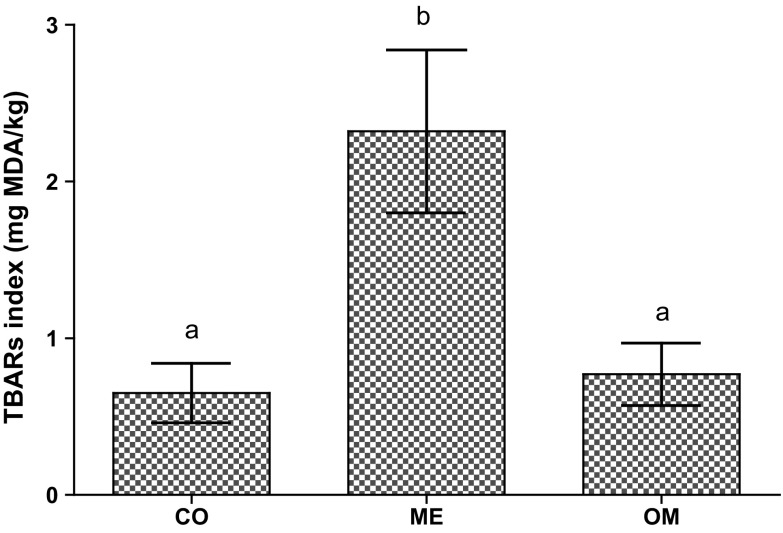
Figure 1 displays the effect of the partial replacement of pork backfat by healthy oils on TBARS values of frankfurter sausages. The batches where pork backfat was replaced by microencapsulated fish oil showed higher TBARS values, reflecting an increase of the lipid oxidation in the fish lipid sausages (with higher levels of unsaturated fat). This fact could be related to the greater susceptibility to lipid oxidation of unsaturated (particularly EPA and DHA) fatty acids, present in high quantities when animal fat was partially replaced by fish oil. This was in disagreement with those reported by Josquin et al. (2012) they reported that frankfurt type sausages elaborated with microencapsulated fish oil presented the lowest TBARS values. This author noticed that encapsulation protected the oxidative sensitive lipids. In the present study, encapsulation did not show the protective desired effect. BHT was not found in microencapsulated sausages, which could be related with the fact that the encapsulation did not allow the BHT to exert its antioxidant effect, or it may have degraded during microencapsulation processes due to the high temperatures used during encapsulation. In fact, the fish oil was maintained at 80 °C during 2 h (homogenisation process) and then was dried at 180 °C. As reported above, the spray-drying process last 3 h. The high temperatures and long-time used in various steps of the process could be related with the high oxidation level of microencapsulated powder, and therefore, in sausages from ME batch. Moreover, other possible explanation was that the microencapsulated powder was maintained at room temperature until sausages manufactured. According to the Jimenez-Martín et al. (2015a), the storage time and temperature also had a great influence in lipid oxidation of fish microencapsulated fish oil.
Fig. 1.
Effect of the partial replacement of pork backfat by healthy oils on TBARs values of Frankfurter sausages. Batches: CO: Control (100 % of backfat); ME: microencapsulated fish oil (50 % backfat; 50 % microencapsulated fish oil); OM: Oil mixture (50 % backfat; 25 % fish oil; 25 % olive oil)
High lipid oxidation has been reported in dry fermented sausage manufacturing with fish oil during dry-ripening process (Muguerza et al. 2004). However, Valencia et al. (2006) did not find oxidation in a product enriched with n-3 PUFAs from fish oil in the presence of antioxidants (BHA + BHT). On the other hand, a recent study evaluated the effect of encapsulation of fish oil in the partial substitution of animal fat in beef burgers and reported higher lipid oxidation index for encapsulated fish oil than the un-encapsulated treatment, which was possible related to oxygen exposure during the encapsulation process, temperature during drying process, susceptibility of fish PUFA to heat and the large surface to oxygen contact (Keenan et al. 2015). It has been stated that the limit of TBARS value that could be considered as the threshold for detection of oxidation flavors by general population was around 2.0 mg/kg sample (Greene and Cumuze 1982). To this regards, TBARS values of the CO and OM batches were lower than the established threshold value, whereas the ME treatment had TBARS values above 2 mg/kg sample.
