Abstract
Serine protease inhibitors from squid ovary (SOSPI) were extracted using different extraction media and conditions. Optimal condition included 0.45 M NaCl for 1 h. After heat treatment at 70 °C for 10 min, the highest specific activity was obtained. Based on Sephadex G-75 gel filtration and activity staining, the major SOSPIs were present as monomer with molecular weight of 9.10 and 10.27 kDa. Impact of SOSPI on autolysis of bigeye snapper surimi was studied. Myosin heavy chain was more retained with coincidentally lower trichloroacetic acid-soluble peptide content as the level of SOSPI increased. When SOSPI at various levels (0.5–3%) was added into surimi, the highest breaking force of both kamaboko and modori gels containing 1% SOSPI was obtained. However, SOSPI had no effect on deformation of kamaboko gel but slightly increased the deformation of modori gel. SOSPI increased water holding capacity of both kamaboko and modori gels in a dose dependent manner, as indicated by the lowered expressible moisture content. SOSPI had no effect on the whiteness of both gels. Thus SOSPI can be used as the protein additive in surimi for increasing breaking force.
Keywords: Squid ovary, Protease inhibitor, Autolysis, Surimi, Gel strength, Bigeye snapper
Introduction
Proteolysis mediated by indigenous proteases has been known as one of the major reasons for gel weakening of surimi. These proteases are activated by the heat, particularly temperature around 50–60 °C (Alvarez et al. 1999). Severe cleavage of myofibrillar proteins at high temperature inhibits the development of three-dimensional network of surimi gel (Kudre and Benjakul 2013a, b). This phenomenon is called modori, which contributes to the loss in gel quality and the market value (Morrissey et al. 1993). To lower the impact of modori phenomenon, several protease inhibitors have been widely used in surimi. Those include beef plasma protein (BPP), porcine plasma protein (PPP), egg white (EW), potato powder and whey protein concentrate, etc. Due to religious obliges of porcine origin product and the outbreak of bovine or mad cow diseases, the inhibitors have been prohibited for use in surimi. High price of EW, egg like flavor and allergic reasons limit its use in surimi. Similarly, potato powder also leads to off-white color (Rawdkuen and Benjakul 2008). Hence, alternative food grade protease inhibitors for surimi industry are still required.
Thailand and other Southeast Asian countries are the major exporters for the marine fisheries, specially squid and cuttlefish (Hoque et al. 2010). In marine capture, 78% are used for human consumption and the rest is discarded or used for non-food purposes, especially for fishmeal production. Among the leftovers, roe and ovary can be utilized as nutritive proteinaceous food. Fish roe are considered as the important source for the bioactive compounds, which exhibit antioxidant, antibacterial and immunomodulatory activities. Intarasirisawat et al. (2011) reported that fish roe is rich in polyunsaturated fatty acids (PUFAs). Fish egg protein hydrolysates showed fat adsorption capacity, foam capacity and emulsifying capacity (Chalamaiah et al. 2010). Furthermore, trypsin inhibitor from yellowfin tuna roe was recovered and used to prevent the modori in surimi (Klomklao et al. 2016). Recently, fish ovarian fluid has been reported to possess proteases inhibitory activity (Minin and Ozerova 2015). During evisceration of female squid, the ovary accounting about 10 to 15% of total weight is discarded by the squid processing industries. The ovary could be used as an alternative source of protease inhibitors for possible use in surimi. Therefore, the objectives of this study were to optimize the recovery of serine protease inhibitor from squid ovary (SOSPI) and to study the impact of SOSPI on autolysis and properties of gel from bigeye snapper surimi.
Materials and methods
Chemicals
All chemicals used were of analytical grade. Na-Benzoyl-dl-arginine-p-nitroanilide (BAPNA), and high molecular weight protein markers were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Trichloroacetic acid (TCA), sodium dodecyl sulfate (SDS), and Coomassie Blue R-250 were procured from Merck (Darmstadt, Germany). Sephadex G75 gel and molecular weight marker (bovine serum albumin, albumin from chicken egg white and cytochrome C) for size exclusion chromatography were obtained from GE Healthcare Life Science (Chicago, IL, USA).
Extraction of serine protease inhibitor from squid ovary
Squid ovary and surimi
The frozen squid (Loligo formosana) viscera were obtained from Sea Wealth Frozen Food, Songkhla, Thailand. The ovary was manually separated. The obtained ovary was chopped using a blender to attain homogeneity, followed by freeze-drying using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). The freeze-dried powder was transferred into polyethylene bag and stored at −40 °C until use.
Grade A frozen surimi from bigeye snapper (Priacanthus macranthus) was obtained from Man A Frozen Foods Co., Ltd. (Songkhla, Thailand) and kept at −20 °C until use but not longer than 2 months.
Effect of different extraction media and times on the recovery of serine protease inhibitor
Distilled water and NaCl solution at different concentrations (0.15, 0.3, 0.45 and 0.6 M) were used for the extraction of serine protease inhibitor from squid ovary powder. The squid ovary powder was mixed with extraction media at a powder/media ratio of 1:30 (w/v). The mixture was homogenized for 1 min at 5000 rpm using a homogenizer (IKA, Labortechnik homogenizer Selangor, Malaysia). The homogenate was stirred for 1 h at room temperature. The mixture was centrifuged at 10,000×g for 30 min (Allegra 25R centrifuge, Beckman Coulter, Palo Alto, CA, USA). The supernatant or extract was collected and determined for trypsin inhibitory activity and specific activity.
