Abstract
Wheat germ has an important enzymatic activity, being lipases the enzymes which cause the highest impact in the reduction of shelf life. The objective of this study was to evaluate the effects of infrared radiation on wheat germ stabilization in an attempt to extend the shelf life. The effects of treatment time, gap (sample distance to IR emitters) and infrared radiation intensity on wheat germ were analyzed through response surface methodology. Final moisture content, final temperature, color of germ and germ oil quality parameters: free fatty acid content changes and total tocopherol content were the responses evaluated using a Box-Behnken design. A combination of an infrared radiation intensity of 4800 W/m2, a 3 min treatment and 0.2 m emitter-sample distance were the best processing condition to stabilize the wheat germ without significantly reduction of the tocopherol content. A confirmatory experiment was conducted with these optimal conditions, and the heat-treated and raw germ samples were stored for 90 days at room temperature in three layer packages to protect them against light and oxygen. The oil quality parameters indicated that the raw germ had a shelf-life of about 15 days, with the heat-treated wheat germ maintaining its quality for at least 90 days under these stored conditions.
Keywords: Wheat germ, Infrared radiation, Lipase, Tocopherol
Introduction
Wheat germ is the name given to the embryo of the wheat seed, which represents approximately 2–3 g/100 g of the weight of the whole grain and contains between 8 and 14 g/100 g of oil (Capitani et al. 2011). Large quantities of wheat flour are produced by the wheat milling industry every year, with the wheat germ fraction representing a low cost by-product with a high concentration of nutrients. In addition, it is an excellent ingredient to improve food formulations due to its protein quality and fatty acid composition, as well as its tocopherol content, vitamin B, dietary fiber, essential amino acids, and functional phytochemicals such as flavonoids and sterols (Marti et al. 2014; Yazicioglu et al. 2014). Vitamin E, a major biological antioxidant, is mainly provided by cereal grains. Engelsen and Hansen (2009) showed that tocopherols are primarily located in the germ fraction, but are also present in the fraction of fine bran. Several factors can influence tocopherol stability, including temperature, light, concentration and substrate. The main cause of vitamin E detriment during the processing of cereals may be through lipid degradation, since whole-grain wheat containing lipids with high proportions of mono- and polyunsaturated fatty acids, while polyunsaturated fatty acids originating from the lipase-catalyzed hydrolysis of cereal lipids are easily peroxidized by lipoxygenase. Considering this fact, tocopherols and tocotrienols are lost due to the lipid peroxidization process, since tocopherols are oxidized by the co-oxidation reaction of lipoxygenase (Hakansson et al. 1987).
Wheat germ has a high content of unsaturated lipids and an important enzymatic activity due to lipases and lipoxygenase (Shurpalekar and Rao 1977), this leads a deterioration in wheat germ oil because of the generation of acidity and volatile compounds such as aldehydes, ketones, alcohols and hydrocarbons. In fact, the production of these molecules reduces wheat germ shelf life to only a few days and limits its capacity to be used as a food ingredient (Sjövall et al. 2000).
Several methodologies have been used to reduce enzymatic activity in wheat germ, including antioxidant addition (Barnes 1948), alkalis (Grandel 1959) and gamma radiation (Jha et al. 2013). Another way of stabilizing wheat germ was also attempted by reducing the moisture content using dry or wet heat, (Galle 1974), with the application of an adequate technique leading to an improvement in flavor and color (Yöndem-Makascioǧlu et al. 2005). In food thermal processing, conduction and convection heat transfer have mainly been used, but radiant heat transfer by infrared radiation (IR) of short wavelength has also been evaluated. In IR heating, heat is supplied by electromagnetic radiation from the IR emitter, with the IR energy emitted by the heater passing through air and being absorbed by the food, before being converted into heat by molecular interaction. Heat is then transferred from the surface to the inside by conduction.
IR radiation can be classified into three regions: near-infrared (NIR), mid-infrared (MIR), and far-infrared (FIR), corresponding to the spectral ranges of 0.75–1.4, 1.4–3, and 3–1000 μm, respectively (Sakai and Hanzawa 1994). The advantages of IR heating for food processing include: (1) a fast heating response in comparison to conventional heating, which reduces the processing time and energy costs; (2) uniform heating; (3) a minimum heat dissipation outside the IR heater. However, an adequate heating control is required to avoid overheating owing to the heating rate. Infrared heating can be used effectively for enzyme inactivation. For example, Krishnamurthy et al. (2008) reported the inactivation of soybean lipoxigenase by IR heating while Yılmaz et al. (2013) showed that an IR treatment on rice bran (600 W, 5 min) maintained the free fatty acid content (FFA) below 5 g oleic acid equivalent/100 g of the oil after 165 days storage.
Wheat germ particles have small diameters of between 0.841 and 1.68 mm, with their thickness being smaller than 1 mm. Based on these characteristics, IR heating appears to be a suitable technology to stabilize the germ. For IR industrial treatment of these raw materials, a high heat transfer capacity, adequate heat penetration, optimal process control and a fast response regulation for heat transfer are desirable characteristics (Hemdane et al. 2015).
No studies concerning wheat germ stabilization by infrared heating have been reported. Nevertheless, the characteristics of this type of technology and the matrix properties suggest that IR should be an adequate methodology to inactivate wheat germ lipase. Therefore, the aim of this study was to extend germ shelf life by evaluating the effects of IR treatment on wheat germ stabilization through analyzing the oil chemical parameters (peroxide value, free fatty acid content, tocopherol content, specific extinction coefficients K232 and K270) and product stability on storage condition.
Materials and methods
Wheat germ, chemicals and reagents
Homogenous commercial batches of wheat germ were supplied by a local milling industry (JOSE MINETTI Y CIA. LTDA. S.A.C.I, Córdoba, Argentina) after grain milling (2013 harvest). To separate the wheat germ from bran and flour particles, the samples were sieved (EJR 2000, Zonytest®), and germ particles retained on a 20 mesh-size sieve (0.841 mm) were stored at –18 °C in a three-layer (polyester, aluminum and polyethylene) package until further use. The sieved germ had 13.47 ± 0.14 g water/100 g dry solids and 13.98 ± 0.36 g total fat/100 g sample (AACC, 2010). The relative abundance of fatty acids (as percentage, Martínez et al. 2006) was: linoleic acid (59.44 ± 6.42), palmitic acid (18.70 ± 2.02), oleic acid (15.77 ± 1.70), α-linolenic acid (6.09 ± 0.66) and palmitoleic acid (traces).
Ethyl acetate and hexane used for tocopherol quantification were of HPLC grade, with the tocopherol standards being obtained from ICN Biomedicals, Inc (Germany). All the other reagents and solvents used were of analytical grade.
