Abstract
The effects of sodium chloride (NaCl) (3.5%) solution and polysaccharides, such as carboxymethyl cellulose (CMC) (0.1, 0.3 and 0.5%) and gum arabic (5, 10 and 15%), on the physicochemical properties, antioxidant capacity and sensory characteristics of bitter gourd juice were investigated. An increase in the concentration of CMC and gum arabic significantly was observed to increase the lightness (L value) and the viscosity (mPas) of bitter gourd juice at all levels. Increased concentrations of gum arabic significantly increased the total soluble solids. The bitter gourd fruit treated with NaCl solution produced the highest lightness (L value) and scavenging activity of free radical 2,2-diphenyl-1-picrylhydrazyl of bitter gourd juice. Increased concentration of gum arabic up to 15% significantly increased the total phenolic content. The addition of 5% gum arabic effectively reduced the bitterness of the bitter gourd juice. Viscosity of the juice resulted in negative correlation for bitterness.
Keywords: Bitter gourd, Sodium chloride, Carboxymethyl cellulose, Gum arabic, Antioxidant capacity, Sensory characteristics
Introduction
Bitter gourd (Momordica charantia), which is also known as karela or bitter melon, is a member of the Cucurbitaceae family. It is commonly grown in tropical regions, such as Malaysia, Thailand, India, China and Africa, as well as the Middle East. Normally, bitter gourd is used to prepare fresh juice or used in culinary preparations. Bitter gourd is a good source of health-inducing components, such as vitamin A (471 IU/100 g sample), potassium (296 mg/100 g sample), vitamin C (84 mg/100 g sample), phosphorus (31 mg/100 g sample) and iron (0.43 mg/100 g sample) (Paul and Raychaudhuri 2010). In addition, it also contains polyphenolic compounds (flavonoids, coumarins, anthroquinones, anthocyanin, carotenoid, gentisic acid, gallic acid, catechin and caffeic acid) (Kubola and Siriamornpun 2008; Horax et al. 2010; Nagarani et al. 2014), saponins, alkaloids, proteins and steroids (Grover and Yadav 2004). Bitter gourd has been used for the treatment of diabetes, gout, jaundice, rheumatism and pneumonia (Joseph and Jini 2013), and possesses other medicinal properties, such as anti-tumor and anti-mutagenic activities (Anilakumar et al. 2015). Even though bitter gourd is very good for health, most people avoid consuming it because of its bitter taste (Paul and Raychaudhuri 2010).
The bitter taste of the bitter gourd is due to the alkaloids (Harinantenaina et al. 2006; Nagarani et al. 2014) and the polyphenolic compounds. Reducing the bitter taste is essential to make them more appealing to consumers. Soaking the vegetables in natrium chloride (NaCl) solution followed by the blanching process could help minimize the bitter taste (Din et al. 2011; Krawinkel and Keding 2006). In addition, applying sucrose also reduces the bitter taste of several food products, such as yogurt fortified with green tea, flavonoid enhanced chocolate bars and bread fortified with grapes (Gaudette and Pickering 2013). Other researchers (Troszynska et al. 2008) also reported that polysaccharides could reduce the bitter taste of foods through the masking effects of the bitter compounds.
The application of carboxymethyl cellulose (CMC) in food formulations could increase the viscosity and clarity as well as produce a gummy texture (Hilal and Ozdemir 2007). In addition, CMC could encapsulate or mask the acidic and bitter compounds in beverages, and, hence, reduce the sourness in orange drinks and the bitterness in coffee (Glicksman 1982). The masking effect of CMC on the antioxidant compounds could protect the compounds from the oxidation process, and, consequently, maintain the quality of the food products.
Gum arabic is a natural plant based gum that is widely used in the food industry as a stabilizing, thickening and emulsifying agent in beverages to enhance the experience in the mouth. It is highly soluble in water and produces lower viscosity compared to other gums. Gum arabic has also been used as a debittering agent due to its ability to mask the bitter compounds and reduce the bitterness of vegetable juice while providing dietary fiber and increasing the nutritional value of the product (Sohi et al. 2004). Gum arabic has also shown antioxidant properties (Ali 2004) that could reduce the oxidation process of the food products.
The present study investigated the effect of bitter gourd juice produced from bitter gourd with and without treatment with a NaCl solution, as well as the addition of CMC and gum arabic at different concentrations on the physicochemical properties, antioxidant capacity and sensory characteristics.
Materials
Fresh matured light green color bitter gourds with firm texture were selected from the local hypermarket, Tesco (Pulau Pinang, Malaysia). The gum arabic and sodium chloride (NaCl) were obtained from Euro Chemo-Pharma (Pulau Pinang, Malaysia). The carboxymethyl cellulose (CMC) and citric acid were purchased from Sim Company Sdn. Bhd. (Pulau Pinang, Malaysia). The caffeine, folin-ciocalteu reagent, iron (III) chloride hexahydrate (FeCl3·6H2O), 2,4,6-tripyridyl-s–triazine (TPTZ) and gallic acid were obtained from Merck KGaA (Darmstadt, Germany). Sodium carbonate (Na2CO3) was obtained from Bendosen Laboratory Chemicals (Bendosen, Norway), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and acetate butter (pH 3.6) were obtained from Sigma-Aldrich Co. (Missouri, USA), Hydrochloric acid (HCl) was obtained from Brightchem Sdn. Bhd. (Selangor, Malaysia), and iron (II) sulfate (FeSO4) was obtained from Fisher Scientific (Leicestershire, UK).