To study the effect of extraction time on recovery of serine protease inhibitor from squid ovary powder, the medium yielding the extract with the highest total and specific activities was selected. The extraction was performed for different times (1, 2, 3, 4 and 5 h). The extracts were examined as previously described.
Effect of heat treatment on the recovery of serine protease inhibitor
The extract containing SOSPI was heated at different temperatures (60, 70, 80, 90 and 100 °C) for 10 min and suddenly cooled on ice. The extracts were then centrifuged at 8000×g for 10 min to remove the coagulated debris. Pre-heated extracts were subjected to analysis. The preheated extract showing the highest total and specific activities was chosen for the further study.
Serine protease inhibitory activity assay
Serine protease inhibitory activity was measured according to the method of Klomklao et al. (2010) with slight a modification. The sample solution (200 µl) was incubated with 200 µl (1 mg/ml) porcine pancreas trypsin at 37 °C for 15 min. Then, 1000 μl of reaction buffer (50 mM Tris–HCl buffer, pH 8.0, containing 10 mM CaCl2) were added. To initiate reaction, 200 µl of BAPNA (2 mg/ml) were added. After incubation for 15 min, 200 µl of 30% acetic acid (v/v) were added to terminate the reaction. The residual activity of trypsin was determined by measuring the release of p-nitroaniline spectrophotometrically at 410 nm (UV-16001, Shimadzu, Kyoto, Japan). One unit of trypsin activity was defined as the enzyme causing an increase of 0.001 absorbance unit/min under the assay condition. One unit of protease inhibitory activity was defined as the amount of inhibitor, which reduced trypsin activity by one unit.
Gel filtration and activity staining
The selected preheated-extract containing SOSPI was separated by size exclusion chromatography using a Sephadex G-75 gel filtration column (GE Healthcare, Bio-Science AB, Uppsala, Sweden). One milliliter of extract (120 mg/ml) was loaded onto the column at room temperature (28–30 °C). After loading the sample, column was eluted with the double distilled milli-Q water and the fractions (3 ml) were collected using a fraction collector (Model 2128, Bio-Rad Laboratories, Hercules, CA, USA) at the flow rate of 1 ml/min. The absorbance was recorded at 280 nm. Blue dextran (200 kDa) was use for void volume measurement. Molecular weight (MW) markers included albumin (66.5 kDa), albumin from chicken egg white (44.3 kDa), and cytochrome C (12.4 kDa). Serine protease inhibitory activity was determined for all fractions. Activity peaks were calculated for MW. Plot between available partition coefficient (Kav) and the logarithm of the MW of protein standards was prepared.
Inhibitory activity staining was conducted with SDS–polyacrylamide gel electrophoresis using casein as substrate according to the method of Gracia-Carreno et al. (1993) with a slight modification. The fractions with the highest trypsin inhibitory activity from the size exclusion chromatography were pooled and separated using a Mini-Protein III unit (Bio-Rad Laboratories, Hercules, CA, USA) under reducing and non-reducing conditions. One gel was fixed and stained for total proteins using Coomassie Blue R-250. Another gel was used for activity staining. The gel was washed with 2.5% Triton X-100 for 15 min twice to remove SDS. The trypsin was allowed to diffuse into the gel by incubating gel with 0.2 mg/ml trypsin for 60 min at 4 °C. The gel was incubated at 60 °C for 60 min. Thereafter, the gel was immersed in 10 mg/ml casein solution for 120 min at 60 °C. The gel was rinsed with distilled water after every treatment. Then the gel was stained with Coomassie blue R-250 to develop inhibitory zone as dark band on a clear background. The MW of inhibitor was calculated.
Effect of SOSPI on autolysis of bigeye snapper surimi
Autolytic activity assay was performed according to the method of Morrissey et al. (1993). SOSPI at various levels (0.5, 1, 2 and 3%, w/w) was mixed with surimi paste (3 g) containing 2.5% NaCl (w/w). The mixture was thoroughly mixed and spread at the bottom of the beaker. Samples without and with SOSPI were incubated at 60 °C for 60 min. Then, the reaction was terminated by adding 27 ml of cold 5% (w/v) trichloroacetic acid (TCA). The mixture was centrifuged at 8000×g for 10 min. TCA-soluble peptide content in the supernatant was determined by the Lowry method (Lowry et al. 1951) and was expressed as µmole of tyrosine equivalent/g sample.
To determine the autolytic pattern of bigeye snapper surimi, autolysis was conducted in the same manner, except that 27 ml of 5% sodium dodecyl sulfate (SDS) (85 °C) were added instead of 5% TCA. The mixtures were homogenized and centrifuged. The supernatant was subjected to SDS-PAGE analysis (Laemmli 1970) using 10% running gel and 4% stacking gel. Quantitative analysis of protein band intensity was performed by image analysis system using a Model GS-700 Imaging Densitometer (Bio-Rad Laboratories, Hercules, CA, USA) with Molecular Analyst Software version 1.4.