IR equipment setup
The IR dryer consisted in an isolated metal chamber (1.0 × 0.25 × 0.30 m) with 15 quartz tubes with individual on–off switches mounted on the top of the chamber. The maximum power density setup was 9000 W/m2, and the sample tray had a surface area of 0.25 m2 and was located in one of three different positions: 0.1, 0.15 and 0.2 m (gap distances) from the emitters and parallel to the top chamber surface. For each trial, 65 g of raw wheat germ was spread homogenously on the sample tray, with this amount being adequate to obtain a homogeneous layer of ~2 mm thick germ. After the heat treatments, germ samples were stored in the three-layer package at −18 °C.
Surface temperature profile analysis
Trials were carried out in order to evaluate the germ temperature profile when was exposed to IR radiation. Wheat germ was treated under different conditions of infrared radiation intensity (IRRI) (3000, 4800 and 9000 W/m2), emitter-sample gap distance (GD) (0.1, 0.15 and 0.2 m) and treatment time (TT) (0–10 min). In order to evaluate the effect of each factor, the temperatures reached by the germ were measured at different times by an IR thermometer (62 MINI, FLUKE®). Two scans along the sample surface were carried out immediately after each heat treatment to obtain the final germ temperature. The space between the sample and thermometer was approximately 0.15 m, with this distance being selected to cover the complete width of the tray during measuring, as recommended by the manufacturer.
A quadratic equation (Eq. 1) was fitted to each temperature curve to analyze the heating ratio, using a nonlinear least squares method (Statgraphic Centurion XV v 15.1.02, USA).
| 1 |
where FT is the final temperature of the wheat germ, TT is the treatment time in minutes, and a, b and c are the parameters of the quadratic function.
Experimental design and response surface analysis
A response surface methodology using a Box-Behnken design (Montgomery 2001) was used to study the effects of the processing parameters on germ temperature and moisture content, lipase activity and oil quality of wheat germ. The selected independent variables used at three different levels were IRRI (X1), GD (X2), and TT (X3). Four replicates of the center point of the design was done to estimate the pure error. The experimental design is presented in Table 1. The evaluated responses were final germ moisture content (Y1), change in free fatty acid content (ΔFA) (Y2), final germ temperature (Y3), color parameters of the germ (L*:Y4; a*:Y5; b*:Y6), total tocopherol content (TTC) (Y7), and whiteness index (WI) (Y8). All determinations were performed in duplicate. Quadratic or lineal polynomials were fitted to express the responses (Yn) as a function of the factors (Eq. 2).
Table 1.
Level values of each factor in the Box-Behnken design
| Treatment | IRRI | GD | TT |
|---|---|---|---|
| 1 | −1 | 0 | −1 |
| 2 | 0 | 0 | 0 |
| 3 | −1 | 0 | 1 |
| 4 | 1 | 0 | 1 |
| 5 | 1 | 1 | 0 |
| 6 | 1 | 0 | −1 |
| 7 | 0 | 1 | −1 |
| 8 | 0 | −1 | −1 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | −1 | −1 | 0 |
| 12 | 0 | −1 | 1 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 1 | 1 |
| 15 | −1 | 1 | 0 |
| 16 | 1 | −1 | 0 |
IR radiation intensity (IRRI): (−1):3000 W/m2; (0): 4800 W/m2; (1): 9000 W/m2
Emitter-sample gap (GD): (−1):0.2 m; (0): 0.15 m; (1): 0.1 m
Treatment time (TT): (−1):1 min; (0): 2 min; (1): 3 min
The generic polynomial fitted for the different responses was the following:
| 2 |
where R is the response, β0 is the constant term, βi symbolizes the coefficients of the linear parameters, βii represents the quadratic parameters, βij signifies the interaction parameters and ε is the random error.
The results were analyzed by multiple regression method to obtain the regression models, with ANOVA being applied to evaluate the quality of the model fitted (Statgraphic Centurion XV v 15.1.02, USA). Only models with high coefficients of determination (extent of the data variability that can be explained by the model, r2) were included in this study, with multiple regression equations including only significant coefficients (p ≤ 0.05). Three-dimensional response surface graphics were generated for each response variable. The calculation of the optimal processing conditions was performed using a desirability methodology (Ferreira et al. 2007), with incorporating the desired values and priorities for each of the variables.
Evaluation of storage stability wheat germ
Heat-treated wheat germ with optimal processing conditions and raw germ samples were evaluated for their stability by a storage test of germ samples carried out at 25 °C for 90 days in a temperature-controlled room. The raw and heat-treated samples were packed in sealed containers with barriers (three-layer packages) against oxygen and light. An exclusive package was made for each time condition in the storage test, and samples were analyzed at 0, 15, 30, 45, 60 and 90 storage days. The color parameters, WI, free fatty acid content (FA), peroxide value (PV), K232 (conjugated dienes) and K270 (conjugated trienes) were evaluated as indicators of storage stability. All determinations were performed in duplicate.
Color determination of the raw and heat-treated germ samples
Color determination of the germ was carried out according to Martínez et al. (2013) with minor modifications. Briefly, the measurements were made on a 1.5 cm thick layer, which was covered with a low reflectance glass. A spectrophotometer (CM600d, Konica-Minolta®) with a D65 illuminant, 10° angle of observer, and specular-included component through a low reflectance glass was used. The color of the treated and raw wheat germ was expressed as CIELAB parameters (L*, a*, b*), and all measurements were made in triplicate. An additional color attribute, the whiteness index (WI), was calculated from L*, a* and b* using Eq. (3) according to Tuncel et al. (2014).
| 3 |
Oil extraction and quality analyses
For analytical determinations, germ oil was obtained as follows: raw and heat-treated samples of wheat germ were twice extracted with hexane (1:10 w/v) for 30 min with magnetic stirring (200 rpm) at room temperature, after which, the hexane portion was centrifuged (5 min, 1000×g) and then evaporated in vacuum conditions at 40 ± 1 °C. Oil samples were stored at −18 °C under a nitrogen atmosphere until being analyzed.
The total tocopherol content was determined by high performance liquid chromatography (HPLC), using hexane:ethyl acetate (70:30 v/v) as mobile phase with a flow rate of 1.0 mL/min. Approximately 0.1 g of oil was vortexed in 0.9 mL of hexane for 30 s in a glass tube protected from light. A 20 μL aliquot of this solution was injected in a HPLC Perkin Elmer Series 200HPLC (Shelton, USA) equipped with a UV detector set at 295 nm using a Supelco HPLC column (Supelcosil LC-NH2-NP, 250 × 4.6 mm ID × 5 μm). An injector with a 20 μL sample loop was used for all sample injections. The quantification of different isomers was carried out by calibration curves using tocopherol standards, with the total tocopherol content expressed in mg/kg oil. All determinations of TTC were performed in duplicate.
