Abstract
The pulsed ultraviolet radiation (UVP) has been used as an alternative strategy for the control of microorganisms in food. However, its application causes the browning of minimally processed fruits and vegetables. In order to control the browning of the ‘Tommy Atkins’ minimally processed mango and treated with UVP (5.7 J cm−2) it was used 1-methylcyclopropene (1-MCP) (0.5 μL L−1), an ethylene action blocker in separate stages, comprising five treatments: control, UVP (U), 1-MCP + UVP (M + U), UVP + 1-MCP (U + M) e 1-MCP + UVP + 1-MCP (M + U + M). At the 1st, 7th and 14th days of storage at 12 °C, we evaluated the color (L* and b*), electrolyte leakage, polyphenol oxidase, total extractable polyphenols, vitamin C and total antioxidant activity. The 1-MCP, when applied before UVP, prevented the loss of vitamin C and when applied in a double dose, retained the yellow color (b*) of the cubes. However, the 1-MCP reduced lightness (L*) of independent mango cubes whatever applied before and/or after the UVP. Thus, the application of 1-MCP did not control, but intensified the browning of minimally processed mangoes irradiated with UVP.
Keywords: Mangifera indica L., Fresh-cut, 1-Methylcyclopropene, Pulsed UV, Browning
Introduction
The handling of minimally processed products as well as the cutting steps and the consequent release of cellular exudate favor contamination by microorganisms (Harris et al. 2003). Chlorine-based sanitizers are usually the most recommended for disinfection of fruits and vegetables. However, these compounds don’t completely oxidize the organic materials, forming undesirable by products in the water treatment as for example chloroform (CHCl3), and other trihalomethanes, which are potentially carcinogenic (Allende et al. 2009).
Ultraviolet radiation (UV) has been adopted as an alternative strategy for controlling contamination of fruits and vegetables by microorganisms. Unlike chlorine compounds, there is no scientific evidence for the production of toxic substances in food disinfected by ultraviolet radiation. On the other hand, UV radiation can degrade toxic compounds from natural (Falguera et al. 2011) or synthetic (Orellana-García et al. 2014) sources. Beyond replacing the chemical compounds of chlorine, this technique has other advantages, such as induction of the synthesis of phenolic compounds, which act as antioxidants in vegetables (Charles et al. 2013). Increase in the concentration of phenolic compounds was observed in tomatoes (Bravo et al. 2012) and in minimally processed apples (Llano et al. 2015) exposed respectively to the ultraviolet radiation applied continuously (UVC) and through pulses (UVP).
However, the minimum processing and UV radiation cause metabolic changes, especially those that induce browning (Saltveit 2004), for it negatively affects the appearance: essential attribute of purchase decision, as observed in tomatoes (Aguiló-Aguayo et al. 2013) and minimally processed apples (Gómez et al. 2010).
This browning is the result of oxidation and polymerization of phenolic compounds (Massolo et al. 2011) that is associated with the synthesis and action of the ethylene. The increase in ethylene concentration is directly related to the activity of the enzyme phenylalanine ammonia lyase (PAL), which catalyzes the biosynthesis of phenylpropanoid compounds (Choi et al. 2005). Browning occurs when the products of these phenylpropanoids metabolism such as phenolic compounds are oxidized on catalyzed reactions fenolases such as PPO (Brecht 1995; Massolo et al. 2011), which also have its synthesis and increased activity in response to ethylene (Massolo et al. 2011).
During the minimum processing occurs mechanical destruction of the membrane system at the cut surface. The ethylene produced in these tissues accelerates the degradation of other cell membranes, disrupting and destroying the tissue (Brecht 1995). This loss of cellular compartmentalization subjects the tissue to increased exposure to oxygen, while providing greater contact between oxidative enzymes (phenolase) and its substrates (phenolic compounds), resulting in the oxidation of these compounds (Massolo et al. 2011), initiating the browning reactions.
Furthermore, in supermarkets, exposition shelves have higher temperatures, around 12 °C, and need alternatives to control browning, for example, blocking the action of ethylene. Avocados, after exposure to ethylene, presented tissues with more browning (Pesis et al. 2002), while minimally processed apples, which had their ethylene action sites blocked had less blackened tissue (Chiabrando and Giacalone 2012).
The 1-MCP is a potent inhibitor of ethylene action controlling senescence (Massolo et al. 2011) as well as the activity of antioxidant enzymes (Zhang et al. 2013). In ‘Tommy Atkins’ mangoes, exposure to 1-MCP before the minimum processing (cutting) reduced ethylene production (Plotto et al. 2003). The 1-MCP also reduced browning in eggplant pulp (Massolo et al. 2011) and minimally processed ‘Empire’ apples (Nock and Watkins 2013).
