Abstract
Post-stroke cognitive decline and dementia pose a significant public health problem, with 30% of stroke survivors suffering from dementia. The reason for this high prevalence is not well understood. Pathogenic B cell responses to the damaged CNS are one possible contributing factor. B-lymphocytes and antibodies are present in and around the stroke core of some human subjects who die with stroke and dementia, and mice that develop delayed cognitive dysfunction after stroke have clusters of B-lymphocytes in the stroke lesion, and antibody infiltration in the stroked hemisphere. The ablation of B-lymphocytes prevents post-stroke cognitive impairment in mice. Multiple drugs that target B cells are FDA approved, and so if pathogenic B cell responses are occurring in a subset of stroke patients, this is potentially treatable. However, it has also been demonstrated that regulatory B cells can be beneficial in mouse models of stroke. Consequently, it is important to understand the relative contribution of B-lymphocytes to recovery versus pathogenicity, and if this balance is heterogeneous in different individuals. Therefore, the purpose of this review is to summarize the current state of knowledge with regard to the role of B-lymphocytes in the etiology of post-stroke dementia.
Keywords: stroke, dementia, vascular dementia, Alzheimer’s Disease, autoimmune, autoantibody, lymphocyte
Introduction
Dementia is a significant problem in stroke survivors. Approximately thirty percent of stroke survivors suffer from cognitive dysfunction or dementia (Leys et al., 2005). Furthermore, stroke increases the risk of cognitive decline in two time windows. It can cause an immediate acute decline in cognition, and also worsen the trajectory of cognitive decline for years after stroke (Levine et al., 2015). Many studies have revealed risk factors that correlate with post-stroke dementia including increasing age, low education level, hypertension, diabetes mellitus, myocardial infarction, cardiac arrhythmias, congestive heart failure, global cerebral atrophy, white matter changes, and recurrent stroke (Leys et al., 2005; Pendlebury and Rothwell, 2009). However, there is compelling emerging data that B cell mediated autoimmunity may be an additional mechanism that plays an important role in the development of dementia after stroke. Stroke has repeatedly been demonstrated to elicit the production of antibodies specific for CNS self-antigens in humans (Dambinova et al., 2003; Kalev-Zylinska et al., 2013; Ortega et al., 2015; Weissman et al., 2011), and we recently demonstrated that B-lymphocytes are required for the development of delayed cognitive impairment in a mouse model of post-stroke dementia (Doyle et al., 2015).
The goal of this minireview is therefore to summarize the evidence that stroke may induce dementia via B-lymphocyte-mediated autoimmunity. We cover B cell biology, stroke-induced activation of B-lymphocytes, early effects of regulatory B-lymphocytes on stroke outcome, autoantibody production, and evidence for chronic autoimmune-mediated effects on cognition.
1.) Introduction to B-lymphocytes
B-lymphocytes are generated in the bone marrow after which they mature into fully competent B cells in the spleen and other secondary lymphoid tissues such as the cervical lymph nodes. Their activation requires antigen recognition by B cell receptors (BCRs) followed by a secondary activation signal, typically in the context of antigen-presenting cells. Upon activation B cells proliferate, form germinal centers, and differentiate into memory B cells or plasma cells. The primary function of plasma cells is the secretion of clone-specific antibodies.
Antibodies contain a clonally unique antigen-binding region joined to a constant immunoglobulin isotype-defining region and there are three antibody diversification mechanisms, all of which may have relevance to the origin of CNS specific antibodies following stroke. The first is VDJ recombination. This is the primary diversification mechanism, and is antigen independent. Antigen receptor gene rearrangement of variable (V), diversity (D) and joining (J) gene segments generates a vast (108) repertoire of antigen receptors with different antibody specificities (Paige and Wu, 1989). In practice, only a portion of this potential repertoire is ever expressed as functional antibody, and the estimate of the available B cell repertoire due to VDJ recombination is closer to 107 (Paige and Wu, 1989). Negative selection is one mechanism that restricts the size of the available B cell repertoire. This mechanism leads to the apoptosis of B cells that recognize self-molecules present in the bone marrow during B cell maturation. Importantly, this mechanism fails to delete all CNS-reactive B cells (Levin et al., 2010).