As the hydrolytic action of lipases on wheat germ generates free fatty acids (FFA) from triglycerides, the increase in FFA (ΔFA) over time was considered to be proportional to lipase activity. To obtain ΔFA, the first step was to moisten the germ to get the optimal water concentration for lipase activity according to Rose and Pike (2006). The germ samples were rewetted from their delivery moisture (approximately 11.5 g water/100 g of wet solids) by adding a measured amount of water to the samples to obtain 17 g water/100 g of wet solids. These mixtures were then put in hermetic plastic containers and left in a cool room at 4 ± 1 °C for 30 min (from now on referred to as conditioned germ). Next, a portion of conditioned germ (8 g) was extracted with hexane (as described above) and the free fatty acid content (g oleic acid/100 g oil) of oil analyzed (FA0). The remaining conditioned germ was maintained at 40 ± 1 °C in a thermostatic bath for 48 h to generate free fatty acids as a result of the lipase activity (Rose and Pike 2006), then, a portion of 8 g of conditioned germ was extracted with hexane, and the free fatty acid content (g oleic acid/100 g oil) of the oil was analyzed (FA48). FA contents were determined in duplicate (AOCS 2009). The increase of acidity (FA48-FA0 = ΔFA, g oleic acid/100 g oil) after 48 h was taken as being a measure of lipase activity (Gili et al. 2013).
The peroxide value was determined according to the AOCS standard method Cd 8–53, and the specific extinction coefficients K232 and K270 were obtained according to AOCS standard method Ti 1a-64, (AOCS 2009), with all these determinations being performed in duplicate.
Statistical analysis
An analysis of variance (ANOVA) was performed on the data, with the means being compared by the LSD (Least significant difference)-Fisher test at a significance level of 0.05, and the relationship between the measured parameters assessed by the Pearson test (significance level p ≤ 0.05) using the Infostat statistical software.
Results and discussion
Surface temperature profile analyses
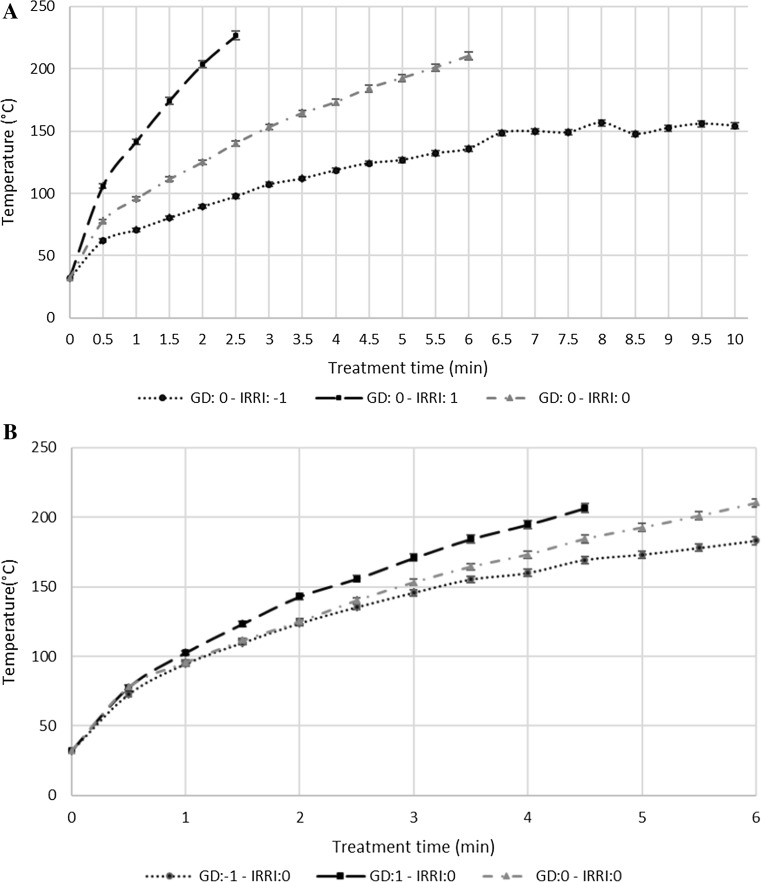
The temperature profiles of germ as a function of IRRI and treatment time (TT) were first determined when the emitter-sample gap distance was maintained at 0.15 m (GD: 0) (Fig. 1a), and presented significant differences (p < 0.05) from 1 min of TT. The maximum temperatures reached for high, medium and low IRRI were 226.6 ± 1.5 °C (2.5 min), 210.2 ± 1.5 °C (6 min) and 156.6 ± 1.5 °C (10 min), respectively. At high and medium levels of IRRI, it was not possible to complete ten minutes of treatment due to the germ starting the carbonization process.
Fig. 1.
Effects of IR radiation intensity (IRRI), emitter-sample gap (GD) and treatment time on final temperature of germ
A second order equation (Eq. 1) was fitted to each curve in order to analyze the rate of heating of each IRRI condition. The adjusted parameters are shown in Table 2 along with their asymptotic standard error (ASE) and the corrected coefficients of determination (r2), with all curves presenting high r2 values. The equation “a” parameter from the adjusted model alters the opening of the fitted parabola, which based on this effect of the “a” parameter on the model can be related to the rate of heating of wheat germ in the IR dryer. The curve GD:0-IRRI:1 revealed the highest absolute value of this parameter (at least 15 times greater than the GD:0-IRRI:-1 curve and 6 times greater than the GD:0-IRRI:0 curve), exhibiting a faster rate of heating and having a notable influence on the final temperature of the samples. The other adjusted parameters (“b” and “c”) had a translation effect on the curves unrelated to the rate of heating.
Table 2.
Parameters determined by fitting the quadratic equation (Eq. 1) to experimental data from preliminary tests with GD = 0 (0.15 m)
| a | A.S.E. | b | A.S.E. | c | A.S.E. | r2 | |
|---|---|---|---|---|---|---|---|
| GD:0-IRRI:1 | −20.0564 | 5.2852 | 124.381 | 13.7652 | 37.8865 | 7.31703 | 0.9925 |
| GD:0-IRRI:0 | −3.18462 | 0.579992 | 45.622 | 3.61042 | 46.0958 | 4.66254 | 0.9875 |
| GD:0-IRRI:-1 | −1.30229 | 0.121809 | 23.8635 | 1.26167 | 44.3776 | 2.72248 | 0.9852 |
| GD:1-IRRI:0 | −5.88454 | 0.983349 | 62.3082 | 4.59657 | 40.8338 | 4.44143 | 0.9897 |
| GD:-1-IRRI:0 | −2.92308 | 0.230542 | 38.0378 | 1.53935 | 57.7273 | 2.1762 | 0.9964 |
A.S.E. asymptotic standard errors
GD emitter-gap distance
The temperature profiles of germ as a function of GD and treatment time (TT) on germ temperature were determined when the IRRI level was maintained at 4800 W/m2 (IRRI:0) (Fig. 1b). As expected, the higher the emitter-sample distance, the lower the heating ratio resulted. The GD:1-IRRI:0 temperature profile was significantly different from the two other profiles from 1 min of heating. On the other hand, the GD:0-IRRI:0 and GD:-1-IRRI:0 temperature profiles were significantly different from each other from 3 min of heating. The maximum temperatures reached for each GD were 206.6 ± 1.5 °C (4.5 min), 210.2 ± 1.5 °C (6 min) and 183.2 ± 1.5 °C (6 min) for the minimum, medium and maximum distances evaluated, respectively.