Methods
Preparation of bitter gourd juice
Fresh bitter gourds were washed with water to remove any dirt on the surface. The seeds were removed and cut into smaller pieces (2 cm thick). The bitter gourd juice was extracted using a centrifugal juice extractor (JE680, Kenwood, UK). Then, carboxymethyl cellulose (CMC) (0, 0.1, 0.3 and 0.5%, w/v) and gum arabic (0, 5, 10 and 15%, w/v) at different concentrations were added into the bitter gourd juice. The juice was then mixed with 0.3% (w/v) citric acid and pasteurized at 90 °C for 10 s (Loong and Goh 2004).
For treatment with sodium chloride (NaCl), the small pieces of bitter gourd were immersed in 3.5% (w/v) NaCl solution at room temperature (25 °C) for 60 min. The fruits were rinsed and blanched at 96 °C for 3 min (Yao and Ren 2011) before extraction using a centrifugal juice extractor (JE680, Kenwood, UK). After that, the juice was acidified with citric acid (0.3% w/v) and pasteurized at 90 °C for 10 s.
Bitter gourd juice (20 mL) was put into a centrifuge tube and centrifuged using a centrifuge machine (4000, Kubota Corporation, Japan) at room temperature for 10 min at 3500 rpm. The supernatants were collected and were analyzed for physicochemical properties, antioxidant capacity and sensory characteristics.
Physicochemical analysis
Determination of color
The color of bitter gourd juice was measured using a colorimeter (CM-3500D, Konica Minolta Co. Ltd., Japan). Calibration was done using a zero calibration box and white calibration plate prior to sample color determination. Bitter gourd juice (15 mL) was placed into a glass container and values of L* (lightness/brightness), a* (redness), -a* (greenness), b* (yellowness) and -b* (blueness) for each sample were measured in triplicate.
Determination of pH and total soluble solid
The pH of the bitter gourd juice was measured using a pH meter (S40 Seven Multi™, Metter-Toledo, Switzerland). Calibration of the pH meter was done prior to sample pH determination. The total soluble solids (TSS) of the sample were measured using a hand-held refractometer (HSR-500, Atago Co. Ltd., Japan). A few drops of the sample were used to determine the TSS content and the value was expressed in °Brix. All samples were measured in triplicate.
Determination of viscosity
The viscosity of the bitter gourd juice was measured using a Viscometer (Vibro-viscometer, A and D Co. Ltd., Japan). A 45 mL sample was used to measure the viscosity using a Viscometer probe, SV-10. All samples were measured in triplicate.
Determination of total phenolic content
The total phenolic content (TPC) of the bitter gourd juice was determined using the method of Zhou and Yu (2006) with slight modification. A 0.1 mL sample was mixed with 50 µL of Folin–Ciocalteau reagent, 0.3 mL of 20% sodium carbonate (Na2CO3) and 1 mL of distilled water in a test tube. The mixture was allowed to react for 2 h at room temperature, in the dark. The mixture (280 µL) was transferred in a 96-well cell culture cluster (Costar, Cortning, NY) and the absorbance was measured at 750 nm using a Microplate reader (ELx800, BioTek Instruments, USA). A standard curve was plotted using gallic acid at concentrations of 150, 200, 250, 300, 350 and 400 ppm. All the samples were measured in triplicate and the result was expressed as mg GAE/100 mL sample.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity
The DPPH radical-scavenging activity of the bitter gourd juice was determined in accordance with the method described by Kubola and Siriamornpun (2008) with some modification. Bitter gourd juice (0.04 mL) was added to 1.2 mL of 0.2 mM DPPH methanolic solution. The mixture was mixed vigorously and allowed to react for 30 min at room temperature in the dark. Then, a 280 µL of mixture was transferred into a 96-well cell culture cluster (Costar, Cortning, NY). The decrease in absorbance value was measured using a Microplate reader (ELx800, BioTek Instruments, USA) at 515 nm. The scavenging activity of the sample was measured according to the percentage of free radical DPPH inhibition and calculated as DPPH inhibition (%) = [(Acontrol − Asample)/Acontrol] × 100. All the samples were measured in triplicate.