Effect of SOSPI on gel properties of bigeye snapper surimi
Preparation of surimi gel
Frozen surimi was partially thawed at 4 °C for 2–3 h, cut into small pieces with an approximate thickness of 1 cm and blended using a blender for 1 min. Salt (2.5%, w/w) was then added. SOSPI was subsequently added into surimi paste to obtain the final concentration of 0.5, 1, 2 and 3% (w/w). The final moisture content of surimi paste was adjusted to 80% (w/w). The mixture was chopped for 2 min. The temperature of surimi paste during chopping was maintained below 7 °C. The paste was stuffed into a polyvinylidine chloride casing with a diameter of 2.5 cm, and both ends were sealed tightly. Modori and kamaboko gels were prepared by incubating the paste at 60 and 40 °C for 30 min, respectively, followed by heating both gels at 90 °C for 20 min. All gels were cooled in iced water for 60 min and stored at 4 °C for 15–18 h prior to analysis.
Analyses
Textural analysis
Textural analysis of surimi gels was carried out using a texture analyzer (Model TA-XT2, Stable Micro Systems, Surrey, UK). Gels were equilibrated at room temperature (28–30 °C) before analysis for 2 h. Cylindrical samples of 2.5 cm in diameter and 2.5 cm in length were prepared. A spherical probe with a diameter of 5 mm was pressed into the cut surface of a gel specimen perpendicularly at a constant depression speed of 60 mm/min until puncture occurred as per the method of Benjakul et al. (2010). Breaking force and deformation were recorded.
Determination of whiteness
Gel samples were subjected to whiteness measurement using a colorimeter (Hunter Lab, Color Flex, Hunter Associates Laboratory, Reston, VA, USA). The instrument was calibrated using white and black standards plates. Illuminant C was used as the light source of measurement. L *, a * and b * values were measured. Whiteness was calculated using the following equation (Park 2000):
where L * is the lightness; a * is the redness/greenness and b * is the yellowness/blueness.
Determination of expressible moisture content
Expressible moisture content was measured according to the method of Rawdkuen and Benjakul (2008). Gel samples of 5 mm thickness were weighed (X) and placed between three pieces of filter papers (Whatman No.1, Whatman International, Ltd., Maidstone, England) at the bottom and two pieces of paper on the top. A standard weight (5 kg) was placed on the top of the sample for 2 min, and then the sample was removed from the papers and weighed again (Y). Expressible moisture content was calculated with the following equation and expressed as the percentage of sample weight:
Statistical analysis
All experiments were run in triplicate. Data were subjected to analysis of variance. Comparison of means was carried out by the Duncan’s multiple range tests (Steel and Torrie 1980). Analysis was performed using a SPSS package (SPSS 22 for Windows, SPSS Inc., Chicago, IL).
Results and discussion
Effect of extraction media and time on the recovery of serine protease inhibitor
Different media yielded the extracts with varying total inhibitor activity and specific activity as shown in the Table 1. The extraction with the NaCl at concentrations of 0.3 and 0.45 M rendered the extracts with higher total inhibitor activity than others (p < 0.05). This was more likely due to the increased solubility of inhibitor at the appropriate salt concentrations used. Chloride ions increased solubility of protease inhibitor by enhancing the electrostatic repulsions after binding to the positively charged groups of the protein. However, the decrease in total activity was found as NaCl solution at high concentration (0.6 M) was used (p < 0.05). There was no difference in total inhibitor activity between water extract and that extracted using 0.6 M NaCl (p > 0.05). At high level of NaCl, the water molecules could be tightly bound to salt, in which the competition between salt ions and protein for water molecules took place. Hence, solubility of protease inhibitor was lowered and some protease inhibitors might be denatured at high NaCl concentration as evidenced by the decrease in inhibitory activity. It was noted that lower protein content was obtained in the extract using NaCl solution with concentration of 0.3–0.6 M (p < 0.05). NaCl might cause the precipitation of other proteins in squid ovary. As a result, proteins were retained at a lower extent in the extract. This was also evidenced by the highest protein content in water extract. When considering specific activity, the extract showed the increases in specific activity when the NaCl solutions with concentrations up to 0.45 M were used (p < 0.05). Nevertheless, at high salt concentration (0.6 M), the decrease in the specific activity was noticeable (p < 0.05). Specific activity of protease inhibitor from yellowfin tuna roe (Klomklao et al. 2014) and Thai legume seeds (Benjakul et al. 2000) decreased as the salt concentration increased. Based on the highest amount of inhibitors recovered and the highest specific activity, 0.45 M NaCl solution was considered as the most appropriate medium for extraction of serine protease inhibitor from squid ovary.
Table 1.