The curve GD:1-IRRI:0 revealed the highest absolute value of the “a” parameter (almost two times greater than the GD:0-IRRI: 0 and GD:-1-IRRI:0 curves), exhibiting a faster rate of heating and having a notable influence on the final temperature of the samples. The other two curves do not reveal any significant differences for the fitted “a” parameter, but indicated a slight influence of GD on the final temperature of the wheat germ for the largest values of GD. Regarding these trials, it was not possible to complete 10 min of heat treatment due to the germ having started the carbonization process.
A previous study on germ stabilization described the inactivation of wheat germ lipase at 175 °C in a forced draft oven (Rose et al. 2008). Taking into account this previous study along with the temperature profiles and the features of the IR equipment of the present investigation, the following ranges of independent variables were selected to reach the optimum processing conditions: IRRI from 3000 to 9000 W/m2, GD from 0.2 to 0.1 m and TT from 1 to 3 min.
Effect of processing conditions
An experimental design of 16 treatments was carried out (4 central points) using the previously mentioned factors and levels (Table 3). For each response variable, a quadratic or linear equation was computed using relevant terms (p ≤ 0.05) to obtain as high r2 values as possible. Based on these equations, the behavior of the response was predicted within the experimental area, and is presented as a response surface. The regression and determination coefficients for the statistically significant (p ≤ 0.05) models are shown in Table 4, with the response surface plots being presented in Fig. 2.
Table 3.
Box-Behnkhen design. Experimental responses of the wheat germ and the oil quality parameters
| Treatment | ΔFAa | TTCb | FM(d.b.)c | FT | L* | a* | b* | WI |
|---|---|---|---|---|---|---|---|---|
| Raw germ | 2.4 ± 0.2a | 3776 ± 4a | 13.5 ± 0.1a | nt | 70.08 ± 0.05b | 5.3 ± 0.3b | 26.8 ± 0.6c | 59.5 ± 0.4a |
| 1 | 1.53 ± 0.04b | 3598 ± 79a | 8.3 ± 0.3b | 49.6 | 71.0 ± 0.2a | 5.2 ± 0.4b | 27.3 ± 0.3b | 59.8 ± 0.2a |
| 2 | 1.27 ± 0.02b | 3557 ± 67a | 3.5 ± 0.3g | 93.8 | 70.3 ± 0.3b | 4.9 ± 0.3b | 29.4 ± 0.2b | 57.9 ± 0.4b |
| 3 | 1.16 ± 0.07b | 3568 ± 148a | 3.87 ± 0.07f | 76 | 70.7 ± 0.3a | 5.2 ± 0.2b | 29 ± 1b | 58.7 ± 0.7b |
| 4 | 0.3 ± 0.1d | 3397 ± 26b | 0.14 ± 0.02j | 174.4 | 34.9 ± 0.2d | 5.7 ± 0.5c | 13.2 ± 0.8d | 33.3 ± 0.1e |
| 5 | 0.30 ± 0.04d | 3617 ± 64a | 1.68 ± 0.08h | 181.6 | 57.9 ± 0.5c | 7.9 ± 0.4a | 27 ± 1c | 49.2 ± 0.8d |
| 6 | 0.99 ± 0.06c | 3680 ± 131a | 4.43 ± 0.06e | 95.6 | 69.5 ± 0.9b | 4.7 ± 0.9b | 28.9 ± 0.5b | 57.7 ± 0.8b |
| 7 | 1.2 ± 0.4b | 3495 ± 56a | 7.02 ± 0.02c | 59.6 | 70.2 ± 0.8b | 4.8 ± 0.2b | 27 ± 1b | 59 ± 1b |
| 8 | 1.19 ± 0.01b | 3735 ± 2a | 8.7 ± 0.4b | 59.8 | 69.6 ± 0.3b | 5.3 ± 0.2b | 26 ± 2c | 59.6 ± 0.8a |
| 9 | 1.3 ± 0.1b | 3521 ± 51a | 4.71 ± 0.08e | 91.4 | 70.7 ± 0.9a | 4.7 ± 0.5b | 29.5 ± 0.3b | 58.2 ± 0.5b |
| 10 | 1.2 ± 0.3b | 3545 ± 135a | 4.30 ± 0.01e | 95.2 | 69.6 ± 0.4b | 5.2 ± 0.1b | 29 ± 1b | 57 ± 1b |
| 11 | 1.49 ± 0.07b | 3369 ± 106b | 6.82 ± 0.07c | 64.4 | 71.0 ± 0.8a | 4.7 ± 0.6b | 29 ± 1b | 59 ± 1b |
| 12 | 0.2 ± 0.2d | 3572 ± 9a | 1.65 ± 0.03h | 115.2 | 69.6 ± 0.9b | 4.8 ± 0.3b | 29.66 ± 0.04b | 57.3 ± 0.7b |
| 13 | 1.3 ± 0.2b | 3548 ± 48a | 3.98 ± 0.04f | 105.4 | 71 ± 1a | 4.6 ± 0.4b | 30.08 ± 0.11b | 57.8 ± 0.7b |
| 14 | 0.35 ± 0.05d | 3367 ± 41b | 1.0 ± 0.3i | 151.8 | 68.8 ± 0.4b | 4.4 ± 0.3c | 27.89 ± 1.49b | 58 ± 1b |
| 15 | 0.81 ± 0.03c | 3631 ± 8a | 5.32 ± 0.01d | 75.4 | 70.0 ± 0.3b | 5.0 ± 0.2b | 28.33 ± 0.09b | 58.4 ± 0.2b |
| 16 | 0.08 ± 0.01d | 3442 ± 29b | 1.5 ± 0.4h | 145.6 | 69.4 ± 0.5b | 4.1 ± 0.2c | 31.02 ± 0.15a | 56.2 ± 0.4c |
L*, a*, b* and Whiteness index (WI): n = 3
Change of free fatty acid content (ΔFA), Total tocopherol content (TTC) and FM (dry basis): n = 2
FT: Instrument accuracy: 1.5 °C
Means with a different letter for each index are significantly different (DGC, p ≤ 0.05); a g oleic acid/100 g oil; b mg/kg oil; c Final moisture (FM), g/100 g dry solids; d Final temperature (FT), °C
Table 4.