In this study, we used 1-MCP in order to inhibit the browning in ‘Tommy Atkins’ minimally processed mangoes treated with UVP maintained at 12 °C.
Materials and methods
Plant material and minimal processing
Mangoes (Mangifera indica L. var ‘Tommy Atkins’) were acquired from accredited supplier in Ceará Supply Center—CEASA (Fortaleza, Ceará, Brazil). The fruits were selected as the shape, size, color, maturity stage (stage 4) and absence of injuries and illnesses. The entire mangoes were first washed with a mild detergent (2 mL L−1) and immersed in sodium hypochlorite solution (200 mg L−1) (Adheclor®) for 5 min; peeled and diced (about 2 cm) with the help of sharp stainless steel knives. They were then rinsed in distilled water and drained for 3 min.
Application of treatments and storage conditions
Ultraviolet radiation was applied at maximum power lamps (900 J), resulting in 5.7 J cm−2 energy, dose selected based on preliminary experiments considering higher browning index (Table 1). The pulses were applied using a UVP camera (SteriBeam, model XeMaticA-2LXL) provided with two lamps filled with high-power xenon gas, width 190 mm, positioned laterally, with a capacity of one pulse every 15 s and 0.3 J cm−2 pulse−1 power (for pulses with 100% lamp power). It was applied to 1-methylcyclopropene (1-MCP, SmarFresh®) at a concentration of 0.5 μL L−1 (Sivakumar et al. 2012) in samples of minimally processed ‘Tommy Atkins’ mangoes (about 250 g). The treatments were divided into: control (without UVP neither 1-MCP); U (irradiation by UVP); M + U (exposure to 1-MCP for 4 h followed by UVP irradiation); U + M (UVP irradiation followed by exposure to 1-MCP for 12 h); and M + U + M (4 h exposure to 1-MCP + UVP irradiation + 12 h exposure to 1-MCP). After application of the treatments, the samples were stored in polyethylene terephthalate containers with lid and kept in cold storage (12 ± 1 °C and RH 85 ± 5%) for 14 days. In the 1st, 7th, and 14th days samples were taken for analysis.
Table 1.
Browning index (BI) of minimally processed mangoes treated with UVP, stored for 14 days at 12 °C
| Fluence (J cm2) | BI |
|---|---|
| 0 | 233 ab |
| 1.8 | 219 bc |
| 3.6 | 199 c |
| 5.7 | 255 a |
Averages followed by the same lowercase letters do not differ by Tukey’s test at 5% probability level
Color
The browning of the external surface of the cubes was measured using a CR-400 Minolta (Konica Minolta Sensing) digital colorimeter calibrated with the white color. The readings were obtained according to the three-dimensional chromaticity coordinates model recommended by the CIELAB (L*, a*, b*). Three measurements were performed in three separate cubes within each sample. The primary colors L* indicates brightness of the light (100) to dark (0) and b* indicates the chromaticity on the axis of blue color (−) to yellow (+).
Extraction and polyphenol oxidase activity (PPO, EC 1.14.18.1)
The extraction of polyphenol was made according Wissemann and Lee (1981) with some modifications. It was homogenized 6 g of pulp in 6 mL 0.05 M phosphate buffer (pH 7.0) containing 0.1 M KCl and 1% polyvinylpyrrolidone (PVP). The homogenate was centrifuged (11,000g for 15 min), with the aid of a Biofuge Stratos (Heraeus Instruments) centrifuge. The supernatant constituted the enzymatic extract. The enzymatic activity was determined by incubating aliquots of 0.03 µL of the extract and 1.85 mL of 0.1 M phosphate buffer (pH 6.0) containing 0.1 M KCl and 0.1 M catechol, for 30 min at 30 °C. The reaction was stopped by adding 0.8 mL of HClO4 2 N. The absorbance readings were performed at 395 nm on a UV–visible U-2910 (Hitachi, Tokyo, Japan) spectrophotometer, and was considered one unit of enzyme activity (AU) PPO as the enzyme activity that produces a change of 0.001 absorbance unit. The PPO activity was expressed as AU min−1 mg−1 protein.