Ferric reducing/antioxidant power (FRAP) assay
The FRAP assay was conducted according to Moyer et al. (2002). The FRAP reagent was freshly prepared by mixing 100 mL of 300 mM acetate buffer (pH 3.6), 10 mL of TPTZ solution (10 mM TPTZ in 40 mM HCl), and 10 mL of iron (III) chloride hexahydrate (FeCl3.6H2O) solution in a ratio of 10:1:1. A 12 mL sample of deoinized water was added to the FRAP reagent and stored at 37 °C until used. To perform the assay, 1.8 mL FRAP reagent, 180 µL deionized water and 60 µL sample were mixed together in a test tube and allowed to react for 4 min at 37 °C in a water bath (Memmert, Germany). The absorbance of the sample (2 mL) was measured at 593 nm using a spectrophotometer (UV-160A, Shimadzu Corp., Japan), with the FRAP reagent as the blank. The standard curve of 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 mM iron (II) sulfate (FeSO4) in methanol with 0.1% (w/v) HCl was prepared. All the samples were measured in triplicate and the results were expressed as µmol FeSO4/mL sample.
Descriptive sensory analysis
A group of ten panelists (1 male, 9 female) aged 23–24 years old were selected for the sensory evaluation test. The panelists were selected based on their ability to detect and recognize four basic tastes; sweetness (1% sucrose), sourness (0.1% citric acid), bitterness (0.05% caffeine) and saltiness (0.5% NaCl) using a simple detection test. The panelists were trained over 9 h (1.5 h per session, 3 times per week for 2 weeks). The training process followed the method described by Ng et al. (2012). The panelists were given several bitter gourd juice formulations and were asked to generate terminology for the juice in terms of sensory attributes (bitter taste, sour taste and viscosity). The description, references and intensity for each sensory attribute are shown in Table 1. The panelists marked the intensity of the reference samples using an unstructured 15 cm line scale. The training process was done several times until all the panellists were familiar with the intensity for each reference material.
Table 1.
Descriptions, references and intensity for sensory attributes
| Attribute | Description | Reference material | Intensity (cm) |
|---|---|---|---|
| Bitterness | Basic taste sensation as found in caffeine solution | 0.05%, 0.15%, 0.20% caffeine solution | 2, 10, 15a |
| Sourness | Basic taste sensation as found in citric acid solution | 0.05%, 0.15%, 0.20% citric acid solution | 2, 10, 15a |
| Viscosity | Thickness of liquid as in carboxymethyl cellulose (CMC) solution | 0.05%, 0.8% CMC solution | 1.6, 13.7 |
aAdapted from Meilgaard et al. (1999). The reference intensity was calculated as the mean rating of the group of ten panelists using a 15 cm unstructured line scale
For the sensory evaluation, bitter gourd juice (15 mL) at different concentrations of carboxymethyl cellulose (CMC) (0.1 and 0.5%, w/v) and gum arabic (5 and 15%, w/v), as well as bitter gourd juice with and without 3.5% (w/v) sodium chloride (NaCl) treatment, were coded with a 3-digit random number and served to each panelist for evaluation. Distilled water was provided for the panelists to rinse their mouth before and between tasting the samples. The panelists evaluated the samples in individual booths in the sensory room (School of Industrial Technology, USM, Penang). Six samples were evaluated in random order for each session. Each sample was evaluated in duplicate on separate testing sessions.
Statistical analysis
SPSS software (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, USA) was used to analyze the data. The results were presented as mean ± standard deviation. The probability value of p < 0.05 was considered significant. Analysis of variance (ANOVA) was performed and mean comparisons were done by Duncan’s multiple range tests. Pearson’s correlation was used to determine the correlation of data between the viscosity of the juice detection by panelists (cm) and Viscometer (mPa), as well as the bitter taste (cm) and the viscosity (cm) of the bitter gourd juice detected by the panelists.
Results and discussions
Color of bitter gourd juice
The color of the bitter gourd juice with and without treated with NaCl solution, as well as the addition of carboxymethyl cellulose (CMC) and gum arabic at different concentrations was expressed as L (Lightness), a* (redness) and b* (yellowness) value (Table 2). All the bitter gourd juices produced were yellowish to brownish red color. The bitter gourd treated with 3.5% NaCl solution showed the highest lightness (L-value) and was significant (p < 0.05) different compared to the other samples of bitter gourd juice. Treating the bitter gourd fruit with NaCl solution effectively prevented the enzymatic browning (Luo et al. 2011) and produced greater lightness (L value) in the bitter gourd juice. The physical process of blanching inactivated the peroxydase (POD) and polyphenol oxydase (PPO) enzyme (Shivhare et al. 2009), which otherwise contributed to the brown color. Increasing the concentrations of CMC and gum arabic resulted in an increase in the lightness of the bitter gourd juice at all levels. This may due to the coating effect on the polyphenolic compound which prevent these from interacting with the peroxydase (POD) and polyphenol oxydase (PPO) that cause the enzymatic browning. Thus, the addition of CMC and gum arabic delayed the enzymatic browning of bitter gourd juice (Aggarwal and Sandhu 2004).
Table 2.