Significant coefficients (95% confidence interval) of the design of the regression fitting model for wheat germ and the oil quality parameters
| Coefficient | ΔFAa | FMb | WIc | FTd |
|---|---|---|---|---|
| Constant | 1.289 | 3.955 | 57.812 | 96.450 |
| A:X1 | −0.416 | −1.916 | −6.073 | 41.475 |
| B:X2 | ns | ns | ns | 10.425 |
| C:X3 | −0.363 | −2.501 | ns | 31.600 |
| AA | ns | ns | ns | ns |
| AB | 0.226 | ns | ns | ns |
| AC | ns | ns | −7.010 | 13.100 |
| BB | −0.447 | ns | ns | ns |
| BC | ns | ns | ns | ns |
| CC | ns | ns | ns | ns |
| r2 | 95.6 | 96.6 | 85.2 | 97.7 |
ns no significant effect (p > 0.05)
ºC; X1 = IR Radiation intensity (W/m2); X2: Emitter-sample gap (m); X3: Treatment time (min)
a Change in free fatty acid content (ΔFA), g oleic acid/100 g oil; b Final moisture (FM), g/g dry solids; c Whiteness index (WI); d Final temperature (FT)
Fig. 2.
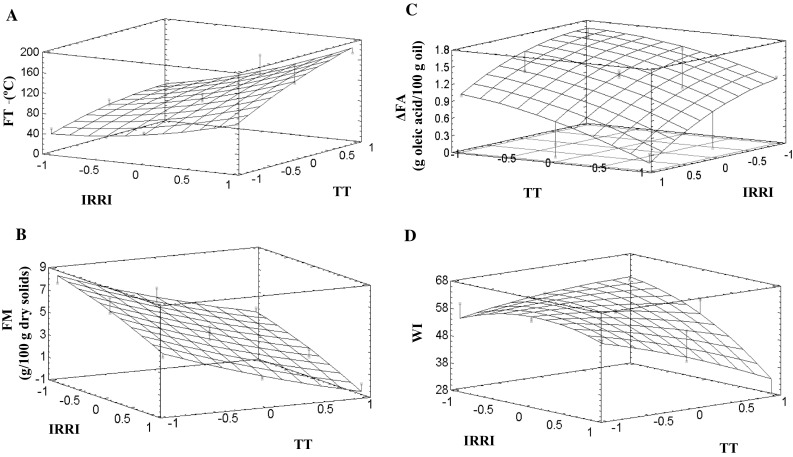
Effects of IR radiation intensity (IRRI) and treatment time (TT) on oil quality and germ parameters at GD = 0
Positive linear effects of IRRI, GD and TT and a positive interactive quadratic effect of IRRI-TT on the germ final temperature were observed (p ≤ 0.05) (Table 4), with the final temperatures of treated germ being between 49.6 and 181.6 °C. As expected, an increase in IRRI and TT and a decrease in the distance between samples and emitters led to a rise in the germ final temperature (Fig. 2a), with several treatments achieving temperatures that were able to inactivate the wheat germ enzymes. The coefficient of determination of the model for germ final temperature was able to explain 97.7% of the data variability.
Although positive linear effects of IRRI and TT on germ final moisture were observed (p ≤ 0.05), no significant effect of GD was found (Table 4). The germ final moisture decreased as a consequence of treatments, especially for the most intense treatments, and reached values near to zero (Fig. 2b). A linear model was adjusted to the final moisture response, and was able to explain 95.1% of the data variability.
The germ sample had an initial free fatty acid content of 1.55 ± 0.01 g oleic acid/100 g oil, which was lower than that informed by Attia and Abou-Gharbia (2011) or Capitani et al. (2011). After 48 h in the temperature-controlled water bath, the free fatty acid content in germ oil showed a significant increase of ~40% (ΔGA of 2.36 ± 0.19 g oleic acid/100 g oil) as a consequence of lipase activity in the germ. Negative linear effects of IRRI and TT as well as a negative quadratic effect of GD were observed on the ΔFA values (Table 4), which were between 0.08 and 1.53 g oleic acid/g oil. The coefficient of determination of the model for ΔFA was able to explain 94.4% of the data variability. As expected, an increase in IRRI, TT and a decrease in the distance between samples and emitters resulted in a decrease in ΔFA (Fig. 2c). Moreover, as predicted, the lowest ΔFA values, associated with reduced lipase activity, were related to the longest times and the highest radiation intensities. These results are in agreement with those reported by Jha et al. (2013) and Yöndem-Makascioglu et al. (2005), who used several technologies such as microwave, fluidized bed and gamma radiation to stabilize the wheat germ. In addition, the present results concur with those of Ding et al. (2015), where IR drying resulted in an effective inactivation of lipase in raw and brown rice.
Color is an important quality attribute of dehydrated foods, since it is related to non-enzymatic browning, which in turn could produce a decrease in the nutritive value due to decreased protein digestibility and loss of essential amino acids, with non-enzymatic browning having been reported to be dependent on the temperature and water activity of the food (Rahman and Labuza 2007). In fact, many authors consider color to be a quality control indicator of processes, because brown pigments increase as browning and caramelization reactions progress. Therefore, the control of color changes seems to be necessary to obtain a good product quality, raw germ had lightness (L*), redness–greenness (a*) and yellowness–blueness (b*) parameter values of 70.08 ± 0.05, 5.27 ± 0.32 and 26.83 ± 0.58, respectively, with L* being consistent with the reported values, while a* and b* were higher (Bansal and Sudha, 2011). The WI for the raw germ was 57.29 ± 0.67, which was significantly affected by processing parameters, with a negative linear effect of IRRI and a negative interactive quadratic effect of IRRI-TT being observed (p ≤ 0.05) (Table 4). Only treatments 1 and 8 had similar values (p > 0.05) to that of raw germ (Table 3), whereas other treatments performed revealed significantly lower values of WI (59.47 ± 0.37) (Fig. 2d). The adjusted model explained 85.2% of the data variability.