Negative selection may fail to successfully delete all CNS-reactive B cells due to the specialized endothelial cells that comprise the blood brain barrier (BBB). These cells are connected with tight junctions and restrict the transit of all soluble proteins greater than 500 Daltons into or out of the brain. This curbs the ability of immature B cells to come into contact with brain antigens in the bone marrow during negative selection, thus hampering the ability of an individual to establish tolerance to brain antigens (Diamond et al., 2009; Levin et al., 2010).
The second antibody diversification mechanism is somatic hypermutation. This is an antigen dependent mechanism that adjusts the affinity of antibodies by altering sequences within the antibody variable genes. Following B cell activation by an antigen, B cells proliferate and the complimentary determining regions (CDR) within the loci of the B cell receptor undergo mutation at a rate up to one million times higher than the rest of the genome. This is mediated by the enzyme activation induced cytidine deaminase (AID), which deaminates cytidines to uracils. Repair of these uracils in DNA triggers the formation of point mutations. B cells that have a higher antigen affinity are positively selected, resulting in antibodies that have an enhanced ability to bind to their target antigen (Murphy et al., 2012).
Although somatic hypermutation is advantageous in the context of infection, the random nature of somatic hypermutation can lead to the generation of autoreactive B cells. For instance, in systemic lupus erythematosus, and mouse models of Lupus, anti-dsDNA autoantibodies develop from non-autoreactive B cells by somatic hypermutation (Schroeder et al., 2013). Although the role of somatic hypermutation in the development of anti-CNS autoantibodies following stroke is unknown, this diversification mechanism compounds the risk of autoimmunity already engendered by the lack of negative selection for CNS-antigens in the bone marrow. Furthermore, anti-dsDNA antibodies that cross-react with neuronal glutamine receptors are produced in the CNS following spinal cord injury (Ankeny et al., 2006). Based on the origin of anti-dsDNA autoantibodies in systemic lupus erythematosus, it is possible that these anti-dsDNA antibodies are also derived from somatic hypermutation. Further studies are necessary to determine the extent to which somatic hypermutation plays a role in the development of CNS reactive antibodies following stroke.
The final antibody diversification mechanism is class switch recombination. This occurs in response to antigen stimulation in the presence of costimulatory signals, and adjusts the effector function of antibodies by switching the constant regions of antibody heavy chain genes. Naïve B cells express both IgM and IgD BCRs on the cell surface, which are the first two heavy chain segments in the immunoglobulin locus. However, following activation B cells can undergo antibody class switching to express IgG, IgA or IgE BCRs on the cell surface, and produce IgG, IgA or IgE antibodies. The specific isotype is determined by cytokines produced by T helper cells (Murphy et al., 2012).
IgM antibodies are expressed early in B cell development and consequently are associated with primary immune responses. They are effective at activating complement and agglutinating microorganisms. The function of secreted IgD antibodies is uncertain, although it has been proposed that membrane bound IgD regulates B cell fate during different stages of B cell maturation and development. IgE antibodies attach to mast cells and can elicit antigen-dependent histamine release. IgA antibodies are found in large quantities in the gut, respiratory and urogenital tract, and provide defense against pathogens found at mucosal surfaces. IgG antibodies are the major isotype found in the blood and are the most versatile antibody isotype, effective at fixing complement, neutralizing toxins and pathogens, and mediating opsonization (Murphy et al., 2012). Significantly, autoantibodies of the IgG, IgM, and IgA isotype have all been detected at an increased frequency in the CSF of stroke patients compared to controls (Pruss et al., 2012b). This indicates that in addition to being activated following stroke, mature B cells may also undergo class switching within the CNS compartment.