Effect of control and treated bitter gourd juice on color values (L, a and b)
| Sample | L* | a* | b* |
|---|---|---|---|
| Bitter gourd treated with 3.5% (w/v) NaCl | |||
| Bitter gourd juice | 22.40a ± 0.09 | 8.44e ± 0.03 | 37.49a ± 0.10 |
| Untreated bitter gourd | |||
| Bitter gourd juice | 16.90e ± 0.11 | 8.14f ± 0.01 | 28.57e ± 0.17 |
| Bitter gourd juice with carboxymethyl cellulose, CMC (w/v) | |||
| 0.1% | 14.25g ± 0.03 | 10.40a ± 0.03 | 24.18f ± 0.07 |
| 0.3% | 16.00f ± 0.04 | 9.56c ± 0.05 | 30.09b ± 0.09 |
| 0.5% | 17.83b ± 0.04 | 7.74h ± 0.02 | 30.11b ± 0.05 |
| Bitter gourd juice with gum arabic (w/v) | |||
| 5% | 14.36g ± 0.08 | 9.67b ± 0.02 | 24.33f ± 0.14 |
| 10% | 17.28d ± 0.02 | 8.90d ± 0.03 | 29.20d ± 0.05 |
| 15% | 17.41c ± 0.06 | 8.08g ± 0.02 | 29.43c ± 0.01 |
Mean ± SD (n = 3) values with different superscript letters within the same column are significantly different at p < 0.05
Bitter gourd treated with 3.5% (w/v) NaCl solution followed by blanching produced bitter gourd juice with a slightly higher (p < 0.05) a* value (redness) than the untreated bitter gourd with NaCl solution (Table 2). During the heating process, the bright green color of bitter gourd changed to slightly brownish. This may be due to the degradation of chlorophyll to pyropheophytin (decarboxymethyoxylated magnesium-free chlorophyll derivative) (Oboh 2005). The central magnesium atom is easily removed during thermal processing, replacing the magnesium with hydrogen and forming the unappealing olive-brown pigments, pheophytins (Loong and Goh 2004). As the concentration of CMC and gum arabic increased, the a* value (redness) of bitter gourd juice significantly decreased (p < 0.05). The results showed that at higher concentrations, hydrocolloids reduced the oxidation process and delayed the enzymatic browning.
The b*value (yellowness) for all samples of bitter gourd juice produced had a similar trend as the L*value (lightness). This showed that the increase in the yellowness also increased the lightness of the bitter gourd juice. Conversely, the increase in yellowness and lightness reduced the redness of the juice. Incorporating CMC and gum arabic minimized the oxidation process and delayed the enzymatic browning, which lessened the color changes from dark green to brownish red.
PH, total soluble solid and viscosity of bitter gourd juice
The pH, total soluble solid (°Brix) and viscosity of untreated bitter gourd juices and that treated with NaCl solution, as well as the addition of CMC and gum arabic at different concentrations, are presented in Table 3. The pH of the bitter gourd juices was in the range of 4.24–4.45. The addition of the citric acid to the juice reduced the pH to less than 4.5, which made it less subject to microbial spoilage, and, hence, appropriate for the pasteurization process.
Table 3.
Effect of control and treated bitter gourd juice on the physicochemical properties of bitter gourd juice
| Sample | pH | Total soluble solids (˚Brix) | Viscosity (mPas) |
|---|---|---|---|
| Bitter gourd treated with 3.5% (w/v) NaCl | |||
| Bitter gourd juice | 4.24 | 3.50d ± 0.00 | 1.43f ± 0.03 |
| Untreated bitter gourd | |||
| Bitter gourd juice | 4.33 | 3.23d ± 0.15 | 1.00f ± 0.03 |
| Bitter gourd juice with carboxymethyl cellulose (CMC) (w/v) | |||
| 0.1% | 4.34 | 3.40d ± 0.00 | 1.36f ± 0.04 |
| 0.3% | 4.41 | 3.37d ± 0.06 | 6.42d ± 0.24 |
| 0.5% | 4.45 | 3.50d ± 0.10 | 13.43b ± 0.12 |
| Bitter gourd juice with gum arabic (w/v) | |||
| 5% | 4.30 | 6.73c ± 1.24 | 3.10e ± 0.03 |
| 10% | 4.33 | 10.67b ± 0.12 | 7.41c ± 0.15 |
| 15% | 4.33 | 15.63a ± 0.15 | 22.6a ± 1.13 |
Mean ± SD (n = 3) values with different superscript letters within the same column are significantly different at p < 0.05
There were no significant (p > 0.05) changes in the total soluble solids (TSS) of bitter gourd juice with and without treated with the NaCl solution, or those containing different concentrations of CMC (Table 3). However, the TSS value of bitter gourd juice at all levels increased significantly (p < 0.05) as the concentration of gum arabic increased. This might be due to the high carbohydrate content (97%) in gum arabic, which were composed of D-galactose, L-arabinose and D-galacturonic acid (Montenegro et al. 2012) that contributed to the high TSS value (Dauqan and Abdullah 2013). Hamad et al. (2013) also showed that Sudanese fermented milk (Robe) supplemented with gum arabic powder at various concentrations significantly increased the TSS value.