The TTC (3776.5 ± 4.6 mg/kg oil) and the relative abundances of α-tocopherol (62.8 ± 0.1%), β-tocopherol (32.5 ± 0.1%), γ-tocopherol (4.69 ± 0.1%) and δ-tocopherol (not detected) of the raw germ were in agreement with the reported values of Capitani et al. (2011). TTC values of the heat-treated samples were between 3745 and 3367 mg/kg oil, with only four treatments (4, 11, 14 and 16) producing significantly lower values of TTC than raw germ (p ≤ 0.05) (Table 3). In spite of these differences between treatments, all treated samples maintained a high level of TTC, with these results being in agreement with Magariño et al. (2012), who found similar values of TTC for raw and heat-treated wheat germ. No significant relations among the process variables or the reduction of TTC could be established. It is important to highlight that the TTC was found in large quantities in the treated germ, with its relative proportions being maintained in the raw germ (data not shown).
Wheat product processing under mild conditions has little or no effect on tocopherols or tocotrienols, although severe conditions may result in a high loss of tocopherols (Hakansson et al. 1987). Vitamin E appears to be less stable in white flour compared with whole grain or flour wheat, which indicates that the particle size of the raw material is influential. Related to this, Hakansson et al. (1987) suggested that this is due to vitamin E in white flour being more exposed to catalytic oxidizers such as iron and copper, but a more plausible explanation could be that the higher concentration of tocopherols in wholemeal flours and the amount of complex substrates and other compounds (such as phenolic acids, lignans and flavonoids, which are mostly localized in the external layers of the kernel) might influence the degradation rate of tocopherols through synergic or competitive effects on their antioxidant activity, as well as by protecting them against oxidation (Hidalgo et al. 2009). Thus, the germ matrix probably provided enough stability to tocopherols under the tested conditions. These results are very important, since the destruction of tocopherols not only decreases the healthy properties of germ, but also plays an important role in the protection of lipid oxidation.
Although previous studies concerning the effect of IR on wheat germ tocopherols were not found, Yılmaz et al. (2013), on studying the effect of infrared radiation on rice bran, reported that tocopherol isomers were significantly affected by applied infrared radiation during different treatments and that α-tocopherol was the most affected isomer.
In addition to helping to explain the behaviour of variables by the contour curves, the models fitted could also be applied for optimization using the desirability function (Ferreira et al. 2007; Jahani et al. 2008). Thus, the latter procedure was carried out to minimize wheat germ final temperature, moisture content and ΔFA, while maximizing the level of TTC and keeping the WI as similar to the original one as possible. The multiple response optimization, with a desirability function value of 0.68, suggested that the best conditions found to promote enzymatic inactivation while maintaining high levels of tocopherol content in the germ were similar to the treatment 12 conditions of the experimental design, thus, this treatment was considered to be the optimum one (4800 W/m2 for IRRI, a 0.2 m emitter-sample gap and 3 min of TT). For these processing parameters, the predicted responses were 118 °C for the final temperature, 0.21 g oleic acid/100 g oil for ΔFA, 3455 mg/kg oil for TTC, 1.45 g water/100 g dry solids for moisture content and 55.3 for WI. These results showed that the error percentage between the estimated value of the model and the experimental observed values were 2.1% for final temperature, 4.9% for ΔFA, 3.3% for TTC, 10.8% for moisture content and 3.5% for WI, indicating a good fit of the model to the experimental data.
Wheat germ storage stability test
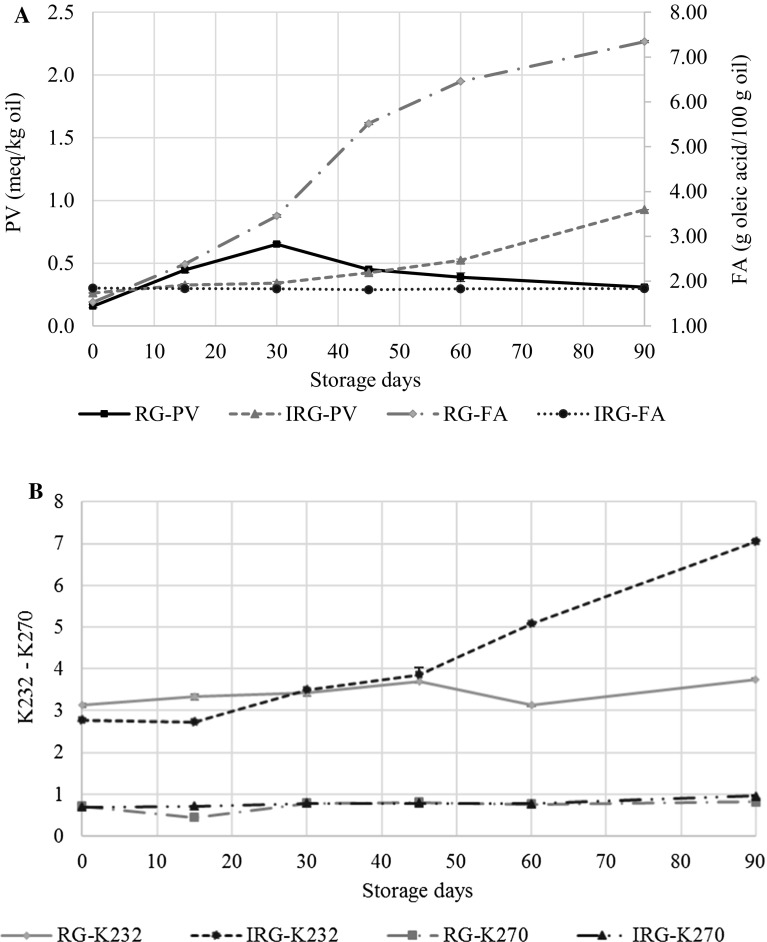
New experiments were performed according to the optimum stabilization conditions, and the raw and optimized heat-treated germ samples were stored and analyzed, with Fig. 3 showing the oxidation and acidity patterns during the storage of these samples for 90 days. As expected, quite different behaviours were revealed, with the initial FA of raw germ being 1.53 ± 0.01 g oleic acid per 100 g of oil and increasing throughout the experiment and reaching 7.34 ± 0.03 g oleic acid per 100 g of oil at 90 days of storage, whereas the FA of heat-treated germ remained constant (1.81–1.83 g oleic acid per 100 g of oil) during 90 days of storage. After 15 days of storage, the raw germ samples had significantly higher FA values than heat-treated germ samples, with the former exceeding the legal limit (2 g/100 g of oleic acid) and indicating deterioration in the nutritional and organoleptic quality of the wheat germ (Codex Alimentarius 1981). On the other hand, the heat-treated germ maintained FA values under the legal limit, thereby indicating a low or limited lipase activity and an acceptable quality of wheat germ oil in this aspect.
Fig. 3.