2.) Antigen-dependent B-lymphocyte activation following stroke
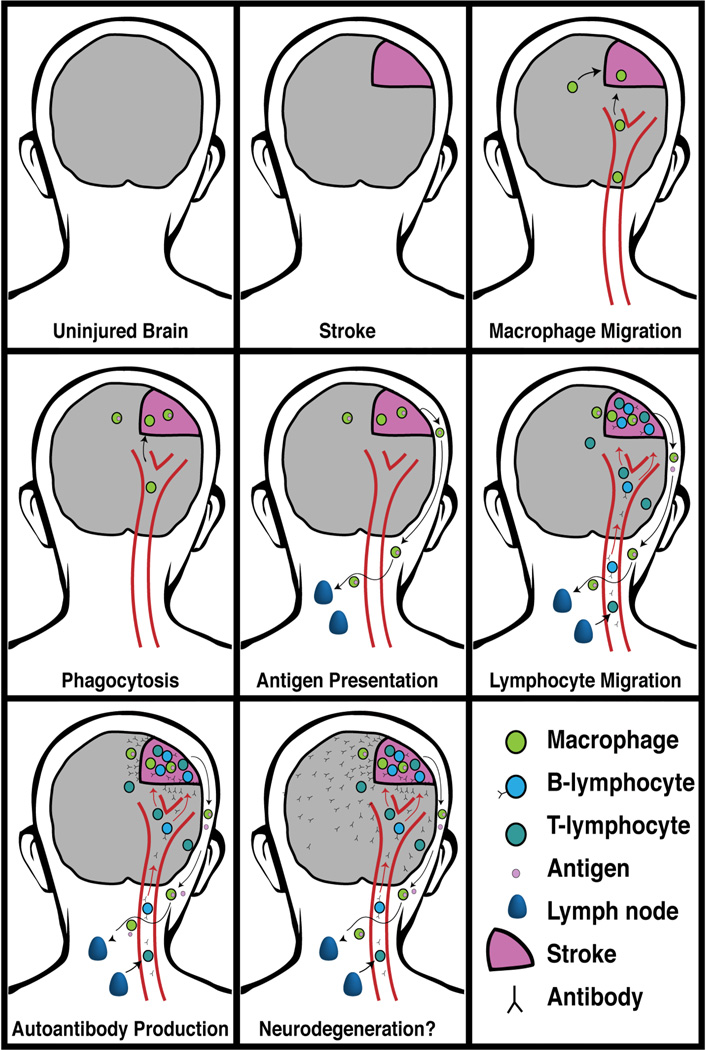
For antigen-dependent B cell activation and autoantibody responses to occur in response to stroke, CNS antigens released from dead and damaged cells must bind to the BCR of autoreactive B-lymphocytes that have escaped negative selection. There are typically fewer than 3 B-lymphocytes/cm2 in the normal human brain (Anthony et al., 2003; Doyle et al., 2015). Therefore, following stroke, CNS antigens likely make first contact with autoreactive B-lymphocytes in the paratracheal, deep cervical lymph nodes (Figure 1).
Figure 1. Autoimmunity after stroke.
Left to right: In response to stroke antigenic material exits the CNS and is taken up by dendritic cells in cervical lymph nodes. Macrophages from the periphery also migrate to the damaged tissue to participate in the clearance of dead cells and cellular debris. We propose that a subset of these phagocytic cells exit the brain via the meningeal lymphatic system and also reach the cervical lymph nodes. Here they come into contact with, and present antigen to, autoreactive B- and T-lymphocytes that have escaped negative selection. Once activated, autoreactive B-lymphocytes proliferate, secrete antibody into the circulation, and home to the infarct. Within the infarct they encounter additional antigen resulting in the further production of autoantibodies within the CNS compartment. Finally, neurodegeneration may occur and contribute to post-stroke cognitive decline and dementia.
Following injury, CNS antigens exit the brain and reach secondary lymphoid tissue, such as the deep cervical lymph nodes, both directly and within macrophage lineage antigen presenting cells that have phagocytosed dead brain tissue. An impaired blood brain interface may facilitate direct exit to cerebral circulation. Antigens and macrophage lineage cells may also exit the brain by draining into Virchow-Robin spaces around cerebral blood vessels. From here, CNS antigens can be transported in interstitial fluid via the glymphatic system to the cerebrospinal fluid (CSF). CSF drains to cervical lymph nodes via lymphatic vessels in the meninges and nasal mucosa thus providing an alternative route for CNS antigens to reach the cervical lymph nodes following stroke (Louveau et al., 2015). CNS antigens are detected in the cervical lymph nodes of stroke patients within 3 days of stroke and their concentration is proportional to the extent of brain damage (Planas et al., 2012).
Once CNS antigens, either free or presented by an antigen-presenting cell, have come into contact with the BCR of autoreactive B-lymphocytes they are then taken up by receptor-mediated endocytosis, fragmented, and presented on the B cell surface by MHCII. Next, secondary activation must occur. How this is achieved following stroke is unknown. It could be via a thymus-dependent or a thymus-independent mechanism.