Treating bitter gourd juice with NaCl solution did not have a significant effect on the viscosity of the bitter gourd juice (Table 3). However, increasing the concentration of CMC caused a significant (p < 0.05) increase in the viscosity of the juice. The formation of a viscous solution may be due to the electrostatic repulsion between the CMC molecules (Liang et al. 2006) as well as between the CMC molecules and the juice haze (Beristain et al. 2006). In addition, the viscosity of the bitter gourd juice also increased significantly (p < 0.05) after incorporating gum arabic at all levels. This is due to the highly branched structure (β-(1–3) galactose), which leads to a compact, relatively small hydrodynamic volume (Montenegro et al. 2012). However, gum arabic has low viscosity at 30% concentration compared to 1% CMC at low shear rates (Williams and Philips 2000). Therefore, the viscosity of the bitter gourd juice at 0.3% (w/v) CMC (6.42 mPas) was similar to the viscosity of the bitter gourd with 10% (w/v) gum arabic (7.41 mPas).
Antioxidant properties of bitter gourd juice
Table 4 shows the total phenolic content and antioxidant activities of bitter gourd juice with and without treatment with NaCl solution, as well as with the addition of carboxymethyl cellulose (CMC) and gum arabic at different concentrations. No significant difference (p > 0.05) was observed in the total phenolic content of the bitter gourd juice from the with and without treated the bitter gourd fruit with the NaCl solution. Conversely, incorporating 0.1% CMC significantly (p < 0.05) increased the total phenolic content of the bitter gourd juice. This showed that the CMC has the ability to mask the phenolic compounds, which prevents the degradation and oxidation process during the processing of bitter gourd juice. However, the addition of gum arabic at 15% caused no significant difference (p > 0.05) in the total phenolic content compared to the bitter gourd juice containing 0.1% CMC. CMC and gum arabic could provide protection of the phenolic compounds from the environmental conditions, such as oxygen and temperature, as well as minimize the oxidation process (Robert and Fredes 2015).
Table 4.
Effect of control and treated bitter gourd juice on the antioxidant property of bitter gourd juice
| Sample | Total phenolic content (mg GAE/100 mL sample) | DPPH (% inhibition) | FRAP (µmol FeSO4/mL sample) |
|---|---|---|---|
| Bitter gourd treated with 3.5% (w/v) NaCl | |||
| Bitter gourd juice | 32.81d ± 1.83 | 63.35a ± 1.25 | 4.95cd ± 0.06 |
| Untreated bitter gourd | |||
| Bitter gourd juice | 30.48d ± 1.37 | 47.87d ± 0.39 | 10.80a ± 0.33 |
| Bitter gourd juice with carboxymethyl cellulose (CMC) (w/v) | |||
| 0.1% | 50.93a ± 1.79 | 47.43d ± 0.99 | 8.61b ± 0.50 |
| 0.3% | 51.48a ± 1.16 | 53.67bc ± 1.80 | 8.56b ± 0.88 |
| 0.5% | 49.63ab ± 2.51 | 55.10bc ± 2.25 | 10.02a ± 0.02 |
| Bitter gourd juice with gum arabic (w/v) | |||
| 5% | 43.89c ± 2.39 | 52.15c ± 1.83 | 4.45d ± 0.17 |
| 10% | 46.91bc ± 1.95 | 54.40bc ± 2.63 | 4.48d ± 0.30 |
| 15% | 53.11a ± 2.49 | 55.58b ± 2.06 | 5.76c ± 0.79 |
Mean ± SD (n = 3) values with different superscript letters within the same column are significantly different at p < 0.05
The percentage of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical inhibition of bitter gourd juice prepared after treating the bitter gourd fruit with NaCl solution followed by the blanching process was significantly (p < 0.05) higher compared to the other samples (Table 4). The presence of NaCl and the heat treatment during the blanching process inhibited the polyphenoloxidase (PPO) enzyme, and, hence, prevented the oxidation process from occurring (Li et al. 2015; Chutintrasri and Noomhorm 2006). Therefore, most of the antioxidant compounds in the juice were available to scavenge the free radicals of DPPH. The DPPH scavenging activity of bitter gourd juice increased significantly (p < 0.05) as the concentration of CMC and gum arabic increased. This suggests that the higher the percentage of CMC and gum arabic used, the more the antioxidant compounds (polyphenolics, vitamins, amino acids) can be protected during processing.
In this study, Ferric reducing/antioxidant power (FRAP) assay was conducted to evaluate the sample’s reducing power based on the reduction of Fe3+-TPTZ to Fe2+-TPTZ by electron-donating antioxidants present within the sample. The results showed that the bitter gourd juice produced after being treated with 3.5% (w/v) NaCl solution followed by the blanching process had a significantly (p < 0.05) lower in FRAP value compared with the untreated bitter gourd (Table 4). This revealed that heat treatment, such as the blanching process can cause alteration to the phytochemical characteristics and chemical composition of vegetables, as has been reported (Amin et al. 2006), to reduce the ascorbic acid as well as the antioxidant components and antioxidant activities. Increasing the concentrations of CMC and gum arabic up to 0.5 and 15% respectively, significantly (p < 0.05) increased the FRAP value. The higher FRAP value may be due to the encapsulation effects of CMC and gum arabic that protected the antioxidant compounds from the oxidation and degradation process during the processing of the juice.