Total inhibitor activity, protein concentration and specific activity of SOSPI as affected by different extraction media
| Extraction media | Concentration (M) | Total inhibitor activity (unit/ml) | Protein concentration (mg/ml) | Specific activity (unit/mg protein) |
|---|---|---|---|---|
| Water | – | 392.39 ± 16.24c | 16.95 ± 0.63a | 23.17 ± 1.40e |
| NaCl | 0.15 | 394.63 ± 1.97c | 4.16 ± 0.08b | 94.85 ± 2.20d |
| 0.3 | 426.85 ± 8.96b | 3.55 ± 0.02c | 120.33 ± 2.75b | |
| 0.45 | 452.46 ± 16.91a | 3.11 ± 0.09c | 145.76 ± 3.46a | |
| 0.6 | 394.21 ± 1.77c | 3.60 ± 0.08c | 109.64 ± 2.16c |
Values are given as mean ± SD (n = 3). Different lowercase superscripts in the same column denote the significant differences (p < 0.05). The extraction time of 1 h was used
Total inhibitor activity was decreased when extraction time was longer than 1 h (p < 0.05) (Table 2). On the other hand, protein content was increased with increasing extraction time (p < 0.05). Very low inhibitor activity was found in the extract when the extraction time was lower than 1 h (data not shown). This was plausibly due to the insufficient time for extraction of inhibitor from squid ovary powder into the extraction medium. Nevertheless, no difference in the protein content was observed as extraction time ranging from 2 to 5 h was used (p > 0.05). For specific activity, the marked decrease was found when extraction time was longer than 1 h (p < 0.05). Decrease was more pronounced with increasing extraction time (p < 0.05). The denaturation of proteins was plausibly caused by the mechanical shearing and adsorption of proteins at liquid–air interface during extraction (Damodaran 1996; Klomklao et al. 2010). The extraction time of 0.5 h was optimum for the isolation of protease inhibitor from the yellowfin tuna fish roe (Klomklao et al. 2014) Extraction time of 0.5, 1 and 3 h was suggested for isolation of trypsin inhibitor from adzuki bean, cowpea and pigeon, respectively (Klomklao et al. 2010; Benjakul et al. 2000). Many factors are involved in the protein solubility and isolation of target protease inhibitors. Those include particle size and nature of tissue, ionic strength, type and concentration of extracting medium and hydration characteristics of protein (Benjakul et al. 2000; Klomklao et al. 2011). Therefore, extraction time of 1 h was selected for the recovery of SOSPI from squid ovary powder.
Table 2.
Total inhibitor activity, protein content and specific activity of SOSPI as affected by different extraction time
| Time (h) | Total inhibitory activity (unit/ml) | Protein concentration (mg/ml) | Specific activity (unit/mg protein) |
|---|---|---|---|
| 1 | 485.40 ± 0.92a | 3.31 ± 0.17b | 146.55 ± 0.28a |
| 2 | 451.73 ± 4.25b | 3.92 ± 0.27a | 115.36 ± 1.08b |
| 3 | 447.01 ± 4.33b | 3.97 ± 0.18a | 114.22 ± 1.11bc |
| 4 | 433.14 ± 2.1c | 3.86 ± 0.26a | 112.07 ± 0.53d |
| 5 | 434.4 ± 3.45c | 3.81 ± 0.25a | 112.66 ± 0.56cd |
Values are given as mean ± SD (n = 3). Different lowercase superscripts in the same column denote the significant differences (p < 0.05) NaCl solution (0.45 M) was used the extraction medium
Effect of heat treatment on the recovery of serine protease inhibitor
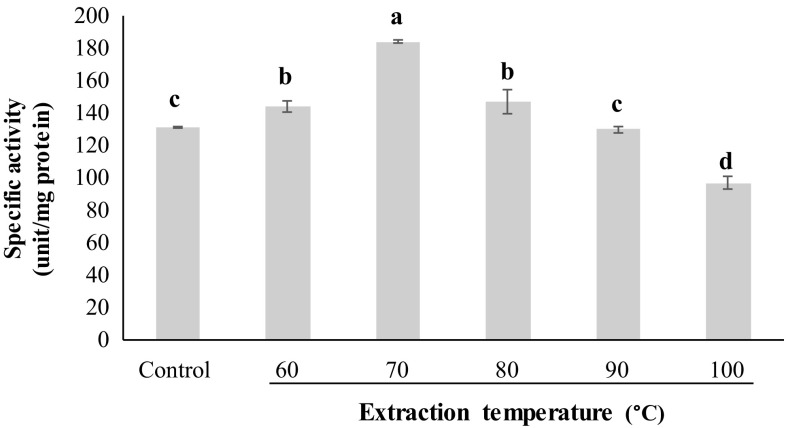
When the extract was subjected to heat treatment at different temperatures, the specific activity was increased when heated up to 70 °C (p < 0.05) (Fig. 1). When heating at temperature higher than 70 °C was applied, the continuous decrease in specific activity was noticeable (p < 0.05). The denaturation of both inhibitors and proteins occurred simultaneously. However, the higher rate of denaturation of inhibitor was pertained in comparison with other proteins. Heating of samples causes the looser structure of inhibitor from compact native form, which is stabilized by the several bonding (Benjakul et al. 2000). The trypsin inhibitor from the egg of skipjack tuna and yellowfin tuna fish showed the decreases in specific activity at temperature above 40 and 70 °C, respectively (Choi et al. 2002; Klomklao et al. 2014). The result revealed that heat treatment at 70 °C for 10 min could be used for partial purification of serine protease inhibitor from squid ovary.
Fig. 1.
Specific activity of the extract from squid ovary preheated at different temperatures. Bars represent the standard deviation (±SD). Different lowercase letters on the bars denote the significant differences (p < 0.05)
Molecular weight distribution of serine protease inhibitors
Gel filtration chromatogram and inhibitor activity profile of SOSPI are shown in Fig. 2a. Two peaks (A280) with molecular weight of 35.09–70.60 and 3.32–16.83 kDa were obtained. Only one activity peak was observed. MW of Sephadex G-75 fraction showing the highest inhibitor activity was estimated to be in the range of 9.05–11.47 kDa. MW of serine protease inhibitor from SOSPI was lower than those of trypsin inhibitor from chickpeas (28 kDa), soybean (19 kDa) and mustard seeds (20 kDa) (Kansal et al., 2008). Trypsin inhibitor from yellow tuna fish roe was reported to have MW of 70 kDa based on size exclusion chromatography (Klomklao et al. 2015). Thus, serine protease inhibitor from SOSPI had the differences in size and the number of amino acids, in comparison with the other serine protease inhibitors.