The effect of the partial replacement of pork backfat by healthy oils on volatile compounds of frankfurter sausages is presented in Table 4. A total of 42 volatile compounds were identified in the three batches by the SPME–GC–MS technique. Nevertheless, aroma perception in meat products depends not only on the concentration and odor thresholds of volatile compounds, but also on their interactions with other food components and among volatile compounds. The compounds were grouped by chemical and their linear retention index, comprising six alkanes, ten terpenes, five ketones, two alcohols, two ethers, two furans, six aldehydes and nine compounds from other classes. Most of the 42 volatile compounds identified have been previously described in frankfurter sausages (Shiratsuchi et al. 1993). The most abundant families were aldehydes (35, 43 and 30% of total volatile compounds, for CO, ME and OM batches, respectively) and terpenes (about 20% in the three batches). These results were in agreement with those reported by Jiménez-Martín et al. (2015b), who observed that aldehydes and terpenes were the groups more abundant in chicken nuggets.
Table 4.
Volatile compounds profile (expressed as AU × 106/g dry matter) of frankfurter type sausages formulated with pork backfat replacement by healthy oils
| Volatile compounds | LRI | R | Batch | Sig. | ||
|---|---|---|---|---|---|---|
| CO | ME | OM | ||||
| Toluene | 781 | m, lri | 5.03 ± 1.43a | 21.67 ± 0.58b | 22.02 ± 3.94b | *** |
| Octane | 800 | m, s, lri | 3.40 ± 1.46a | 9.93 ± 3.01b | 49.10 ± 3.68c | *** |
| Decane | 1000 | m, s, lri | 0.97 ± 0.22a | 4.91 ± 1.64b | 1.96 ± 0.24a | *** |
| Undecane | 1100 | m, s, lri | 1.65 ± 0.35a | 9.58 ± 1.69c | 4.85 ± 1.31b | *** |
| Dodecane | 1200 | m, s, lri | 3.50 ± 1.27 | 4.39 ± 1.44 | 3.55 ± 1.35 | ns |
| Tridecane | 1300 | m, s, lri | 1.48 ± 0.36 | 1.52 ± 0.52 | 1.16 ± 0.46 | ns |
| Total alkanes | 14.22 ± 2.43a | 46.53 ± 8.11b | 81.96 ± 5.03c | *** | ||
| p-Xylene | 915 | m, lri | 1.98 ± 0.12a | 3.66 ± 1.14b | 2.97 ± 0.46b | ** |
| α-Pinene | 971 | m, lri | 17.49 ± 1.65b | 13.39 ± 0.47a | 22.16 ± 0.98c | *** |
| β-Myrcene | 1031 | m, lri | 1.37 ± 0.10a | 3.24 ± 0.11c | 1.86 ± 0.33b | *** |
| α-Terpinene | 1059 | m, lri | 17.65 ± 2.16a | 22.50 ± 4.62b | 24.10 ± 1.51b | ** |
| D-Limonene | 1069 | m, lri | 17.47 ± 1.81a | 39.91 ± 2.60b | 41.69 ± 2.90b | *** |
| γ-Terpinene | 1096 | m, lri | 10.10 ± 1.23 | 11.04 ± 1.12 | 11.43 ± 0.85 | ns |
| α-Terpinolene | 1122 | m, lri | 4.26 ± 0.09a | 17.05 ± 5.60b | 4.67 ± 0.36a | *** |
| α-Cubebene | 1353 | m, lri | 2.79 ± 0.60 | 2.62 ± 0.43 | 3.18 ± 0.27 | ns |
| Caryophyllene | 1394 | m, lri | 2.75 ± 0.61 | 3.07 ± 0.36 | 3.10 ± 0.25 | ns |
| α-Curcumene | 1425 | m, lri | 0.95 ± 0.24 | 1.39 ± 0.61 | 1.09 ± 0.23 | ns |
| Total terpenes | 70.10 ± 5.53a | 117.90 ± 24.54b | 118.15 ± 19.70b | *** | ||
| Acetoin | 766 | m, lri | 5.39 ± 0.13 | 4.89 ± 0.27 | 5.34 ± 0.55 | ns |
| Cyclohexanone, 2,2,6-trimethyl | 1103 | m, lri | 0.00 ± 0.00a | 3.19 ± 0.21b | 0.00 ± 0.00a | *** |
| Acetophenone | 1147 | m, lri | 0.00 ± 0.00a | 3.93 ± 1.25c | 1.27 ± 0.35b | *** |
| 2-Nonanone | 1151 | m, s, lri | 1.06 ± 0.05a | 12.43 ± 4.14b | 2.94 ± 0.34a | *** |
| 3,5-Octadien-2-one | 1167 | m, lri | 0.00 ± 0.00a | 37.39 ± 13.19b | 2.07 ± 0.54a | *** |
| Total ketones | 5.80 ± 0.53a | 60.67 ± 12.62b | 10.71 ± 0.89a | *** | ||