Peroxide value (PV), free fatty acids content (FA), conjugated dienes (K232) and trienes (K270) evolution during the storage time of raw (RG) and heat-treated (IRG) germ
The TTC values during the storage test were between 4133 ± 51 and 4181 ± 68 for raw wheat germ and between 3395 ± 45 and 3899 ± 10 for treated wheat germ, revealing a slight, but significant decrease (p ≤ 0.05) after 90 days of storage of treated samples. It is important to highlight that, after storage, the TTC values of treated germ maintained a high concentration.
The WI ranged between 58.31–59.12 and 54.91–53.21 for raw and heat-treated germ samples, respectively, with the heat-treated sample being slightly darker (p ≤ 0.05), but these differences were small with no changes being detected as a consequence of storage for 90 days.
Raw germ oil samples showed peroxide values from 0.16 ± 0.01 (0 day) to 0.36 ± 0.01 (90 days) meq O2/kg oil, whereas the heat-treated values varied between 0.26 ± 0.01 (0 day) and 0.93 ± 0.01 (90 days) meq O2/kg oil, in spite of the thermal treatment. Thus, PV well below the legal limits (15 meq O2/kg oil) for all 90 days of storage.
The process of lipid peroxidation starts with the generation of radicals, which appear in the oils when polyunsaturated fatty acids (PUFAs) are in contact with oxidation initiators such as metals, light or heat. The generated lipid radicals then rapidly react with molecular oxygen to generate peroxyl radicals, which possess a wide reactivity and produce hydroperoxides as primary oxidation products (Hernández Sánchez et al. 2016). The end of lipid oxidation occurs regularly when lipid radicals interact with one another to form stable non-radical products, or bind to other non-lipid macromolecules in food systems such as tocopherols (Elisia et al. 2013). It was suggested that the lipid radicals generated in wheat germ oil may have been stabilized by the high content of TTC of heat-treated samples combined with the effect of barriers from the package, thereby avoiding a vast generation of hydroperoxides during the storage test.
K232 and K270 are spectrophotometric measures that are used along with PV to evaluate oil primary oxidation. In the present sturdy, the K232 and K270 values of the raw germ were 3.12 ± 0.03 and 0.72 ± 0.02, respectively. As expected, K232 was higher than K270, with the wheat germ oil fatty acid profile being in agreement with Elisia et al. (2013). The K232 values of the heat-treated germ samples after 60 days of storage were significantly higher than those of raw germ samples (Fig. 3), whereas K270 did not reveal any significant differences, which could have been due to the low content of linolenic acid in germ oil.
The K232 and K270 values of the germ seem to be high compared with the literature values of raw cold-pressed oils reported by Martínez and Maestri (2015). These high values together with the low values of PV suggest a reduced activity of lipid radicals, thus producing stabilized conjugated dienes and trienes and avoiding hydroperoxide generation. As previously described, the reduced activity of the lipid radicals may be explained by the stabilizing action of wheat germ tocopherols or other antioxidant molecules from the matrix.
Conclusion
It was found that the infrared radiation technology resulted in reduced lipase activity and moisture content of the wheat germ in a short time, while preserving the high tocopherol content and maintaining the oil quality indicators within acceptable tolerances.
The surface response methodology utilized in the present investigation allowed the optimum IR process conditions for maximum lipase inactivation to be estimated, which were given by: IR Radiation intensity 4800 W/m2, 3 min of treatment time and a 0.2 m emitter-sample gap.
The evaluation of the oil quality parameters of the raw and optimized heat-treated germ demonstrated the effectiveness of the infrared treatment for reducing the generation of free fatty acid by wheat germ lipase, and permitted a good quality germ to be obtained for at least 90 days of storage at controlled room temperature in sealed packages.
Acknowledgements
The authors would like to thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Secretaría de Ciencia y Tecnología of Universidad Nacional de Córdoba (SeCyT-UNC) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) for financial support. We thank Dr. Paul Hobson, native speaker, for revision of the manuscript.
Abbreviations
- IR
Infrared radiation
- FFA
Free fatty acid content
- WI
Whiteness index
- FA0
Free fatty acid content before incubation
- FA48
Free fatty acid content after 48 h of incubation
- ΔFA
Change in free fatty acid content
- TTC
Total tocopherol content
- PV
Peroxide value
- IRRI
Infrared radiation intensity
- GD
Emitter-sample gap distance
- TT
Treatment time
- RSM
Response surface methodology
Contributor Information
Renato D. Gili, Email: renatogili@agro.unc.edu.ar
Pablo M. Palavecino, Email: pmpalavecino@agro.unc.edu.ar
M. Cecilia Penci, Phone: ++ 54 351 462 9520, Email: cpenci@gmail.com
Marcela L. Martinez, Email: marcelamartinez78@hotmail.com
Pablo D. Ribotta, Email: pribotta@agro.unc.edu.ar
References
- AACC . Approved methods of the American association of cereal chemistry. 11. Minnesota: St. Paul; 2010. [Google Scholar]
- Alimentarius Codex. Codex standard for edible fats and oils not covered by individual standards. Rome: FAO/WHO; 1981. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. 5. Champaign: AOCS Press; 2009. [Google Scholar]
- Attia RS, Abou-Gharbia HA. Evaluation and stabilization of wheat germ and its oil characteristics. Alex J Fd Sci Technol. 2011;8(2):31–39. [Google Scholar]
- Bansal S, Sudha ML. Nutritional, microstructural, rheological and quality characteristics of biscuits using processed wheat germ. Int J Food Sci Nut. 2011;62(5):474–479. doi: 10.3109/09637486.2010.549116. [DOI] [PubMed] [Google Scholar]
- Barnes HM. 5-PentadecylIresorcinol as an oxidation inhibitor of glycerides. US Pat. 1948;2(448):207. [Google Scholar]
- Capitani M, Mateo CM, Nolasco SM. Effect of temperature and storage time of wheat germ on the oil tocopherol concentration. Braz J Chem Eng. 2011;28(02):243–250. doi: 10.1590/S0104-66322011000200008. [DOI] [Google Scholar]