Thymus-dependent secondary activation requires contact between B cells and T helper cells that bind to the antigen-MHC II complex on the B cell surface along with costimulatory molecules, and tends to result in the production of more specific antibodies than thymus independent activation. In the case of autoimmunity, this requires the presence of autoreactive T helper cells that have also escaped negative selection. The resulting activated T cell provides a reciprocal secondary signal to fully activate the B cell. This is the most likely scenario for B cell activation following stroke because CNS injury is known to activate autoreactive T cells (Popovich et al., 1996). Furthermore, stroke releases molecules from the damage-associated molecular pattern (DAMP) family such as hyaluronan, heat shock proteins, and high mobility group box-1 protein (HMGB1), which can bind to toll-like receptors and increase the expression of costimulatory molecules on B cells (Kono and Rock, 2008).
In thymus-independent activation, the secondary B cell activation signal is provided to an antigen-binding B cell directly by thymus-independent antigens, such as pathogen associated molecular patterns (PAMPs) like lipopolysaccharide and bacterial flagellin, or by extensive cross-linking of the BCR by an antigen with repeating epitopes. Infection is a common comorbidity following stroke, the risk of which is increased due to stroke-induced immunosuppression, and so in some patients infection could facilitate thymus-independent B cell activation. PAMPs also increase the expression of costimulatory molecules on B cells and so infection is also likely to increase the risk of thymus-dependent secondary activation of B-lymphocytes.
3.) Antibody production after stroke
In both animal models and in humans, stroke has repeatedly been demonstrated to elicit the production of antibodies specific for CNS self-antigens (Dambinova et al., 2003; Kalev-Zylinska et al., 2013; Ortega et al., 2015; Weissman et al., 2011). Of relevance to stroke recovery, stroke can lead to the intrathecal production of IgG antibodies (Doyle et al., 2015; Pruss et al., 2012b). This is strong evidence that the generation and activation of autoimmune memory B cell responses can occur in the CNS following stroke.
The intrathecal production of IgG antibodies following stroke has been observed for over 45 years but it is only recently that the frequency has become clear. Pruss et al recently published a retrospective study of 318 patients with ischemic stroke that underwent lumbar puncture (Pruss et al., 2012b). They discovered immunoglobulin synthesis in the CSF of 24.8% of the patients. 17.9% had CSF-specific oligoclonal IgG bands, and 6.9% had increased CSF/serum antibody indices for IgM and IgA. Control individuals with no evidence of CNS disease had a much lower oligoclonal band frequency of 2.5%. Other studies have reported a similar oligoclonal band frequency of 3.9% in non-stroke patients (Pruss et al., 2012b). The prevalence of class switched antibody in the CSF of stroke patients combined with their absence in serum indicates that the immunoglobulins are being produced in the CNS.
Although the initial activation of B-lymphocytes by CNS antigens likely occurs and persists in deep cervical lymph nodes and other secondary lymphoid tissue, the lesion may subsequently become an additional site of B cell activation and autoantibody production in the weeks after stroke. A significant proportion of patients who die years after a stroke have inflammatory infiltrates in their brain (Mena et al., 2004), and in mice, beginning 2 weeks following stroke, clusters of B-lymphocytes appear in the stroke lesion (Doyle et al., 2015). These B-lymphocytes appear in conjunction with T-lymphocytes and plasma cells, and the B- and T-lymphocytes are compartmentalized with respect to each other. We demonstrated this in 4 different stroke models. The compartmentalization of the B- and T-lymphocytes suggests that structures similar to ectopic B cell follicles are capable of forming in stroke lesions.
Ectopic B cell follicles are also observed outside the CNS in both pathogenic and non-pathogenic chronic inflammatory states, and correlate with increased severity of autoimmune disease or infection (Carragher et al., 2008). Ectopic lymphoid aggregates consisting of interfollicular T cell zones and B cell follicles with germinal centers have been found in rheumatoid arthritis, Sjogren’s syndrome, Crohn’s disease, Hashimoto thyroiditis, and in the meninges in multiple sclerosis (MS) (Serafini et al., 2004). In mice, the extent of B cell follicle formation in the lesion is strain specific. Follicles are smaller and are less prevalent in BALB/cJ mice than C57BL/6J mice (unpublished observations). In humans, the extent to which they form in the brain in response to stroke is unknown. However, there are increased numbers of B cells in the brain in approximately two-thirds of stroke survivors with dementia (Doyle et al., 2015).