| 1-Octen-3-ol | 1052 | m, lri | 3.99 ± 0.38a | 21.52 ± 6.55c | 8.90 ± 0.64b | *** |
| 4-Terpinenol | 1223 | m, lri | 36.67 ± 4.45a | 44.91 ± 6.49b | 39.57 ± 3.53ab | * |
| Total alcohols | 40.66 ± 4.82a | 65.70 ± 4.99c | 48.47 ± 4.04b | *** | ||
| Eugenol methyl ether | 1388 | m, lri | 7.48 ± 1.65a | 9.92 ± 2.16b | 8.35 ± 1.64ab | ns |
| Isoeugenol methyl ether | 1454 | m, lri | 0.36 ± 0.12b | 0.60 ± 0.09b | 0.00 ± 0.00a | *** |
| Total ethers | 7.79 ± 1.79a | 10.41 ± 2.45b | 8.35 ± 1.64ab | ns | ||
| Furan, 2-ethyl | 668 | m, lri | 0.00 ± 0.00a | 0.00 ± 0.00a | 8.77 ± 1.71b | *** |
| Furan, 2-pentyl | 1037 | m, lri | 6.06 ± 0.93a | 11.50 ± 3.79b | 7.49 ± 2.08a | *** |
| Total furan | 6.06 ± 0.93a | 11.50 ± 3.79b | 16.41 ± 2.08c | *** | ||
| Pentanal | 700 | m, s, lri | 10.87 ± 0.89b | 0.00 ± 0.00a | 34.44 ± 2.88c | *** |
| Hexanal | 850 | m, s, lri | 96.55 ± 10.10a | 225.60 ± 64.17b | 113.80 ± 5.51a | *** |
| 2-Pentenal, 2-methyl | 897 | m, lri | 0.00 ± 0.00a | 8.21 ± 2.87b | 0.00 ± 0.00a | *** |
| 2-Hexenal | 930 | m, s, lri | 0.00 ± 0.00a | 5.16 ± 2.07b | 0.00 ± 0.00a | *** |
| Heptanal | 968 | m, s, lri | 5.67 ± 0.60a | 35.80 ± 2.87c | 11.14 ± 1.13b | *** |
| Propanal, 2-methyl-3-phenyl | 1288 | m, lri | 1.46 ± 0.11a | 3.01 ± 0.07b | 1.71 ± 0.64a | ** |
| Total aldehydes | 115.66 ± 11.56a | 277.57 ± 56.10c | 161.57 ± 5.51b | *** | ||
| 1H-Pyrrole, 1-methyl | 772 | m, lri | 6.49 ± 0.95a | 8.81 ± 1.86b | 8.39 ± 0.72b | * |
| 1-Butanol, 3-methyl-, acetate | 930 | m | 0.00 ± 0.00a | 0.00 ± 0.00a | 1.98 ± 0.24b | *** |
| o-Cymol | 1074 | m | 40.68 ± 3.83 | 40.53 ± 4.78 | 44.61 ± 3.56 | ns |
| Eucalyptol | 1079 | m | 8.67 ± 0.74a | 15.70 ± 3.93b | 10.97 ± 1.13a | ** |
| Hexane, 1-nitro | 1130 | m | 3.94 ± 0.71 | 3.67 ± 0.10 | 4.40 ± 0.39 | ns |
| 2,3-Dimethylhydroquinone | 1252 | m | 0.63 ± 0.17a | 0.46 ± 0.08a | 1.22 ± 0.12b | *** |
| Myristicine | 1468 | m | 2.33 ± 0.79a | 4.33 ± 0.91b | 2.82 ± 0.54a | * |
| Elemicin | 1479 | m | 1.30 ± 0.29a | 1.87 ± 0.43b | 1.66 ± 0.27ab | * |
| Butylated hydroxytoluene | 1453 | m | 0.00 ± 0.00a | 0.00 ± 0.00a | 8.43 ± 0.68b | *** |
| Total others | 64.64 ± 4.75a | 74.87 ± 2.86b | 82.93 ± 5.02c | *** | ||
| Total compounds | 327.59 ± 22.10a | 644.88 ± 70.64c | 525.68 ± 64.79b | *** | ||
Batches: CO Control (100% of backfat); ME microencapsulated fish oil (50% backfat; 50% microencapsulated fish oil); OM Oil mixture (50% backfat; 25% fish oil; 25% olive oil)
a–c Mean values in the same row (corresponding to the same parameter) not followed by a common letter differ significantly (P < 0.05)
Sig. significance: * (P < 0.05), ** (P < 0.01), *** (P < 0.001), ns (not significant); AU area units resulting of counting the total ion chromatogram (TIC) for each compound; LRI linear retention index calculated for DB-624 capillary column (J&W scientific: 30 m × 0.25 mm id, 1.4 mm film thickness) installed on a gas chromatograph equipped with a mass selective detector; R Reliability of identification; LRI volatiles identified by comparing their LRI with those reported in the literature (Domínguez et al. 2014; Jiménez-Martín et al. 2015b); ms: mass spectrum agreed with mass database (NIST05); s: mass spectrum and retention time identical with an authentic standard