Quantitative descriptive analysis (QDA)
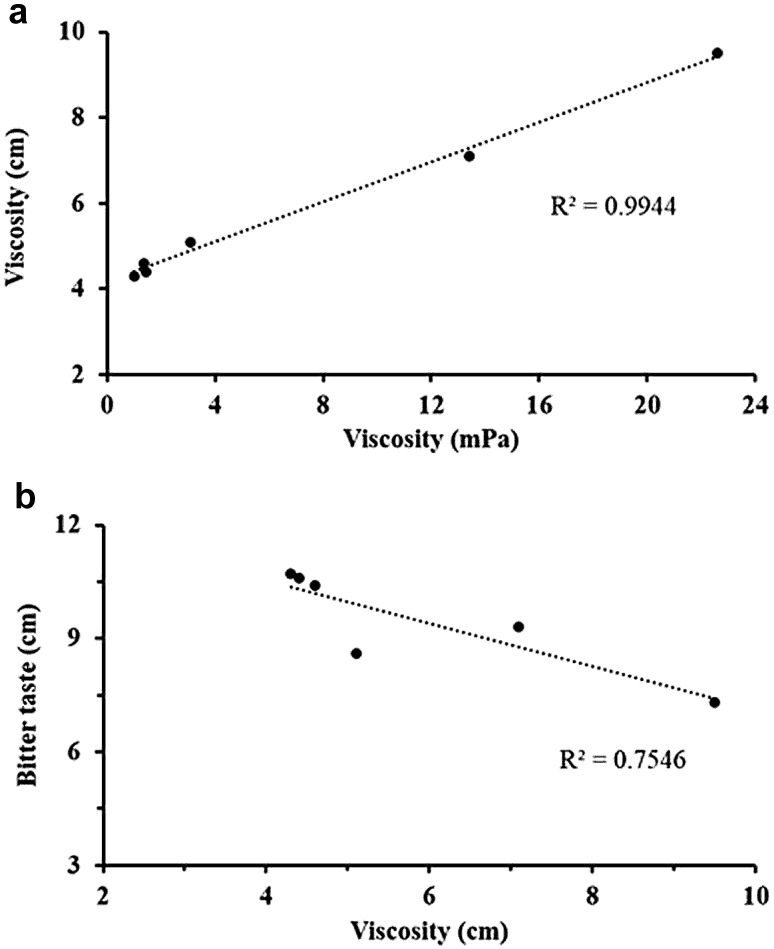
The sensory characteristics (viscosity, bitterness and sourness) of bitter gourd juice are presented in Table 5. The results clearly showed that an increase in the concentration of CMC and gum arabic significantly (p < 0.05) increased the viscosity of the bitter gourd juice. However, the panelists did not detect the differences in the viscosity of the bitter gourd juice produced from the bitter gourd fruit with and without treated with 3.5% NaCl solution, as well as the juice containing 0.1% CMC and 5% gum arabic. The viscosity of the bitter gourd juice measured by the panelists (cm) showed a highly positive correlation (r = 0.997) with the viscosity measured using a viscometer (Fig. 1a).
Table 5.
Effect of control and treated bitter gourd juice on the sensory attributes of bitter gourd juice
| Sample | Viscosity | Bitterness | Sourness |
|---|---|---|---|
| Bitter gourd treated with 3.5% (w/v) NaCl | |||
| Bitter gourd juice | 4.4c ± 0.7 | 10.6a ± 1.1 | 5.5b ± 0.7 |
| Untreated bitter gourd | |||
| Bitter gourd juice | 4.3c ± 0.6 | 10.7a ± 1.7 | 8.8a ± 1.1 |
| Bitter gourd juice with carboxymethyl cellulose, CMC (w/v) | |||
| 0.1% | 4.6c ± 1.6 | 10.4ab ± 1.7 | 8.2a ± 0.7 |
| 0.5% | 7.1b ± 0.3 | 9.3ab ± 1.1 | 5.7b ± 0.4 |
| Bitter gourd juice with gum arabic (w/v) | |||
| 5% | 5.1c ± 1.4 | 8.6bc ± 0.6 | 8.2a ± 0.5 |
| 15% | 9.5a ± 0.6 | 7.3c ± 1.4 | 5.4b ± 0.9 |
Mean ± SD (n = 10) values with different superscript letters within the same column are significantly different at p < 0.05
Fig. 1.
a The correlation between the viscosity detection by panelists (cm) and that using a viscometer (mPa), and b the correlation between the viscosity (cm) and the bitter taste (cm) detection by panelists
Generally, the bitter taste of vegetables could be reduced or eliminated by the treatment of NaCl (Din et al. 2011) and the blanching process (Kim et al. 2013). In addition, polysaccharides, such as CMC, have also been used in the food industry to encapsulate the bitter taste of polyphenols and tannins in beverages to reduce their astringent effects on the salivary glands (Troszynska et al. 2010). Table 5 shows that no significant (p > 0.05) difference was observed in the bitterness of the bitter gourd juice from the untreated bitter gourd or that treated with NaCl solution and the addition of CMC up to 0.5%. However, the addition of 5% gum arabic significantly (p < 0.05) reduced the bitterness of the bitter gourd juice. The results showed that the bitter taste of the bitter gourd juice had a negative correlation (r = −0.869) and was significant (p < 0.05) with the viscosity detected by the panelists (Fig. 1b).