- Ding C, Khir R, Pan Z, Zhao L, Tu K, El-Mashad H, McHugh TH. Improvement in shelf life of rough and brown rice using infrared radiation heating. Food Bioprocess Technol. 2015;8(5):1149–1159. doi: 10.1007/s11947-015-1480-5. [DOI] [Google Scholar]
- Elisia I, Young JW, Yuan YV, Kitts DD. Association between tocopherol isoform composition and lipid oxidation in selected multiple edible oils. Food Res Int. 2013;52(2):508–514. doi: 10.1016/j.foodres.2013.02.013. [DOI] [Google Scholar]
- Engelsen MM, Hansen A. Tocopherol and tocotrienol content in commercial wheat mill streams. Cereal Chem. 2009;86(5):499–502. doi: 10.1094/CCHEM-86-5-0499. [DOI] [Google Scholar]
- Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, dosSantos WNL. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Galle KL (1974) Process for production stabilized wheat germ. US Pat 3783164 A
- Gili RD, Palavecino PM, Penci MC, Ribotta PD (2013) Estabilización y aprovechamiento de germen de trigo empleando radiación infrarroja. In: Asociación Argentina de Tecnólogos Alimentarios (ed) Proceedings of XIV Congreso Argentino de Tecnología de Alimentos
- Grandel F (1959) Process of making germ flakes. US Pat 2879167 A
- Hakansson B, Jagerstad M, Oste R, Akesson B, Jonssson L. The effects of various thermal processes on protein quality, vitamins and selenium content in whole-grain wheat and white flour. J Cereal Sci. 1987;6:269–282. doi: 10.1016/S0733-5210(87)80064-4. [DOI] [Google Scholar]
- Hemdane S, Leys S, Jacobs PJ, Dornez E, Delcour JA, Courtin CM. Wheat milling by-products and their impact on bread making. Food Chem. 2015;187:280–289. doi: 10.1016/j.foodchem.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Hernández Sánchez MDR, Cuvelier ME, Turchiuli C. Effect of α-tocopherol on oxidative stability of oil during spray drying and storage of dried emulsions. Food Res Int. 2016;88(A):32–41. doi: 10.1016/j.foodres.2016.04.035. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brandolini A, Pompei C. Kinetics of tocols degradation during the storage of einkorn (Triticum monococcum L. ssp. monococcum) and breadwheat (Triticum aestivum L. ssp. aestivum) flours. Food Chem. 2009;116:821–827. doi: 10.1016/j.foodchem.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Jahani M, Alizadeh M, Pirozifard M, Qudsevali A. Optimization of enzymatic degumming process for rice bran oil using response surface methodology. Lwt-Food Sci Technol. 2008;41(10):1892–1898. doi: 10.1016/j.lwt.2007.12.007. [DOI] [Google Scholar]
- Jha PK, Kudachikar VB, Kumar S. Lipase inactivation in wheat germ by gamma irradiation. Int J Radiat Phys Chem. 2013;86:136–139. doi: 10.1016/j.radphyschem.2013.01.018. [DOI] [Google Scholar]
- Krishnamurthy K, Khurana HK, Soojin J, Irudayaraj J, Demirci A. Infrared heating in food processing: an overview. Compr Rev Food Sci Food Saf. 2008;7(1):2–13. doi: 10.1111/j.1541-4337.2007.00024.x. [DOI] [Google Scholar]
- Magariño M, Mateo CM, Nolasco SM (2012) Efecto de variables de almacenamiento del aceite sobre los tocoferoles de germen de trigo. In: Proceedings of IV Congreso Internacional Ciencia y Tecnología de los Alimentos. http://cicytac.cba.gov.ar/edicionesAnt/2012/Libro%20de%20Res%C3%BAmenes%20CICyTAC%202012.pdf. Accessed 29 Jan 2016
- Marti A, Torri L, Casiraghi MC, Franzetti L, Limbo S, Morandin F, Pagani MA. Wheat germ stabilization by heat-treatment or sourdough fermentation: effects on dough rheology and bread properties. Lwt-Food Sci Technol. 2014;59(2):1100–1106. doi: 10.1016/j.lwt.2014.06.039. [DOI] [Google Scholar]
- Martínez ML, Maestri DM. Aceites vegetales no tradicionales. 1. Córdoba: Editorial Brujas; 2015. [Google Scholar]
- Martínez ML, Mattea MA, Maestri DM. Varietal and crop year effects on lipid composition of walnut (Juglans regia) genotypes. J Am Oil Chem Soc. 2006;83(9):791–796. doi: 10.1007/s11746-006-5016-z. [DOI] [Google Scholar]
- Martínez ML, Marín MA, Ribotta PD. Optimization of soybean heat-treating using a fluidized bed dryer. J Food Sci Technol. 2013;50(6):1144–1150. doi: 10.1007/s13197-011-0434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 5. New York: Wiley; 2001. [Google Scholar]
- Rahman M, Labuza TP. Water activity and food preservation. In: Rahman M, editor. Handbook of food preservation. 2. Boca Raton: Taylor and Francis; 2007. pp. 447–476. [Google Scholar]
- Rose DJ, Pike OA. A simple method to measure lipase activity in wheat and wheat bran as an estimation of storage quality. J Am Oil Chem Soc. 2006;83(5):415–419. doi: 10.1007/s11746-006-1220-0. [DOI] [Google Scholar]
- Rose D, Lynn D, Dunn M, Pike O. Enhanced lipid stability in whole wheat flour by lipase inactivation and antioxidant retention. Cereal Chem. 2008;85(2):218–223. doi: 10.1094/CCHEM-85-2-0218. [DOI] [Google Scholar]
- Sakai N, Hanzawa T. Applications and advances in far-infrared heating in Japan. Trends Food Sci Technol. 1994;5:357–362. doi: 10.1016/0924-2244(94)90213-5. [DOI] [Google Scholar]
- Shurpalekar SR, Rao PH. Wheat Germ. Adv Food Res. 1977;23:187–304. doi: 10.1016/S0065-2628(08)60329-8. [DOI] [PubMed] [Google Scholar]
- Sjövall O, Virtalaine T, Lapveteläinen A, Kallio H. Development of rancidity in wheat germ analyzed by headspace gas chromatography and sensory analysis. J Agric Food Chem. 2000;48(8):3522–3527. doi: 10.1021/jf981309t. [DOI] [PubMed] [Google Scholar]
- Tuncel NB, Yılmaz N, Kocabıyık H, Uygur A. The effect of infrared stabilized rice bran substitution on physicochemical and sensory properties of pan breads: part I. J Cereal Sci. 2014;59(2):155–161. doi: 10.1016/j.jcs.2013.12.003. [DOI] [Google Scholar]
- Yazicioglu B, Sahin S, Sumnu G. Microencapsulation of wheat germ oil. J Food Sci Technol. 2014;52(6):3590–3597. doi: 10.1007/s13197-014-1428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yılmaz N, Tuncel NB, Kocabıyık H. Infrared stabilization of rice bran and its effects on γ-oryzanol content, tocopherols and fatty acid composition. J Sci Food Agric. 2013;94:1568–1576. doi: 10.1002/jsfa.6459. [DOI] [PubMed] [Google Scholar]
- Yöndem-Makascıoǧlu F, Gürün B, Dik T, Suzan Kıncal N. Use of a spouted bed to improve the storage stability of wheat germ followed in paper and polyethlyene packages. J Sci Food Agric. 2005;85(8):1329–1336. doi: 10.1002/jsfa.2102. [DOI] [Google Scholar]