Fig. 2.
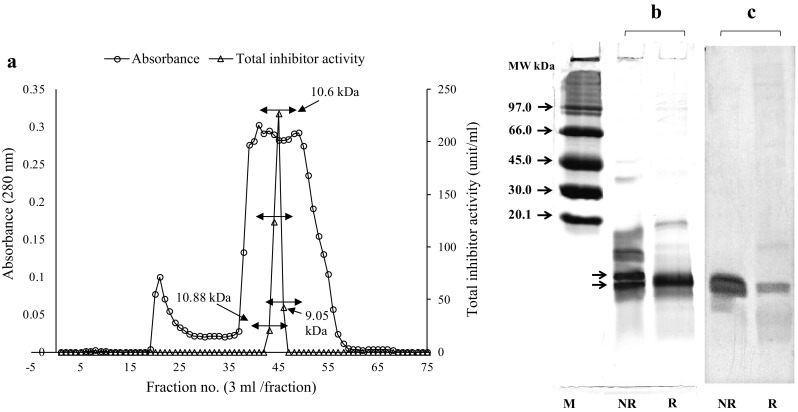
Sephadex G-75 elution profile and inhibitory activity (a), SDS-PAGE (b) and inhibitory activity staining (c) of active Sephadex G-75 fraction (pooled active fractions) of squid ovary extract. M Low molecular weight marker, NR non-reducing condition, R reducing condition (↔ pooled fractions)
Pooled active fractions containing inhibitor obtained from gel filtration were subjected to SDS-PAGE and inhibitory activity staining under both reducing and non-reducing conditions (Fig. 2b, c). Based on SDS-PAGE, there were two major bands with MW 10.27 and 9.10 kDa when examined under non-reducing conditions. Additionally, proteins with MW of 14.10, 36.73 and 46.85 kDa were also found. Under reducing condition, those protein bands disappeared. The result suggested that those proteins were more likely stabilized by disulfide bond. However, protein of MW 9.10 kDa was still retained under the reducing condition (Fig. 2b).
Inhibitor activity staining study reveals that two protein bands with apparent MW of 10.27 and 9.10 kDa under non-reducing conditions were serine protease inhibitors (Fig. 2c). Apparent MW of inhibitor was supported by the results from size exclusion chromatography, which showed MW in the range of 9.05–11.47 kDa. The results also indicated that serine protease inhibitors were present as monomer. Under reducing conditions, inhibitor band with MW 10.27 kDa disappeared, indicating the loss of inhibitory activity in the presence of βME. Thus, the inhibitor with MW of 10.27 kDa more likely contained intramolecular disulfide bonds. Kunitz and Bowman inhibitor have seven and two disulfide bonds, respectively. When these disulfide bonds were reduced, the loss in inhibitor activity against trypsin was found (Benjakul et al. 2000). Trypsin inhibitor from yellow tuna fish roe of MW 70 kDa contained two subunits with MW of 30 and 40 kDa, which are stabilized by disulfide bond. The results indicated that SOSPI had two inhibitors with MW of 10.27 and 9.10 kDa, in which the former was stabilized by disulfide bond.
Effect of SOSPI on autolysis of bigeye snapper surimi
Autolysis of bigeye snapper surimi in the absence and presence of SOSPI at various levels was expressed in term of TCA-soluble peptides content (Fig. 3a). Heat activated proteases in surimi have been known to hydrolyze the muscle proteins (Kudre and Benjakul 2013a, b). TCA soluble peptides indicate the degradation of muscle proteins during heat induced gelation of surimi. The highest content of TCA soluble peptides was observed in the control (without SOSPI). The TCA-soluble peptide content was decreased when surimi paste was added with SOSPI in a dose-dependent manner. The result indicated the inhibition of proteolysis in surimi paste by SOSPI. The inhibitory activity of SOSPI was confirmed by SDS-PAGE results (Fig. 3b). MHC and actin were found as the major proteins in surimi paste. When the surimi paste was incubated at 60 °C for 60 min, the MHC band completely disappeared. Nevertheless, the addition of SOSPI was able to maintain MHC band to a higher extent as SOSPI levels increased. MHC band intensity increased by 5.8, 8.9, 9.4 and 31.3% as compared to control (without SOSPI) when the levels of SOSPI increased from 0.5 to 3%. Actin band density almost remained unchanged. Actin was resistant toward the proteolysis (Rawdkuen and Benjakul 2008). MHC of sardine surimi was more retained when the protein isolate from mung bean, black bean and bambara groundnut were incorporated (Kudre and Benjakul 2013a). Furthermore, whey protein concentrate was also reported to exhibit inhibitory effect toward proteolysis of various tropical fish (Rawdkuen and Benjakul 2008).
Fig. 3.