Combined with the intrathecal presence of oligoclonal bands in the CSF following stroke, these results provide further evidence that B-lymphocyte activation occurs in the CNS outside of the cervical lymph nodes in some stroke patients. However, the full extent to which this takes place still needs to be determined. Furthermore, it is possible that B cell follicle formation may occur in the meninges following stroke. The meninges are increasingly being revealed to be a missing link between the brain and the immune system (Aspelund et al., 2015; Louveau et al., 2015). The location of B cell activation in the CNS following stroke is an important avenue for future research and is potentially achievable in living patients using PET imaging with radiolabelled anti-CD20 antibodies. A similar strategy has already shown promise for detecting B cells in rheumatoid arthritis sufferers (Tran et al., 2011), and has been validated in a mouse model of B cell non-Hodgkin’s lymphoma (Natarajan et al., 2013).
While there is much evidence to indicate that B-lymphocyte activation and resultant antibody production occurs in the CNS following stroke, B-lymphocytes could also impair stroke recovery and contribute to the delayed onset of post-stroke dementia if activation is restricted to peripheral locations such as the cervical lymph nodes. This is because stroke results in chronic impairment of the blood brain interface, thus providing a route of entry for brain-reactive autoantibodies produced outside the CNS to gain access to the brain during recovery (Levin et al., 2010).
4.) B-lymphocyte activation following stroke leads to the production of anti-CNS autoantibodies
There are multiple mechanisms through which autoantibodies in the CNS could be detrimental, including antibody-dependent cell-mediated cytotoxicity (ADCC), stimulation of complement, inhibition of signal transduction, or direct induction of apoptosis. In multiple sclerosis, autoantibodies against myelin sheaths cause neuronal loss (Gold et al., 2012). However, current evidence suggests that the primary harmful mechanism of action of most other autoantibodies in the CNS is inhibition of signal transduction and interference with neuronal function (Vincent et al., 2011). For example, antibodies to the acetylcholine receptor interfere with neuronal function in myasthenia gravis (Ha and Richman, 2015), and antibodies that bind to voltage-gated calcium channels interfere with neuronal function in Lambert-Eaton myasthenic syndrome (Gold et al., 2012; Hulsbrink and Hashemolhosseini, 2014; Vincent et al., 2011). Antibodies that bind to the N-methyl-D-aspartate receptor (NMDAR) interfere with NMDAR function and cause anti-NMDAR encephalitis, characterized by seizures, psychiatric manifestations, dyskinesias, mutism, and a reduction in consciousness (Barry et al., 2015; Ding et al., 2015; Vincent et al., 2011). Similarly, antibodies that bind to the GluR1/2 subunits of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) cause amnesia, seizures, limbic encephalitis, and psychosis in AMPAR-Antibody limbic encephalitis (Vincent et al., 2011). A summary of the most common diseases associated with CNS autoantibodies is provided in Table 1.
Table 1.
Diseases with B cell and associated CNS autoantibody involvement
| Disease | Major Antigen(s) | Selected References |
|---|---|---|
|
Multiple Sclerosis |
Myelin basic protein (MBP), Myelin oligodendrocyte glycoprotein (MOG), Myelin-associated glycoprotein (MAG) |
(Gold et al., 2012; Probstel et al., 2015) |
|
Systemic Lupus Erythematosus |
dsDNA, N-methyl-d-aspartate receptor subunit 2A (NR2A), N-methyl-d-aspartate receptor subunit 2B (NR2B) |
(Brimberg et al., 2015; Diamond and Volpe, 2012) |
|
Myasthenia gravis |
Acetylcholine receptor (AChR) | (Ha and Richman, 2015) |
|
Lambert-Eaton myasthenic syndrome |
P/Q-type voltage-gated calcium channels (VGCC) | (Gold et al., 2012; Hulsbrink and Hashemolhosseini, 2014) |
|
Anti-NMDAR encephalitis |
N-methyl-D-aspartate receptor (NMDAR) | (Barry et al., 2015; Ding et al., 2015) |
|
AMPAR Antibody limbic encephalitis |
GluR1/2 subunits of the α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid receptor (AMPAR) |
(Vincent et al., 2011) |
|
Limbic encephalitis |
B-1 subunit of GABA Type B receptor (GABABR1) | (Melzer et al., 2015; Vincent et al., 2011) |
|
Neuromyelitis optica |
Aquaporin 4 (AQP4) | (Gold et al., 2012; Zekeridou and Lennon, 2015) |
| Stroke | Subunit 1 (GluN1) and subunit 2 (GluN2) of the NMDAR, Neurofilament (NF), Glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), Myelin basic protein (MBP), Proteolipid protein (PLP) |