Total extractable polyphenols (TEP)
For extract preparation it was used the methodology described by Larrauri et al. (1997). It was homogenized 5 g of pulp and 4 mL of 50% methanol with the aid of a Turrax T 25 (IKA Labortechnick) digital tissues homogenizer. The homogenate was allowed to stand for 1 h. After that, the mixture was centrifuged in a Biofuge Stratos (Heraeus Instruments) centrifuge at 25,000g for 15 min. The supernatant was filtered and transferred to a flask of 10 mL and the precipitate dissolved in 70% acetone. After 1 h at rest, the mixture was centrifuged under the same conditions of the first centrifugation. The second supernatant was added to the first 10 mL volumetric balloon, completing the volume with distilled water, thus obtaining the extract. The determination of total extractable polyphenols followed the method described by Obanda and Owuor (1997). A total of 1 mL of Folin-Ciocalteu reagent, 2 mL of 20% NaHCO3 and 2 mL of distilled water was added to assay tubes containing 300 µL of each sample extract. Then the samples were shaken and allowed to stand for at least 30 min in the dark. After that, the absorbance reading was performed on spectrophotometer at 700 nm using a standard curve of gallic acid (GA) and the results expressed in mg of GA 100 g−1 pulp.
Electrolyte leakage (EL)
It was performed according to the methodology described by Serek et al. (1995). Cylinders (1.0 cm diameter by 1.0 cm length) taken from the middle region of pulp were washed in deionized water and dried lightly with absorbent paper. Then they were then incubated in glass jars with a lid containing 20 mL of deionized water and allowed to stand for 2 h. After standing it was measured the initial electrical conductivity of the solution (IEC), with the help of a conductivity meter (Digimed, Model DM—32). Following that, the samples were stored for 12 h in ultra freezer (−80 °C). After this period, the jars were left on the bench until the solution came into equilibrium with ambient temperature (27 ± 1 °C). It was measured electrical conductivity again, expressing the amount of leaked electrolytes (TEC). The electrolyte leakage (EL) was calculated by the following formula: , the results were expressed as a percentage of the total conductivity.
Vitamin C
It was determined immediately after processing of the pulp, by titration with Tillman solution (2.6 dichlorophenol—indophenol 0.02%, DFI) until a permanent pink color. It was used 1 g of pulp in 50 mL of oxalic acid (0.5%) and the results were expressed in mg ascorbic acid (AA) 100 g−1 of fresh weight (FW) (Strohecker and Henning 1967).
Total antioxidant activity (TAA)
To obtain the extract it was used the same methodology described for extraction of total phenolics. The TAA was obtained by free radical capture method ABTS·+, with modifications (Re et al. 1999). The ABTS·+ was generated by the interaction of 7 mM ABTS with 2.45 mM potassium persulphate. The solution incubated for 16 h and after that time it was diluted in ethanol to obtain a solution with absorbance of 0.70 (±0.01). After adding 40 μL of the diluted sample to 1960 μL of the solution containing ABTS, absorbance was determined in a spectrophotometer at 734 nm after 20 min of reaction. As a standard solution, it was used the synthetic antioxidant Trolox in concentrations from 100 to 1000 μM in ethanol. The results were expressed as trolox μM g−1 of FW.
Experimental design and statistical analysis
The experiment was analyzed in split plot with treatment in the plot (5 treatments) and the retention time in the subplot (3 retention periods). The experimental design was completely randomized, with four replications, totaling 60 plots, each plot constituted of approximately 250 g of minimally processed mango. The data were submitted to analysis of variance and the means compared by Scoott-Knott test at 5% probability. The Pearson correlation coefficient was estimated for all variables at the level of significance of 1 and 5% by the t-Student test.
Results and discussion
There was a significant interaction between treatment and conservation time for the L* and b* values, electrolyte leakage (EL), total extractable polyphenols (TEP), vitamin C content and total antioxidant activity (TAA).