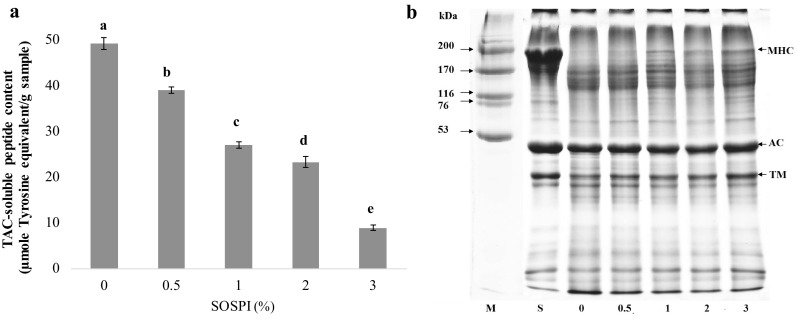
TCA-soluble peptide contents (a) and protein pattern of bigeye snapper surimi incubated at 60 °C for 60 min (b) in the absence and presence of the extract from squid ovary. MHC myosin heavy chain, AC actin, TM tropomyosin. M high molecular weight marker, S surimi. Numbers are denoted as concentration of SOSPI (% w/w). Bars represent the standard deviation. Different lowercase letters on the bars denote the significant differences (p < 0.05)
Effect of SOSPI on gel properties of bigeye snapper surimi
Effect of SOSPI on the textural properties of surimi gels
Breaking force and deformation of modori (60/90 °C) and kamaboko gel (40/90 °C) of bigeye snapper surimi added with SOSPI at different levels (0–3%) are shown Table 3. For modori gel, the lowest breaking force and deformation were observed in gels without SOSPI addition (p < 0.05). Modori gel added with SOSPI up to 1% showed the increases in both breaking force and deformation (p < 0.05). However, gel added with SOSPI at concentrations above 1% showed no marked difference in breaking force (p > 0.05). There was no difference in deformation between samples added with SOSPI ranging from 0.5 to 3% (p > 0.05). With addition of 1% SOSPI, breaking force and deformation of modori was increased by 40% and 40.7%, respectively, compared with the control (without SOSPI). Results suggested that SOSPI had the potential to enhance the gel strength of bigeye snapper surimi via inhibition of heat activated protease. When muscle proteins, especially myosin heavy chain, were more retained, those proteins were able to undergo gel formation more effectively. Long chain proteins could form the inter-junction or gel network more effectively. This was evidenced by the increases in breaking force when the SOSPI was added. The surimi from bigeye snapper contain serine proteases as the major indigenous enzyme associated with myofibrillar proteins (Benjakul and Visessanguan 2003; Benjakul et al. 2004a), which are responsible for the degradation of myofibrillar proteins in surimi. Trypsin inhibitor from yellowfin tuna roe and protein isolate from bambara ground nut increased the breaking force and deformation in the modori surimi gel from bigeye snapper and threadfin bream, respectively (Klomklao et al. 2015; Oujifard et al. 2012).
Table 3.
Breaking force, deformation, whiteness and expressible moisture content (EMC) of modori and kamaboko gels from bigeye snapper surimi added with SOSPI at different levels
| Gel samples | SOSPI (%) | Breaking force (g) | Deformation (mm) | Whiteness | EMC (%) |
|---|---|---|---|---|---|
| Modori gel | 0 | 185.14 ± 10.69c | 4.59 ± 0.31b | 80.21 ± 0.56a | 5.03 ± 0.38a |
| 0.5 | 230.28 ± 11.41b | 5.94 ± 0.11a | 79.96 ± 0.52a | 4.12 ± 0.51ab | |
| 1 | 259.91 ± 16.09a | 6.46 ± 0.42a | 80.03 ± 0.23a | 4.01 ± 0.8ab | |
| 2 | 283.15 ± 4.03a | 6.27 ± 0.11a | 79.07 ± 0.12a | 3.27 ± 1.06b | |
| 3 | 274.06 ± 5.21a | 6.47 ± 0.12a | 79.73 ± 0.41a | 3.22 ± 0.39b | |
| Kamaboko gel | 0 | 716.72 ± 13.09c | 11.6 ± 0.6a | 81.34 ± 1.23a | 2.71 ± 0.13a |
| 0.5 | 757.52 ± 25.1b | 10.86 ± 0.27a | 81.44 ± 0.24a | 2.32 ± 0.06b | |
| 1 | 791.06 ± 23.16a | 11.37 ± 0.45a | 81.35 ± 1.08a | 2.29 ± 0.07b | |
| 2 | 811.58 ± 10.65a | 11.76 ± 0.19a | 82.18 ± 0.54a | 2.23 ± 0.04bc | |
| 3 | 665.51 ± 12.19d | 11.25 ± 0.2a | 81.85 ± 0.27a | 2.12 ± 0.05c |
Values are mean ± SD (n = 3). Different lowercase superscripts in the same column under the same gel denote the significant differences (p < 0.05)
For kamaboko gels, breaking force increased (p < 0.05) as SOSPI amount increased up to 1%, in which the increase by 10.37% was attained, compared with the control. There was no difference in breaking force between kamaboko gels added with 1 and 2% SOSPI (p > 0.05). Nevertheless, the decrease in breaking force was observed as SOSPI at 3% was incorporated (p < 0.05). SOSPI generally showed higher inhibition toward protein degradation in modori gel than in kamaboko gel. This confirmed the role of heat activated protease in bigeye snapper surimi. When those proteases with optimal temperature of 60 °C were inactivated by SOSPI, the proteins were more retained and the stronger gel network could be formed. The result was supported by the higher increase in breaking force in modroi gel than kamaboko gel, as compared to the controls (Table 3). In addition, non-disulfide covalent bonds such as ε-(γ-glutamyl) lysine linkages formed in kamaboko gel during setting at 40 °C were resistant to proteolysis (Kumazawa et al. 1995). The setting phenomenon in kamaboko might therefore proceed the degradation inhibition by SOSPI. This was indicated by the lower increase in breaking force of kamaboko gel when SOSPI was incorporated, compared with modori gel. Bambara groundnut protein isolate, porcine plasma protein and casein at high concentration also alleviated the weakening of gel in threadfin bream and walleye pollack surimi mediated by indigenous proteases (Oujifard et al. 2012; Benjakul et al. 2004a, b; Yamashita et al. 1995). Apart from the inhibitory activity against the proteolysis, SOSPI at a proper level might act as a filler, thereby increasing gel strength of kamaboko gel.