Statistical analysis showed that the partial replacement of pork backfat by microencapsulated fish oil and by fish/olive oil emulsion highly affected the content of the volatile compounds. To this regard, ME batch presented the highest (P < 0.001) total volatile compounds (644.88 AU × 106/g DM) and the CO group had the lowest values (327.59 AU × 106/g DM), while OM treatment had intermediate values (525.68 AU × 106/g DM). This fact was mainly related to the ME and OM batches displayed higher aldehydes (277 vs. 161 vs. 115 AUx106/g DM for ME, OM, and CO treatments, respectively), terpenes (117 vs. 118 vs. 70 AU × 106/g DM for ME, OM, and CO groups, respectively), alkanes (46 vs. 82 vs. 14 AU × 106/g DM for ME, OM, and CO batches, respectively) and the other compounds (75 vs. 83 vs. 65 AU × 106/g DM for ME, OM, and CO treatments, respectively) amounts than CO group.
In this study, two large groups of volatile compounds were distinguished. A group of compounds derived from spices and other of compounds derived from lipid oxidation. The terpenes constituted a high proportion in the odor concentrate of the sausages (as commented above, about 20% of total volatile compounds). Hence, the spicy flavor, one of the principal flavor attributes of sausage, could be attributed to these terpenes (Shiratsuchi et al. 1993). Our results were in agreement with those found by Shiratsuchi et al. (1993), who noticed that 38% of the total volatile compounds were terpenes, due to the use of spices as main ingredient. Among the terpenes identified, the most abundant were limonene followed by α-terpinene and α-pinene (about 65–70% of total terpenes). The partial replacement of pork backfat caused a significant (P < 0.001) increase in the total terpenes (Table 4). These differences were related to the fact that modified sausages had higher p-xylene, β-myrcene, α-terpinene and limonene amounts than the CO batch.
With regards to volatile compounds derived from lipid oxidation, the main chemical group was of aldehydes. In this study, the major aldehyde detected in the three studied batches was hexanal (one of the main markers of lipid oxidation in meat products) which represent around 75% of total aldehydes, followed by heptanal and pentanal. Our results agreed with those reported by Josquin et al. (2012), who observed that hexanal and propanal were the most abundant aldehydes in Frankfurt sausages prepared with partial replacement of backfat by fish oil. However, Jiménez-Martín et al. (2015b) did not find difference in hexanal and heptanal content between control and fish oil enriched nuggets.
These results confirmed that the sausages prepared with microencapsulated fish oil presented higher lipid oxidation than control sausages or sausages elaborated with fish/olive oils emulsion. As commented above, this fact could be related with the microencapsulation process and with the storage conditions of microencapsulated powder. Moreover, the contents of volatile compounds derived from lipid oxidation are in line with the results obtained for the TBARS values. As was found for the TBARS values, the sausages from ME batch showed a highest formation of aldehydes, alcohols, ketones and furans. In fact, significant correlations were found between TBARS and hexanal (r = 0.878, P < 0.001), total aldehydes (r = 0.952, P < 0.001), total ketones (r = 0.960, P < 0.001) and total alcohols (r = 0.76, P < 0.01).