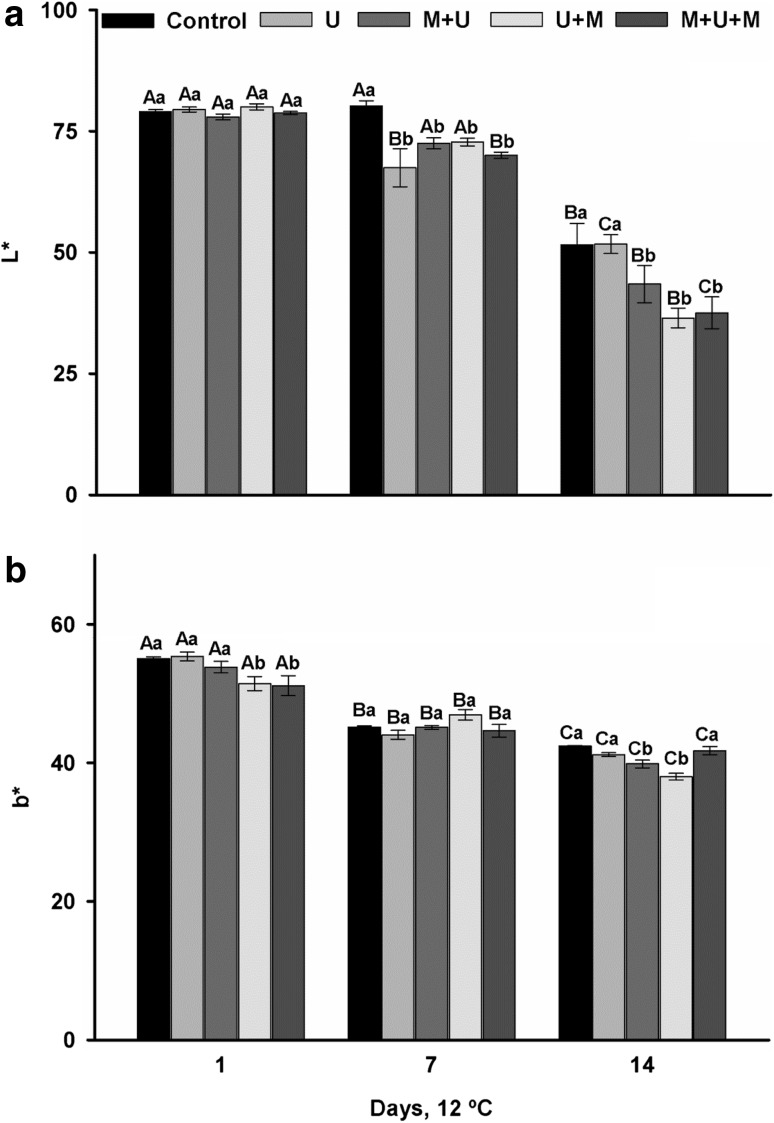
The L* values decreased in all treatments during the storage period (Fig. 1a). The decrease of the L* value indicates browning of the cubes and, according to these figures, the blackout was first noticed from the seventh day of cold storage in all treatments except the control treatment cubes. At 14 days, there was a higher browning (lower L* value) in the treatments exposed to 1-MCP (M + U, U + M and M + U + M), this result being the opposite result to the expected with the use of this inhibitor.
Fig. 1.
Color (L* and b*) of minimally processed mangoes treated with UVP (U), 1-MCP + UVP (M + U), UVP + 1-MCP (U + M) and 1-MCP + UVP + 1-MCP (M +U + M), stored for 14 days at 12 °C
Induction of browning of mango cubes by 1-MCP has not been clarified. Some authors have also noticed browning in ‘Empire’ apples by 1-MCP (Jung and Watkins 2011; Lee et al. 2012a, b), and have devoted efforts to understand it, addressing aspects involved in antioxidant metabolism (Lee et al. 2012a) and metabolomic analysis (Lee et al. 2012b), but the results were inconclusive. It is believed that the blackening occured due to inhibition of ethylene production by the fruit, resulting in the abnormal metabolism. This inhibition can occur at low temperature (0–4 °C) with or without the application of 1-MCP, or at higher temperatures (>4 °C) when treated with 1-MCP (Jung and Watkins 2011). UV light can induce oxidative stress (Jiang et al. 2010), leading to the browning of tissues. Ethylene is involved in this process and can both induce symptoms (Vranová et al. 2002) or repair or protect the tissues from these stresses (Larkindale and Knight 2002). The inhibition of ethylene by 1-MCP in this study probably blocked defense responses triggered by ethylene that act protecting tissues against browning reactions.
As in the L* coordinate, the value of the coordinate b* decreases with storage time, indicating loss of yellow color of the mango cubes (Fig. 1b). However, the difference in b* values between treatments after 14 days of conservation, although significant, was small, reaching 7% between the lowest (U + M) and highest (M + U + M) value, not being the loss of yellow color visually observed. The yellow color was preserved when the mango cubes were subjected to 1-MCP before and after being irradiated.
It has been proposed that the polyphenol oxidase (PPO) enzyme is responsible for inducing the browning in minimally processed fruit, oxidizing phenolic compounds to quinones (Massolo et al. 2011). However, this work has not verified any difference between treatments for the activity of this enzyme. However, there was an increase in PPO activity, starting from the seventh day of storage (Table 2).