Effect of SOSPI on whiteness of surimi gels
Whiteness of modori and kamaboko added with SOSPI at different levels is shown in Table 3. No difference in whiteness was observed in both modori and kamaboko gels, regardless of SOSPI concentrations (p > 0.05). Color characteristic of surimi gels were mainly dependent on the type and amount of additives added in surimi gels (Benjakul et al. 2004a, b). However, other protein additives including Thai legumes and whey protein concentrate resulted in the decreased whiteness of surimi gel from sardine and tropical fishes, respectively (Kudre and Benjakul 2013a, b; Rawdkuen and Benjakul 2008). When comparing the whiteness of modori and kamaboko gels, kamaboko gels had a slightly higher whiteness, compared with modori gel (p < 0.05). Incubation of modori gel at higher temperature (60 °C) might induce the browning reaction, particularly Maillard reaction to a higher extent, compared to kamaboko gel, with setting at lower temperature (40 °C). The overall results suggested that the SOSPI had no negative effect on the color of both kamaboko and modori surimi gels.
Effect of SOSPI on the expressible moisture content (EMC) of surimi gel
Modori gel showed the decrease in EMC (p < 0.05), when SOSPI at level above 1% was added (p < 0.05), suggesting the ability of SOSPI in enhancing water holding capacity (WHC) of modori gel (Table 3). For kamaboko gel, the continuous decrease in EMC was noticeable with increasing concentration. SOSPI was suggested to assist in retaining more water in gel matrix which is the requirement for the better gel quality. Whey protein concentrate also decreased EMC in the kamaboko gel from the bigeye snapper (Rawdkuen and Benjakul 2008). Protein isolates from black bean, mung bean and bambara groundnut were able to lower EMC of kamaboko gels from sardine and threadfin bream surimi (Kudre and Benjakul 2013a; Kudre et al. 2013; Oujifard et al. 2012). In the present study, SOSPI rich in proteinaceous matters might exhibit high WHC, thus reducing water loss. In comparison between the controls (without SOSPI) of both gels, modori showed higher EMC than kamaboko gel, indicating the higher water holding capacity of the latter. The degradation of muscle proteins in modori gel caused the gel matrix poorer in holding the water. Furthermore, the addition of SOSPI directly prevented the degradation of surimi proteins, resulting in the strong gel network with capacity of water holding. The increased water holding capacity of gel was in accordance with the increased breaking force.
Conclusion
Serine protease inhibitors from the squid ovary were successfully isolated from squid ovary using 0.45 M of NaCl for 1 h, followed by heat treatment at 70 °C for 10 min. Protease inhibitors having apparent MW of 9.10 and 10.27 kDa were detected. It was able to inhibit the proteolysis of surimi paste from bigeye snapper and improved the gelling properties of both modori and kamaboko gel. Thus, serine protease inhibitor from squid ovary could be used as alternative additive for improving the gelling properties of surimi.
Acknowledgements
This research was supported by the Thailand’s Education Hub for Southern Region of ASEAN Countries (TEH-AC, 2015) scholarship and Prince of Songkla University. The TRF Distinguished Research Professor Grant was also acknowledged.