In present study, some BHT was detected only in the headspace of the OM treatment. As mentioned above, the BHT was used as antioxidant in fish oil (used in both, ME and OM batches). Therefore, BHT was not detected in the ME group could be related with the fact that the encapsulation did not allow the BHT to exert its antioxidant effect. Another possible explanation was that BHT may have degraded during microencapsulation processes. Consequently, the lower quantities of aldehydes, alcohols, and ketones in the OM batches compared to those observed in the ME treatment may be attributed to the antioxidant effect of BHT, and tocopherols contributed by olive oil. Furthermore, the high temperature used for encapsulation of fish oil, with high PUFA levels which was susceptible to oxidation, could be related to increase in lipid oxidation in the ME batch.
Conclusion
It was concluded that frankfurter sausages could be manufactured using a olive and or fish oils to give a product with healthy lipid content (high oleic and long chain n-3 PUFA). Therefore, the sausages prepared with these oils met dietary recommendations made by various international organizations. The pH, fat content and energy value were lower in the sausages formulated with microencapsulated fish oil (ME) than the control and olive/fish oil mixture (OM). According to nutritional point of view, the OM batch presented the highest MUFA and n-3 PUFA values and the lowest SFA contents. Regarding lipid oxidation (TBARS and volatile compounds derived from lipid oxidation) the ME treatment showed the higher content compared to the other ones. With this in mind, the microencapsulation process could also be improved. However, additional studies of the effect of partial replacement of pork backfat with oils with better nutritional property on sensory properties and textural characteristics are required in future.
Acknowledgements
This work was supported by the Xunta de Galicia (Grant number FEADER 2013/34). The authors thank INIA for granting Ruben Agregán with a predoctoral scholarship (CPR2014-0128) and Biomega Natural Nutrients S.L. for the fish oil. The authors are members of the MARCARNE network, funded by CYTED (ref. 116RT0503).
References
- Andrés SC, Zaritzky NE, Califano AN. Innovations in the development of healthier chicken sausages formulated with different lipid sources. Poult Sci. 2009;88(8):1755–1764. doi: 10.3382/ps.2008-00495. [DOI] [PubMed] [Google Scholar]
- AOCS . AOCS Official Procedure Am 5-04. Rapid determination of oil/fat utilizing high temperature solvent extraction. Urbana: American Oil Chemists Society; 2005. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Phys. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Caceres E, Garcia ML, Selgas MD. Effect of preemulsified fish oil—as source of PUFA n-3—onmicrostructure and sensory properties of mortadella, a Spanish bologna-type sausage. Meat Sci. 2008;80(2):183–193. doi: 10.1016/j.meatsci.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Choi YS, Choi JH, Han DJ, et al. Effects of replacing pork back fat with vegetable oils and rice bran fiber on the quality of reduced-fat frankfurters. Meat Sci. 2010;84(3):557–563. doi: 10.1016/j.meatsci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Delgado-Pando G, Cofrades S, Ruiz-Capillas C, Jiménez-Colmenero F. Healthier lipid combination as functional ingredient influencing sensory and technological properties of low-fat frankfurters. Eur J Lipid Sci Tech. 2010;112(8):859–870. doi: 10.1002/ejlt.201000076. [DOI] [Google Scholar]
- Domínguez R, Gómez M, Fonseca S, Lorenzo JM. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014;97(2):223–230. doi: 10.1016/j.meatsci.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Domínguez R, Crecente S, Borrajo P, Agregán R, Lorenzo JM. Effect of slaughter age on foal carcass traits and meat quality. Animal. 2015;9(10):1713–1720. doi: 10.1017/S1751731115000671. [DOI] [PubMed] [Google Scholar]
- Domínguez R, Pateiro P, Munekata PES, Campagnol PCB, Lorenzo JM. Influence of partial pork backfat replacement by fish oil on nutritional and technological properties of liver pâté. Eur J Lipid Sci Tech. 2016 [Google Scholar]