Table 2.
Polyphenol oxidase (PPO) activity of minimally processed mangoes treated with UVP (U), 1-MCP + UVP (M + U), UVP + 1-MCP (U + M) and 1-MCP + UVP + 1-MCP (M + U + M), stored for 14 days at 12 °C
| Treatments | PPO (AU min−1 µg prot.) |
|---|---|
| Control | 108.7 a |
| U | 148.1 a |
| M + U | 152.2 a |
| U + M | 159.9 a |
| M + U + M | 180.3 a |
| Days, 12 ± 1 °C | |
| 1 | 76.7 a |
| 7 | 191.0 b |
| 14 | 181.7 b |
| CV (%) | 10.5 |
| Average | 149.8 |
Averages followed by the same lowercase letters do not differ by Scoott-Knott’s test at 5% probability level
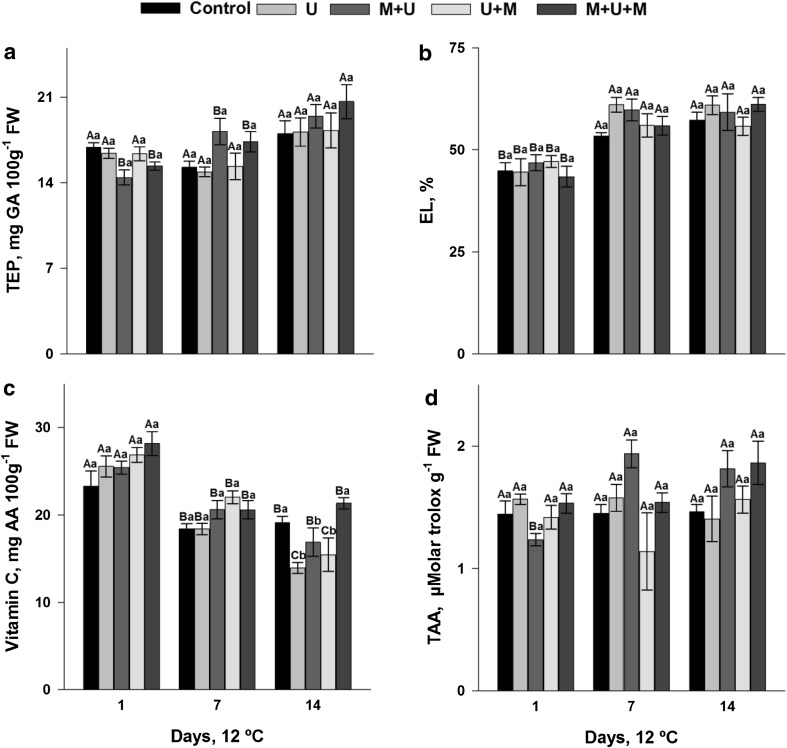
Similarly, TEP content was not influenced by the treatments (Fig. 2a). But it was observed an increase in TEP during the retention period, on the cubes that were exposed to 1-MCP before UVP (M + U at 7 days and M + U + M at 14 days). The changes that occur in compounds of phenolic origin have been reported in several minimally processed fruits and may have irregular behavior during storage (Llano et al. 2015).
Fig. 2.
Total extractable polyphenols (TEP), electrolyte leakage (EL), vitamin C and total antioxidant activity (TAA) of minimally processed mangoes treated with UVP (U), 1-MCP + UVP (M +U), UVP + 1-MCP (U + M) and 1-MCP + UVP + 1-MCP (M + U + M), stored for 14 days at 12 °C
At 7 and 14 days of refrigerated storage, all treatments increased EL (Fig. 2b). EL increased during the storage period indicates the loss of integrity (selective permeability) of the cellular membrane system, and this may have favored the browning of mango cubes tissue. These results can be confirmed by the Pearson’s correlation coefficient analysis in which we observed a positive correlation between EL and PPO (0.830) and negative between EL and L* (−0674) (Table 3).
Table 3.