References
- Alvarez C, Couso I, Tejada M. Thermal gel degradation (modori) in sardine surimi gels. J Food Sci. 1999;64(4):633–637. doi: 10.1111/j.1365-2621.1999.tb15099.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W. Transglutaminase-mediate setting in bigeye snapper surimi. Food Int Res. 2003;36:253–266. doi: 10.1016/S0963-9969(02)00167-9. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Thummaratwasik P. Isolation and characterization of trypsin inhibitors from some Thai legume seeds. J Food Biochem. 2000;24:107–127. doi: 10.1111/j.1745-4514.2000.tb00689.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Chantarasuwan C. Effect of porcine plasma protein and setting on gel properties of surimi produced from fish caught in Thailand. LWT-Food Sci Technol. 2004;37:177–185. doi: 10.1016/j.lwt.2003.07.002. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Tueksuban J, Tanaka M. Effect of some protein additives on proteolysis and gel-forming ability of lizardfish (Saurida tumbil) Food Hydocoll. 2004;18:395–401. doi: 10.1016/S0268-005X(03)00127-9. [DOI] [Google Scholar]
- Benjakul S, Yarnpakdee S, Visessanguan W, Phatcharat S. Combination effects of whey protein concentrate and calcium chloride on the properties of goatfish surimi gel. J Texture Stud. 2010;41:341–357. doi: 10.1111/j.1745-4603.2010.00228.x. [DOI] [Google Scholar]
- Carreno GF, Dimes LE, Haard NF. Substrate-gel electrophoresis for composition and molecular weight of proteinase or proteinaceous proteinase inhibitors. Anal Biochem. 1993;214:65–69. doi: 10.1006/abio.1993.1457. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Rao GN, Rao DG, Jyothirmayi T. Protein hydrolysates from meriga (Cirrhinus mrigala) egg and evaluation of their functional properties. Food Chem. 2010;120:52–657. doi: 10.1016/j.foodchem.2009.10.057. [DOI] [Google Scholar]
- Choi JH, Park PJ, Kim SW. Purification and characterization of a trypsin inhibitor from the egg of skipjack tuna Katsuwonus pelamis. Fish Sci. 2002;68:1367–1373. doi: 10.1046/j.1444-2906.2002.00576.x. [DOI] [Google Scholar]
- Damodaran S. Amino acids, peptides, and proteins. In: Fennema OR, editor. Food chemistry. New York: Marcel Dekker; 1996. pp. 322–429. [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J Food Eng. 2010;96:66–73. doi: 10.1016/j.jfoodeng.2009.06.046. [DOI] [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Chemical compositions of the roes from skipjack, tongol and bonito. Food Chem. 2011;124:1328–1334. doi: 10.1016/j.foodchem.2010.07.076. [DOI] [Google Scholar]
- Kansal R, Kumar M, Kuhar K, et al. Trypsin protease inhibitor from Cicer arietinum L. and its efficacy against Helicoverpa armigera. Brazilian Soc. Plant Physiol. 2008;20(4):313–322. [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H, Osako K, Tanaka M. A heat-stable trypsin inhibitor in adzuki bean (Vigna angularis): effect of extraction media, purification and biochemical characteristics. Int J Food Sci Technol. 2010;45:163–169. doi: 10.1111/j.1365-2621.2009.02117.x. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H, Chaijan M. Extraction, purification and properties of trypsin inhibitor from Thai mung bean (Vigna radiata (L.) R. Wilczek) Food Chem. 2011;129:1348–1354. doi: 10.1016/j.foodchem.2011.05.029. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H. Optimum extraction and recovery of trypsin inhibitor from yellowfin tuna (Thunnus albacores) roe and its biochemical properties. Int J Food Sci Technol. 2014;49:168–173. doi: 10.1111/ijfs.12294. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H, Osako K, Simpson BK. Trypsin inhibitor from yellowfin tuna (Thunnus albacores) roe: effects on gel properties of surimi from bigeye snapper (Priacanthus macracanthus) LWT-Food Sci Technol. 2015;65:122–127. doi: 10.1016/j.lwt.2015.07.074. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Kishimura H, Osako K, Benjamin KS. Purification and characterization of trypsin inhibitor from yellowfin tuna (Thunnus albacores) roe. J Food Biochem. 2016;40(2):140–147. doi: 10.1111/jfbc.12204. [DOI] [Google Scholar]
- Kudre T, Benjakul S. Effect of legume seed protein isolates on autolysis and gel properties of surimi from sardine (Sardinella albella) Int J Chem Environ Biol Sci. 2013;1(1):2320–4087. [Google Scholar]
- Kudre TG, Benjakul S. Combining effect of microbial transglutaminase and bambara groundnut protein isolate on gel properties of surimi from sardine (Sardinella albella) Food Biophys. 2013;8:240–249. doi: 10.1007/s11483-013-9292-5. [DOI] [Google Scholar]
- Kudre T, Benjakul S, Kishimura H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella) LWT-Food Sci Technol. 2013;50:511–518. doi: 10.1016/j.lwt.2012.08.018. [DOI] [Google Scholar]
- Kumazawa Y, Numazawa N, Seguro M. Motoki Suppression of surimi gel setting by transglutaminase inhibitors. J Food Sci. 1995;60:715–717. doi: 10.1111/j.1365-2621.1995.tb06213.x. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Fan AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:256–275. [PubMed] [Google Scholar]
- Minin A, Ozerova SG. Fish ovarian fluid contains protease inhibitors. Russ J Dev Biol. 2015;46(1):33–37. doi: 10.1134/S1062360415010063. [DOI] [PubMed] [Google Scholar]
- Morrissey MT, Wu JW, Lin D, An H. Protease inhibitor effects on torsion measurements and autolysis of Pacific whiting surimi. J Food Sci. 1993;58:1050–1054. doi: 10.1111/j.1365-2621.1993.tb06109.x. [DOI] [Google Scholar]
- Oujifard A, Benjakul S, Ahmad M, Seyfabadi J. Effect of bambara groundnut protein isolate on autolysis and gel properties of surimi from threadfin bream (Nemipterus bleekeri) LWT-Food Sci Technol. 2012;47(261):266. [Google Scholar]
- Park JW. Ingredient technology and formulation development. In: Park JW, editor. Surimi and surimi seafood. New York: Marcel Dekker; 2000. pp. 343–392. [Google Scholar]
- Rawdkuen S, Benjakul S. Whey protein concentrate: autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chem. 2008;106:1077–1084. doi: 10.1016/j.foodchem.2007.07.028. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2. New York: McGraw-Hill; 1980. [Google Scholar]
- Yamashita T, Araki H, Seki Y. Effects of casein on gelling properties of surimi paste. Fish Sci. 1995;62(3):421–426. [Google Scholar]