The bitter gourd juice prepared after treating the bitter gourd with NaCl solution followed by the blanching significantly (p < 0.05) reduced the sour taste (Table 5). This could be due to the neutralization of the ascorbic acid in the juice by the NaCl and/or the ascorbic acid leached out during the blanching process. Increasing the concentration of CMC and gum arabic effectively (p < 0.05) decreased the sourness of the bitter gourd juice. However, no significant difference (p > 0.05) was observed in the sourness of the bitter gourd juice between the untreated bitter gourd and that treated with NaCl solution, or that to which 0.1% CMC and 5% gum arabic was added, for which the intensity was 8.8, 8.2 and 8.2, respectively.
Conclusion
Bitter gourd juice produced from untreated bitter gourd and that treated with 3.5% NaCl solution, as well as the addition with CMC and gum arabic at different concentrations, led to significant changes in the color (L, a and b) of the juice. Increasing the concentrations of gum arabic significantly (p < 0.05) increased the total soluble solids and the viscosity of the bitter gourd juice. The bitter gourd treated with NaCl solution increased the scavenging activity of DPPH but decreased the FRAP value. However, increasing the concentrations of CMC and gum arabic significantly increased the scavenging activity of DPPH and the FRAP value. The panelists could detect a reduction (p < 0.05) in the bitterness of the bitter gourd juice after the addition of 5% gum arabic.
Acknowledgements
The researchers would like to express their gratitude to Universiti Sains Malaysia for its financial support (304/PTEKIND/6313061) for this research.
References
- Aggarwal P, Sandhu KS. Effect of hydrocolloids on the limonin content of kinnow juice. JFAE. 2004;2(1):44–48. [Google Scholar]
- Ali BH. Does GA have an antioxidant action in rat kidney? Ren Fail. 2004;26(1):1–3. doi: 10.1081/JDI-120028536. [DOI] [PubMed] [Google Scholar]
- Amin I, Norazaidah Y, Emmy HKI. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006;94:47–52. doi: 10.1016/j.foodchem.2004.10.048. [DOI] [Google Scholar]
- Anilakumar KR, Kumar GP, Ilaiyaraja N. Nutritional, pharmacological and medicinal properties of Momordica charantia. Int J Food Sci Nutr. 2015;4:75–83. doi: 10.11648/j.ijnfs.20150401.21. [DOI] [Google Scholar]
- Beristain CI, Cruz Sosa F, Lobato-Calleros C, Pedroza-Islas R, Rodriguez Huezo ME, Verde-Calvo JR. Applications of soluble dietary fibers in beverage. Rev Mex Ing Quim. 2006;5:81–95. [Google Scholar]
- Chutintrasri B, Noomhorm A. Thermal inactivation of polyphenoloxidase in pineapple puree. LWT Food Sci Technol. 2006;39(5):492–495. doi: 10.1016/j.lwt.2005.04.006. [DOI] [Google Scholar]
- Dauqan E, Abdullah A. Utilization of gum arabic for industries and human health. Am J Appl Sci. 2013;10(10):1270–1279. doi: 10.3844/ajassp.2013.1270.1279. [DOI] [Google Scholar]
- Din A, Aftab S, Bukhari H, Salam A, Ishfaq B. Development of functional and dietetic beverage from bitter gourd. Food Technol. 2011;13:355–360. [Google Scholar]
- Gaudette NJ, Pickering GJ. Modifying bitterness in functional food systems. Crit Rev Food Sci Nutr. 2013;53(5):464–481. doi: 10.1080/10408398.2010.542511. [DOI] [PubMed] [Google Scholar]
- Glicksman M. Food hydrocolloids. Boca Raton: CRC Press Inc.; 1982. pp. 3–101. [Google Scholar]
- Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Enthnopharmacol. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Hamad HE, Sulieman AME, Salih ZA. Quality aspects of the sudanese fermented milk (Robe) supplemented with gum arabic powder. Discourse J Agric Food Sci. 2013;1(1):8–13. [Google Scholar]
- Harinantenaina L, Tanaka M, Takaoka S, Oda M, Mogami O, Uchida M, Asakawa Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem Pharm Bull. 2006;54(7):1017–1021. doi: 10.1248/cpb.54.1017. [DOI] [PubMed] [Google Scholar]
- Hilal S, Ozdemir F. Effect of some hydrocolloids on the serum separation of different formulated ketchups. J Food Eng. 2007;81:437–446. doi: 10.1016/j.jfoodeng.2006.11.022. [DOI] [Google Scholar]
- Horax R, Hettiarachchy N, Chen P. Extraction, quantification, and antioxidant activities of phenolics from pericarp and seeds of bitter melons (Momordica charantia) harvested at three maturity stages (immature, mature, and ripe) J Agric Food Chem. 2010;58:4428–4433. doi: 10.1021/jf9029578. [DOI] [PubMed] [Google Scholar]