Pearson correlation coefficient between the parameters L*, b*, electrolyte leakage (EL), polyphenol oxidase (PPO), vitamin C, total extractable polyphenols (TEP) and total antioxidant activity (TAA)
| L* | b* | EL | PPO | Vitamin C | TEP | TAA | |
|---|---|---|---|---|---|---|---|
| L* | 1 | ||||||
| b* | 0.832** | 1 | |||||
| EL | −0.674* | −0.874** | 1 | ||||
| PPO | −0.510 ns | −0.766** | 0.830** | 1 | |||
| Vitamin C | 0.679* | 0.854** | −0.804** | −0.655** | 1 | ||
| TEP | −0.422 ns | −0.392 ns | 0.407 ns | 0.156 ns | −0.212 ns | 1 | |
| TAA | −0.422 ns | −0.392 ns | 0.407 ns | 0.156 ns | −0.212 ns | 1** | 1 |
ns not significant
* and ** significant at 1 and 5% probability, respectively
The positive correlation between PPO and EL indicates that the higher the leakage of electrolyte, the greater the activity of this enzyme; and the negative correlation suggests that the greater the leakage of solutes, lower the L* coordinate value, i.e., greater darkening. Thus, in intact cells, the PPO is separated from their substrates due to the compartmentalization of the membranes (Liu et al. 2011). Therefore, the loss of membrane integrity facilitates contact of this enzyme with its phenolic substrates and therefore favors the browning especially the last day of cold storage. However, another process unidentified associated with loss of membrane integrity during the retention period, enhances the browning of cubes treated with 1-MCP.
In all treatments, the content of vitamin C decreased after 7 days of storage (Fig. 2c). At 14 days, only the cubes treated with U and U + M suffered further decrease in vitamin C content. This reduction of the vitamin C content during the retention period is common in fruits and whole or minimally processed vegetables, and this is mainly due to their participation in oxidative reactions. Vitamin C acts to inactivate reactive oxygen species (ROS), particularly in the inactivation of hydrogen peroxide (H2O2) produced during oxidative stress (Deutsch 1998).
Vitamin C acts preventing the enzymatic browning of fruits, inhibiting competitively the catalytic site of the PPO. Furthermore, vitamin C is able to reduce the quinones generated by PPO to their original structure, diphenol, limiting the darkening by a process known as “reaction off” (Reuck et al. 2011). Thus, reduction in the potential inhibition promoted by vitamin C in the site of the PPO (higher PPO activity, Table 2) and the ability of forming diphenols from quinones were probably hampered by a decrease in vitamin C content of the treatments, which led to darkening of the cubes (lowest value of L*, Fig. 1a) during the storage period. These results were confirmed by the Pearson correlation coefficient test (Table 3) in which it was observed negative correlation between vitamin C and PPO (−0.655) and positive between vitamin C and the coordinate L* (0.679).
Despite an overall reduction in the content of vitamin C, it was observed after 14 days of storage high levels of vitamin C in cubes that received a double dose of 1-MCP (M + U + M) (Fig. 2c). This indicates that the application of 1-MCP maintained the vitamin C content of fresh-cut mangoes and treated with UVP.
The treatments did not affect the TAA of mango cubes (Fig. 2d). There was an increase in the TAA, at 7 and 14 days, the cubes in which the application of 1-MCP happened prior to irradiation with UVP (M + U) (Fig. 2d). Phenolic compounds were responsible, in this study, for the increase in antioxidant capacity of mango cubes, since the cubes treated with M + U the TEP and TAA concentration increased similarly during cold storage (Fig. 2a, d). It was possible to confirm these results verifying the correlation analysis, in which it is possible to observe strong positive correlation between TAA and TEP (1) (Table 3). The collaboration of phenolic compounds in the antioxidant capacity of fruits has been reported in other studies, such as whole avocados (Zhang et al. 2013) and minimally processed mangoes (González-Aguilar et al. 2007).
Conclusion
The exposition of minimally processed ‘Tommy Atkins’ mango to 1-MCP before and/or after the application of UVP radiation did not control the darkening during the 14 days of storage at 12 °C. Contrary to expectations and in an unclear way, browning of minimally processed mango cubes tissue was higher in cubes with 1-MCP. However, the results of this study are consistent with other research that launched the hypothesis that the darkening is due to inhibition of the production of ethylene by the fruit, resulting in abnormal metabolism. Studies with ethylene receptors, or more refined techniques of molecular biology, such as the breakdown of metabolic profile or the differential gene expression of minimally processed mangoes, may help to unravel the possible causes of browning induced by application of 1-MCP.
Acknowledgements
The authors thank CNPQ/INCT—Tropical Fruits and CAPES for the financial support in carrying out this work.
