Abstract
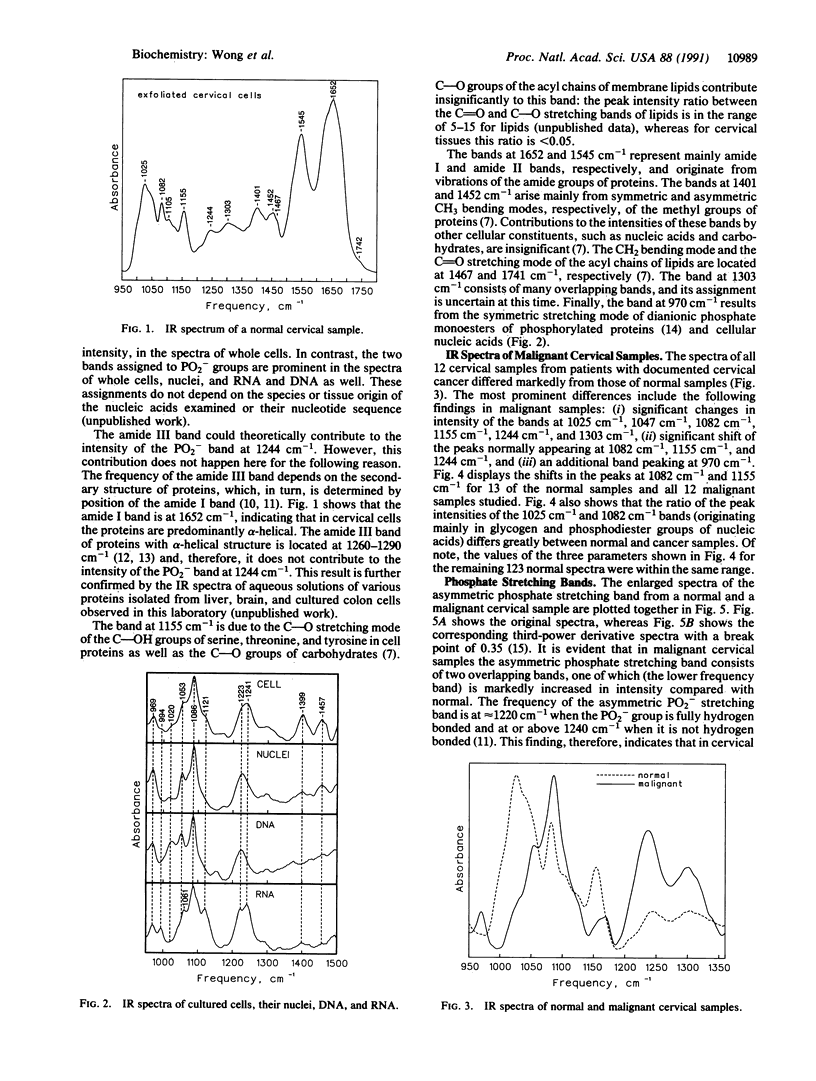
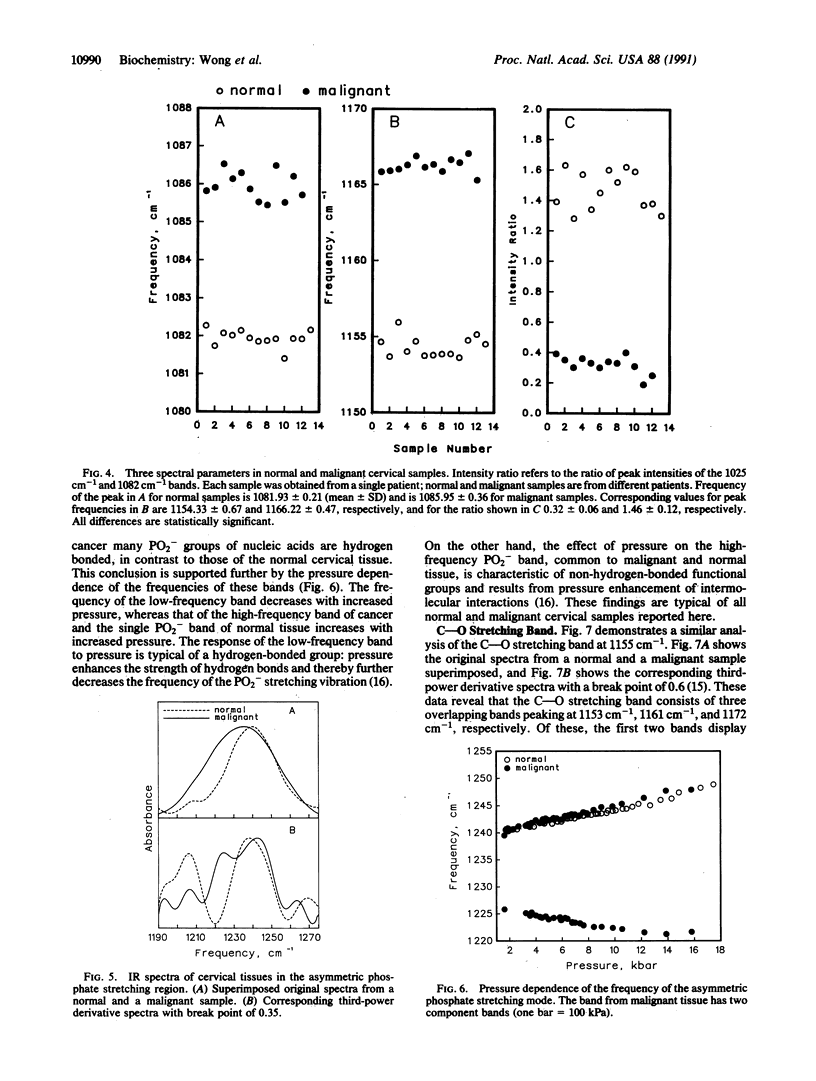
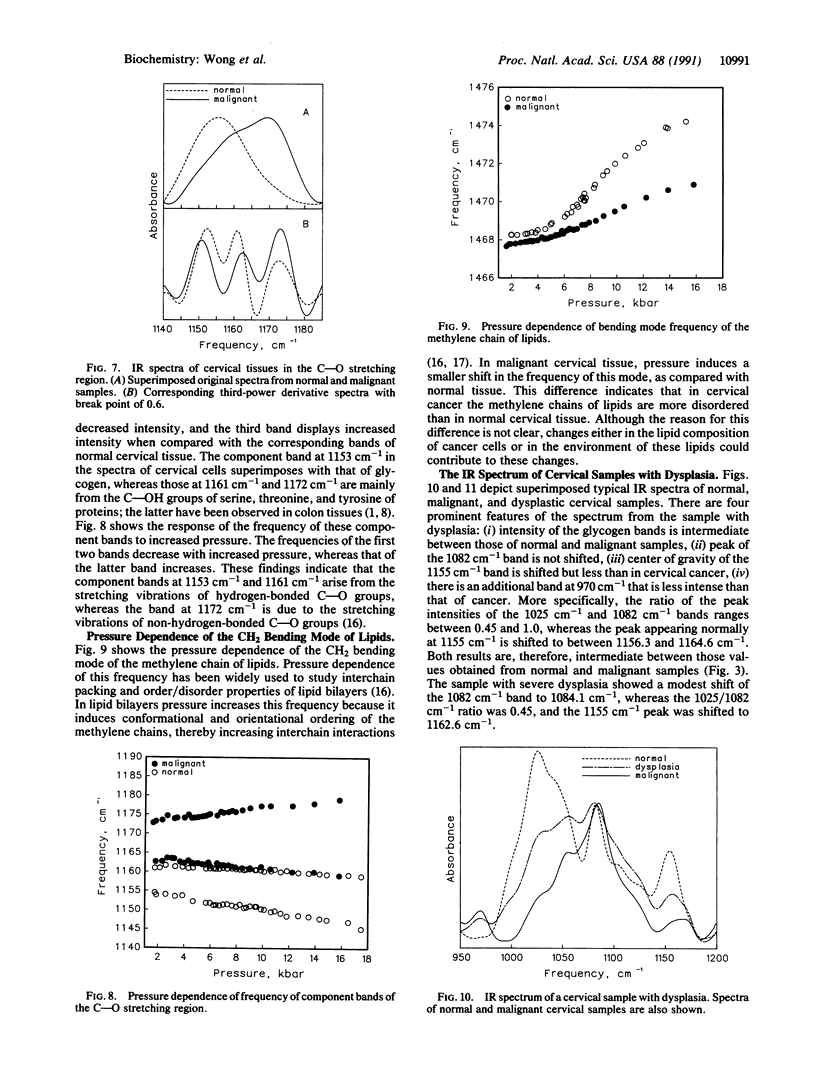
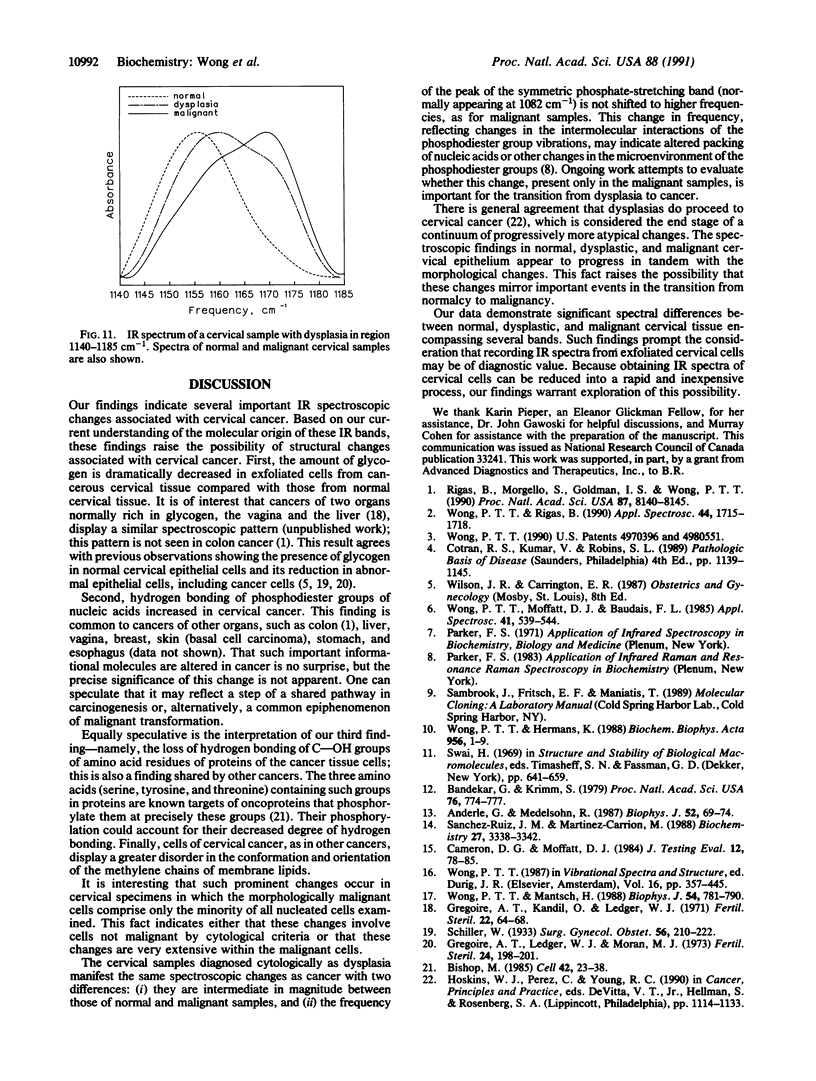
Infrared spectra were obtained from exfoliated cervical cells from 156 females, of whom 136 were normal, 12 had cervical cancer, and 8 had dysplasia. The spectra of the normal women, essentially identical, differed from those obtained from patients with either cancer or dysplasia. In malignant samples we noted (i) significant changes in the intensity of the glycogen bands at 1025 cm-1 and 1047 cm-1, the bands at 1082 cm-1 and 1244 cm-1, the C--O stretching band at 1155 cm-1, and the band at 1303 cm-1, (ii) significant shifts of the peaks normally appearing at 1082 cm-1, 1155 cm-1, and 1244 cm-1, and (iii) an additional band at 970 cm-1. Further study of several of these bands, including the pressure dependence of their frequencies, revealed that in the malignant cervical tissue there were extensive changes in the degree of hydrogen bonding of phosphodiester groups of nucleic acids and C--OH groups of proteins, as well as changes in the degree of disorder of methylene chains of lipids. The IR spectra of samples with dysplasia demonstrated the same changes