- Domínguez R, Agregán R, Gonçalves A, Lorenzo JM. Effect of fat replacement by olive oil on the physico-chemical properties, fatty acids, cholesterol and tocopherol content of pâté. Grasas Aceites. 2016;67(2):e133. doi: 10.3989/gya.0629152. [DOI] [Google Scholar]
- European Commission (2010) Regulation (EU) No. 116/2010 of 9 February 2010 amending Regulation (EC) No. 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Official Journal of the European Union, L37, 16e18
- Greene BE, Cumuze TH. Relationship between TBA numbers and inexperienced panelists’ assessments of oxidized flavor in cooked beef. J Food Sci. 1982;47(1):52–54. doi: 10.1111/j.1365-2621.1982.tb11025.x. [DOI] [Google Scholar]
- Herrero AM, Carmona P, Pintado T, Jiménez-Colmenero F, Ruiz-Capillas C. Lipid and protein structure analysis of frankfurters formulated with olive oil-in-water emulsion as animal fat replacer. Food Chem. 2012;135(1):133–139. doi: 10.1016/j.foodchem.2012.04.114. [DOI] [Google Scholar]
- Huang CL, Sumpio BE. Olive oil, the mediterranean diet, and cardiovascular health. J Am Coll Surg. 2008;207(3):407–416. doi: 10.1016/j.jamcollsurg.2008.02.018. [DOI] [PubMed] [Google Scholar]
- ISO 1442 . International standards meat and meat products - Determination of moisture content. Geneva: International Organization for Standardization; 1997. [Google Scholar]
- ISO 936 . International standards meat and meat products - Determination of ash content. Geneva: International Organization for Standardization; 1998. [Google Scholar]
- ISO 937 . International standards meat and meat products - Determination of nitrogen content. Geneva: International Organization for Standardization; 1978. [Google Scholar]
- Jiménez-Colmenero F. Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci Tech. 2007;18:567–578. doi: 10.1016/j.tifs.2007.05.006. [DOI] [Google Scholar]
- Jiménez-Colmenero F, Herrero A, Pintado T, Solas MT, Ruiz-Capillas C. Influence of emulsified olive oil stabilizing system used for pork backfat replacement in frankfurters. Food Res Int. 2010;43(8):2068–2076. doi: 10.1016/j.foodres.2010.06.010. [DOI] [Google Scholar]
- Jiménez-Martín E, Gharsallaoui A, Pérez-Palacios T, Carrascal JR, Rojas TA. Suitability of using monolayered and multilayered emulsions for microencapsulation of ω-3 fatty acids by spray drying: effect of storage at different temperatures. Food Bioprocess Tech. 2015;8(1):100–111. doi: 10.1007/s11947-014-1382-y. [DOI] [Google Scholar]
- Jiménez-Martín E, Pérez-Palacios T, Carrascal JR, Rojas TA. Enrichment of chicken nuggets with microencapsulated omega-3 fish oil: effect of frozen storage time on oxidative stability and sensory quality. Food Bioprocess Tech. 2015;9(2):285–297. doi: 10.1007/s11947-015-1621-x. [DOI] [Google Scholar]
- Josquin NM, Linssen JP, Houben JH. Quality characteristics of Dutch-style fermented sausages manufactured with partial replacement of pork back-fat with pure, pre-emulsified or encapsulated fish oil. Meat Sci. 2012;90(1):81–86. doi: 10.1016/j.meatsci.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Keenan DF, Resconi VC, Smyth TJ, Botinestean C, Lefranc C, Kerry JP, Hamill RM. The effect of partial-fat substitutions with encapsulated and unencapsulated fish oils on the technological and eating quality of beef burgers over storage. Meat Sci. 2015;107:75–85. doi: 10.1016/j.meatsci.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Lee KH, Joaquin H, Lee CM. Improvement of moistness and texture of high omega-3 fatty acid mackerel nuggets by inclusion of moisture-releasing ingredients. J Food Sci. 2007;72:119–124. doi: 10.1111/j.1750-3841.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Munekata P, Pateiro M, Campagnol PCB, Dominguez R. Healthy Spanish salchichón enriched with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Food Res Int. 2016 doi: 10.1016/j.foodres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Mansour EH, Khalil AH. Characteristics of low-fat beef burgers as influenced by various types of wheat fibres. J Sci Food Agric. 1999;79(4):493–498. doi: 10.1002/(SICI)1097-0010(19990315)79:4<493::AID-JSFA4>3.0.CO;2-5. [DOI] [Google Scholar]