References
- Aguiló-Aguayo I, Charles F, Renard CM, Page D, Carlin F. Pulsed light effects on surface decontamination, physical qualities and nutritional composition of tomato fruit. Postharvest Biol Technol. 2013;86:29–36. doi: 10.1016/j.postharvbio.2013.06.011. [DOI] [Google Scholar]
- Allende A, Mcevoy J, Tao Y, Luo Y. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control. 2009;20:230–234. doi: 10.1016/j.foodcont.2008.05.009. [DOI] [Google Scholar]
- Bravo S, Garcia-Alonso J, Martin-Pozuelo G, Gomez V, Santaella M, Navarro-Gonzalez I, Periago MJ. The influence of post-harvest UV-C hormesis no lycopene, carotene, and phenolic content and antioxidant activity of breaker tomatoes. Food Res Int. 2012;49:296–302. doi: 10.1016/j.foodres.2012.07.018. [DOI] [Google Scholar]
- Brecht JK. Physiology of lightly processed fruits and vegetables. Hortic Sci. 1995;30:18–22. [Google Scholar]
- Charles F, Vidal V, Olive F, Filgueiras H, Sallanon H. Pulsed light treatment as new method to maintain physical and nutritional quality of fresh-cut mangoes. Innov Food Sci Emerg Technol. 2013;18:190–195. doi: 10.1016/j.ifset.2013.02.004. [DOI] [Google Scholar]
- Chiabrando V, Giacalone G. Effect of antibrowning agents on color and related enzymes in fresh-cut apples during cold storage. J Food Process Preserv. 2012;36:133–140. doi: 10.1111/j.1745-4549.2011.00561.x. [DOI] [Google Scholar]
- Choi Y, Tomás-Barberán FA, Saltveit ME. Wound-induced phenolic accumulation and browning in lettuce (Lactuca sativa L.) leaf tissue is reduced by exposure to n-alcohols. Postharvest Biol Technol. 2005;37:47–55. doi: 10.1016/j.postharvbio.2005.03.002. [DOI] [Google Scholar]
- Deutsch JC. Ascorbic acid oxidation by hydrogen peroxide. Anal Biochem. 1998;255:1–7. doi: 10.1006/abio.1997.2293. [DOI] [PubMed] [Google Scholar]
- Falguera V, Pagán J, Garza S, Garvín A, Ibarz A. Ultraviolet processing of liquid food: a review: part 2—effects on microorganisms and on food components and properties. Food Res Int. 2011;44:1580–1588. doi: 10.1016/j.foodres.2011.03.025. [DOI] [Google Scholar]
- Gómez PL, Alzamora SM, Castro MA, Salvatori DM. Effect of ultraviolet-C light dose on quality of cut-apple: microorganism, color and compression behavior. J Food Eng. 2010;98:60–70. doi: 10.1016/j.jfoodeng.2009.12.008. [DOI] [Google Scholar]
- González-Aguilar GA, Villegas-Ochoa MA, Martínez-Téllez MA, Gardea AA, Ayala-Zavala JF. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J Food Sci. 2007;72:197–202. doi: 10.1111/j.1750-3841.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Farber JN, Beuchat LR, Parish ME, Suslow TV, Garrett EH, Busta FF. Outbreaks associated with fresh produce: incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Comp Rev Food Sci Food Saf. 2003;2:78–141. doi: 10.1111/j.1541-4337.2003.tb00031.x. [DOI] [Google Scholar]
- Jiang T, Jahangir MM, Jiang Z, Lu X, Ying T. Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Technol. 2010;56:209–215. doi: 10.1016/j.postharvbio.2010.01.011. [DOI] [Google Scholar]
- Jung SK, Watkins CB. Involvement of ethylene in browning development of controlled atmosphere-stored ‘Empire’ apple fruit. Postharvest Biol Technol. 2011;59:219–226. doi: 10.1016/j.postharvbio.2010.08.019. [DOI] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrauri JA, Rupérez P, Saura-Calixto F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J Agric Food Chem. 1997;45:1390–1393. doi: 10.1021/jf960282f. [DOI] [Google Scholar]
- Lee J, Mattheis JP, Rudell DR. Antioxidant treatment alters metabolism associated with internal browning in ‘Braeburn’ apples during controlled atmosphere storage. Postharvest Biol Technol. 2012;68:32–42. doi: 10.1016/j.postharvbio.2012.01.009. [DOI] [Google Scholar]
- Lee J, Rudell DR, Davies PJ, Watkins CB. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics. 2012;8:742–753. doi: 10.1007/s11306-011-0373-5. [DOI] [Google Scholar]