with cancer samples, except that the changes were of lesser magnitude and the phosphodiester peak normally appearing at 1082 cm-1 did not shift. These spectroscopic changes appear to progress in tandem with the morphological changes that lead normal cervical epithelium to cancer through the premalignant stage of dysplasia. The diagnostic potential of IR spectroscopy is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderle G., Mendelsohn R. Thermal denaturation of globular proteins. Fourier transform-infrared studies of the amide III spectral region. Biophys J. 1987 Jul;52(1):69–74. doi: 10.1016/S0006-3495(87)83189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandekar J., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of beta-turns. Proc Natl Acad Sci U S A. 1979 Feb;76(2):774–777. doi: 10.1073/pnas.76.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Gregoire A. T., Kandil O., Ledger W. J. The glycogen content of human vaginal epithelial tissue. Fertil Steril. 1971 Jan;22(1):64–68. doi: 10.1016/s0015-0282(16)37989-4. [DOI] [PubMed] [Google Scholar]
- Gregoire A. T., Ledger W. D., Morgan M. J. The glycogen content of the human female genital tract in cycling, menopausal, and women with endometrial and cervical carcinoma. Fertil Steril. 1973 Mar;24(3):198–201. doi: 10.1016/s0015-0282(16)39553-x. [DOI] [PubMed] [Google Scholar]
- Rigas B., Morgello S., Goldman I. S., Wong P. T. Human colorectal cancers display abnormal Fourier-transform infrared spectra. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8140–8144. doi: 10.1073/pnas.87.20.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ruiz J. M., Martinez-Carrion M. A Fourier-transform infrared spectroscopic study of the phosphoserine residues in hen egg phosvitin and ovalbumin. Biochemistry. 1988 May 3;27(9):3338–3342. doi: 10.1021/bi00409a033. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Heremans K. Pressure effects on protein secondary structure and hydrogen deuterium exchange in chymotrypsinogen: a Fourier transform infrared spectroscopic study. Biochim Biophys Acta. 1988 Aug 31;956(1):1–9. doi: 10.1016/0167-4838(88)90291-9. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Mantsch H. H. Reorientational and conformational ordering processes at elevated pressures in 1,2-dioleoyl phosphatidylcholine: a Raman and infrared spectroscopic study. Biophys J. 1988 Nov;54(5):781–790. doi: 10.1016/S0006-3495(88)83016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