- Joseph B, Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac J Trop Dis. 2013;3(2):93–102. doi: 10.1016/S2222-1808(13)60052-3. [DOI] [Google Scholar]
- Kim MH, Kim JM, Yoon KY. Effects of blanching on antioxidant activity and total phenolic content according to type of medicinal plants. Food Sci Biotechnol. 2013;22(3):817–823. doi: 10.1007/s10068-013-0150-5. [DOI] [Google Scholar]
- Krawinkel MB, Keding GB. Bitter gourd (Momordica Charantia): a dietary approach to hyperglycemia. Nutr Rev. 2006;64:331–337. doi: 10.1111/j.1753-4887.2006.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110(4):881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Li Y, Wills RBH, Golding JB. Sodium chloride, a cost effective partial replacement of calcium ascorbate and ascorbic acid to inhibit surface browning on fresh-cut apple slices. LWT Food Sci Technol. 2015;64:503–507. doi: 10.1016/j.lwt.2015.05.010. [DOI] [Google Scholar]
- Liang C, Hu X, Ni Y, Wu J, Chen F, Liao X. Effect of hydrocolloids on pulp sediment, white sediment, turbidity and viscosity of reconstituted carrot juice. Food Hydrocoll. 2006;20(8):1190–1197. doi: 10.1016/j.foodhyd.2006.01.010. [DOI] [Google Scholar]
- Loong MN, Goh HK. Colour degradation of acidified vegetable juice. Int J Food Sci Technol. 2004;39(4):437–441. doi: 10.1111/j.1365-2621.2004.00802.x. [DOI] [Google Scholar]
- Luo YG, Lu SM, Zhou B, Feng H. Dual effectiveness of sodium chlorite for enzymatic browning inhibition and microbial inactivation on fresh-cut apples. LWT Food Sci Technol. 2011;44(7):1621–1625. doi: 10.1016/j.lwt.2011.02.015. [DOI] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. 3. Boca Raton: CRC Press; 1999. [Google Scholar]
- Montenegro MA, Boiero ML, Valle L, Borsarelli CD (2012) Gum Arabic: more than an edible emulsifier. Products and applications of biopolymers, vol 220. Retrieved from http://www.intechopen.com/books/products-and-applications-of-biopolymers/gum-arabic-more-than-an-edible-emulsifier
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad R. Antocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- Nagarani G, Abirami A, Siddhuraju P. Food prospects and nutraceutical attributes of Momordica species: a potential tropical bioresources—a review. Food Sci Hum Wellness. 2014;3(3–4):117–126. doi: 10.1016/j.fshw.2014.07.001. [DOI] [Google Scholar]
- Ng M, Lawlor JB, Chandra S, Chaya C, Hewson L, Hort J. Using quantitative descriptive analysis and temporal dominance of sensations analysis as complementary methods for profiling commercial blackcurrant squashes. Food Qual Prefer. 2012;25(2):121–134. doi: 10.1016/j.foodqual.2012.02.004. [DOI] [Google Scholar]
- Oboh G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT Food Sci Technol. 2005;38:513–517. doi: 10.1016/j.lwt.2004.07.007. [DOI] [Google Scholar]
- Paul A, Raychaudhuri SS. Medicinal uses and molecular identification of two Momordica charantia varieties—a review. Electron J Biol. 2010;6(2):43–51. [Google Scholar]
- Robert P, Fredes C. The encapsulation of anthocyanins from berry-type fruits. Trends Foods Mol. 2015;20:2875–5888. doi: 10.3390/molecules20045875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivhare US, Gupta M, Basu S, Raghavan GSV. Optimization of blanching process for carrots. J Food Process Eng. 2009;32(4):587–605. doi: 10.1111/j.1745-4530.2007.00234.x. [DOI] [Google Scholar]
- Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004;30(5):429–448. doi: 10.1081/DDC-120037477. [DOI] [PubMed] [Google Scholar]
- Troszynska A, Narolewska O, Wolejszo A, Ostaszyk A. Effect of carboxymethyl cellulose (CMC) on perception of astringency of phenolic compounds. Pol J Food Nutr Sci. 2008;58(2):241–245. [Google Scholar]
- Troszyńska A, et al. The effect of polysaccharides on the astringency induced by phenolic compounds. Food Qual Prefer. 2010;21:463–469. doi: 10.1016/j.foodqual.2009.12.005. [DOI] [Google Scholar]
- Williams PA, Philips GO. Gum arabic. In: Philips GO, Williams PA, editors. Handbook of hydrocolloids. New York: Woodhead Publishing Limited; 2000. pp. 155–168. [Google Scholar]
- Yao Y, Ren G. Effect of thermal treatment on phenolic composition and antioxidant activities of two celery cultivars. LWT Food Sci Technol. 2011;44:181–185. doi: 10.1016/j.lwt.2010.07.001. [DOI] [Google Scholar]
- Zhou K, Yu L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci Technol. 2006;39(10):1155–1162. doi: 10.1016/j.lwt.2005.07.015. [DOI] [Google Scholar]