- Liu H, Song L, You Y, Li Y, Duan X, Jiang Y, Joyce DC, Ashraf M, Lu W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol Technol. 2011;60:24–30. doi: 10.1016/j.postharvbio.2010.11.008. [DOI] [Google Scholar]
- Llano KA, Marsellés-Fontanet AR, Martín-Belloso O, Soliva-Fortuny R. Impact of pulsed light treatments on antioxidant characteristics and quality attributes of fresh-cut apples. Innovat Food Sci Emerg Technol. 2015 [Google Scholar]
- Massolo JF, Concellón A, Chaves AR, Vicente AR. 1-Methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol Technol. 2011;59:10–15. doi: 10.1016/j.postharvbio.2010.08.007. [DOI] [Google Scholar]
- Nock JF, Watkins CB. Repeated treatment of apple fruit with 1-methylcyclopropene (1-MCP) prior to controlled atmosphere storage. Postharvest Biol Technol. 2013;79:73–79. doi: 10.1016/j.postharvbio.2013.01.002. [DOI] [Google Scholar]
- Obanda M, Owuor PO. Flavonol composition and caffeine content of green leaf as quality potencial indicators of Kenyan black teas. J Sci Food Agri. 1997;74:209–215. doi: 10.1002/(SICI)1097-0010(199706)74:2<209::AID-JSFA789>3.0.CO;2-4. [DOI] [Google Scholar]
- Orellana-García F, Álvarez MA, López-Ramón V, Rivera-Utrilla J, Sánchez-Polo M, Mota AJ. Photodegradation of herbicides with different chemical natures in aqueous solution by ultraviolet radiation. Effects of operational variables and solution chemistry. Chem Eng J. 2014;255:307–315. doi: 10.1016/j.cej.2014.06.047. [DOI] [Google Scholar]
- Pesis E, Ackerman M, Ben-Arie R, Feygenberg O, Feng X, Apelbaum A, Prusky D. Ethylene involvement in chilling injury symptoms of avocado during cold storage. Postharvest Biol Technol. 2002;24:171–181. doi: 10.1016/S0925-5214(01)00134-X. [DOI] [Google Scholar]
- Plotto A, Bai J, Baldwin EA, Brecht JK. Effect of pretreatment of intact ‘Kent’ and ‘Tommy Atkins’ mangoes with ethanol vapor, heat or 1-methylcyclopropene on quality and shelf life of fresh-cut slices. Proc Florida State Hortic Soc. 2003;116:394–400. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying improved ABTS radical cátion decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reuck K, Sivakumar D, Korsten L. Integrated application of 1-methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharvest Biol Technol. 2011;52:71–77. doi: 10.1016/j.postharvbio.2008.09.013. [DOI] [Google Scholar]
- Saltveit ME. Effect of 1-methylcyclopropene on phenylpropanoid metabolism, the accumulation of phenolic compounds, and browning of whole and fresh-cut ‘Iceberg’ lettuce. Postharvest Biol Technol. 2004;34:75–80. doi: 10.1016/j.postharvbio.2004.05.001. [DOI] [Google Scholar]
- Serek M, Tamari G, Sisler EC, Borochov A. Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropene, a new inhibitor of ethylene action. Physiol Plant. 1995;94:229–232. doi: 10.1111/j.1399-3054.1995.tb05305.x. [DOI] [Google Scholar]
- Sivakumar D, Van Deventer F, Terry LA, Polenta GA, Korsten L. Combination of 1-methylcyclopropene treatment and controlled atmosphere storage retains overall fruit quality and bioactive compounds in mango. J Sci Food Agric. 2012;92:821–830. doi: 10.1002/jsfa.4653. [DOI] [PubMed] [Google Scholar]
- Strohecker R, Henning HM. Analisis de vitaminas: métodos comprobados. Madrid: Paz Montalvo; 1967. [Google Scholar]
- Vranová E, Inzé D, Van Breusegem F. Signal transduction during oxidative stress. J Exp Bot. 2002;53:1227–1236. doi: 10.1093/jexbot/53.372.1227. [DOI] [PubMed] [Google Scholar]
- Wissemann KW, Lee CY. Characterization of polyphenoloxidase from Ravat 51 and Niagara grapes. J Food Sci. 1981;46:506–508. doi: 10.1111/j.1365-2621.1981.tb04897.x. [DOI] [Google Scholar]
- Zhang Z, Huber DJ, Rao J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol Technol. 2013;76:58–64. doi: 10.1016/j.postharvbio.2012.09.003. [DOI] [Google Scholar]