- Marchetti L, Andrés SC, Califano AN. Low-fat meat sausages with fish oil: optimization of milk proteins and carrageenan contents using response surface methodology. Meat Sci. 2014;96:1297–1303. doi: 10.1016/j.meatsci.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Marquez EJ, Ahmed EM, West RL, Johnson DD. Emulsion stability and sensory quality of beef frankfurters produced at different fat and peanut oil levels. J Food Sci. 1989;54:867–873. doi: 10.1111/j.1365-2621.1989.tb07901.x. [DOI] [Google Scholar]
- Morales-Irigoyen EE, Severiano-Perez P, Rodriguez-Huezo ME, Totosaus A. Textural, physicochemical and sensory properties compensation of fat replacing in pork liver pate incorporating emulsified canola oil. Food Sci Tech Int. 2012;18(4):413–421. doi: 10.1177/1082013211428218. [DOI] [PubMed] [Google Scholar]
- Muguerza E, Fista G, Ansorena D, Astiasaran I, Bloukas JG. Effect of fat level and partial replacement of pork backfat with olive oil on processing and quality characteristics of fermented sausages. Meat Sci. 2002;61:397–404. doi: 10.1016/S0309-1740(01)00210-8. [DOI] [PubMed] [Google Scholar]
- Muguerza E, Ansorena D, Astiasarán I. Functional dry fermented sausages manufactured with high levels of n-3 fatty acids: nutritional benefits and evaluation of oxidation. J Sci Food Agr. 2004;84(9):1061–1068. doi: 10.1002/jsfa.1786. [DOI] [Google Scholar]
- Ospina JC, Cruz A, Pérez JA, Fernández J. Development of combinations of chemically modified vegetable oils as pork backfat substitutes in sausages formulation. Meat Sci. 2010;84:491–497. doi: 10.1016/j.meatsci.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ospina JC, Rojano B, Ochoa O, Pérez JA, Fernández J. Development of frankfurter - type sausages with healthy lipid formulation and their nutritional, sensory and stability properties. Eur J Lipid Sci Tech. 2015;117:122–131. doi: 10.1002/ejlt.201400157. [DOI] [Google Scholar]
- Pelser WM, Linssen JP, Legger A, Houben JH. Lipid oxidation in n− 3 fatty acid enriched Dutch style fermented sausages. Meat Sci. 2007;75(1):1–11. doi: 10.1016/j.meatsci.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Reddy KJ, Jayathilakan K, Pandey MC. Olive oil as functional component in meat and meat products: a review. J Food Sci Technol. 2015;52(11):6870–6878. doi: 10.1007/s13197-015-1852-x. [DOI] [Google Scholar]
- Ritzoulis C, Petridis D, Derlikis EM, Fytianos K, Asteriou P. Utilization of inverse water-in-oil emulsions as fat replacers in frankfurter model sausages: influence of fat emulsion content on the organoleptic and mechanical properties. J Texture Stud. 2010;41:62–74. doi: 10.1111/j.1745-4603.2009.00213.x. [DOI] [Google Scholar]
- Rodríguez-Carpena JG, Morcuende D, Estévez M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: effect on lipid composition, oxidative stability and quality traits. Meat Sci. 2012;90(1):106–115. doi: 10.1016/j.meatsci.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Salcedo-Sandoval L, Cofrades S, Pérez CRC, Solas MT, Jiménez-Colmenero F. Healthier oils stabilized in konjac matrix as fat replacers in n-3 PUFA enriched frankfurters. Meat Sci. 2013;93(3):757–766. doi: 10.1016/j.meatsci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H, Shimoda M, Minegishi Y, Osajima Y. Isolation and identification of volatile flavor compounds in nonfermented coarse-cut sausage. Flavor as a quality factor of nonfermented sausage. J Agric Food Chem. 1993;41(4):647–652. doi: 10.1021/jf00028a027. [DOI] [Google Scholar]
- Simopoulos AP. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev Int. 2004;20(1):77–90. doi: 10.1081/FRI-120028831. [DOI] [Google Scholar]
- Valencia I, Ansorena D, Astiasaran I. Stability of linseed oil and antioxidants containing dry fermented sausages: a study of the lipid fraction during different storage conditions. Meat Sci. 2006;73(2):269–277. doi: 10.1016/j.meatsci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Vyncke W. Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel (Scomber scombrus L) Fette Seifen Anstrichmittel. 1975;77:239–240. doi: 10.1002/lipi.19750770610. [DOI] [Google Scholar]
- WHO (2010) World Health Organization [Internet] Population nutrient intake goals for preventing diet-related chronic diseases. ([Accessed 2010 Jun 20] http://www.who.int/nutrition/topics/5_population_nutrientm/en/index12.html)
- Zhang W, Xiao S, Samaraweera H, Lee EJ, Ahn DU. Improving functional value of meat products. Meat Sci. 2010;86:15–31. doi: 10.1016/j.meatsci.2010.04.018. [DOI] [PubMed] [Google Scholar]