(Bornstein et al., 2001; Janardhan et al., 2004; Kalev-Zylinska et al., 2013) (Mecocci et al., 1995; Shibata et al., 2012) |
The list of anti-CNS antibodies produced following stroke includes antibodies against the NMDAR. In humans, stroke has been demonstrated to elicit the production of antibodies that recognize subunit 1 (GluN1) and subunit 2 (GluN2) of the NMDAR (Dambinova et al., 2003; Kalev-Zylinska et al., 2013; Weissman et al., 2011). In a study of 48 ischemic stroke patients and 96 healthy controls, Kalev-Zylinska et al discovered that antibodies that bind to GluN1 are present in the serum of 44% of stroke patients and only 3% of age matched controls (Kalev-Zylinska et al., 2013). NMDARs are important for synaptic plasticity and memory, and during ischemic stroke high glutamate levels cause NMDAR overactivation, excitotoxicity, neuronal cell death, and thus the release of NMDAR antigens (Doyle et al., 2008). NMDAR antigens are detected in the cervical lymph nodes of stroke patients within days of stroke (Planas et al., 2012). Importantly, anti-NMDA receptor antibodies have repeatedly been connected to cognitive dysfunction in humans (Pruss et al., 2012a), and the administration of antibodies that bind to the NMDAR to mouse brain slices in vitro leads to elevated calcium levels in neurons and excitotoxic cell death (DeGiorgio et al., 2001).
CNS injury also leads to the production of anti-nuclear antibodies that can cross-react with the NMDAR and cause neuronal dysfunction (Ankeny et al., 2006; DeGiorgio et al., 2001). Anti-DNA antibodies that cross-react with the NMDA receptor are implicated in neurological dysfunction in spinal cord injury, MS and lupus (Ankeny et al., 2006; Schuller et al., 1978; Williamson et al., 2001), and have been observed in the serum of vascular dementia patients (Kanai et al., 2007; Mecocci et al., 1993).
In addition to antibodies specific for peptides of the NMDAR, and antibodies that can cross-react with the NMDAR, stroke has also been found to generate antibodies that recognize neurofilament (NF), an intermediate filament present in neurons (Bornstein et al., 2001; Pruss et al., 2012b). For example, in patients presenting with their first-ever stroke, levels of anti-neurofilament antibodies are elevated in serum for at least 6 months following stroke compared to controls (Bornstein et al., 2001; Janardhan et al., 2004).
The list of autoantibodies that have been found in stroke patients also includes antibodies against the astrocyte proteins glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100B), as well as antibodies against the white matter antigens myelin basic protein (MBP) and proteolipid protein (PLP) (Bornstein et al., 2001; Mecocci et al., 1995; Shibata et al., 2012). Autoantibodies against these brain proteins have frequently been associated with vascular dementia, but it is unknown if they are a direct cause of cognitive dysfunction, or rather evidence that an antigen-specific immune reaction has taken place following stroke.
While it appears that there are dominant antigens that elicit autoantibody production following stroke, the repertoire of CNS autoantibodies that have been detected following stroke is diverse, and varies from stroke patient to stroke patient (unpublished observations).
5.) Evidence that B cell activation and autoantibody production leads to post-stroke cognitive impairment
There is mounting evidence that stroke triggers a slow neurodegenerative process that may have an autoimmune etiology. Post stroke cognitive impairment is very common, impacting thirty to fifty percent of stroke survivors (Barba et al., 2000; Bejot et al., 2011; Leys et al., 2005). There is a negative correlation between MMSE score and plasma C-reactive protein concentration in stroke survivors (Rothenburg et al., 2010), and there is a demonstration of a decline in cognitive trajectory after stroke in large population studies (Levine et al., 2015). Furthermore autoantibodies are common in Alzheimer’s Disease and vascular lesions are present in over half of all Alzheimer’s Disease cases (Schneider et al., 2007).
While there is a paucity of direct experimental evidence that demonstrates that autoantibody production leads to post-stroke cognitive impairment, we can extrapolate from studies of spinal cord injury. Ankeny et al have shown that B cells produce antibodies that impair recovery in a mouse model of spinal cord injury (SCI). In this study Ankeny et al injected antibodies purified from mice that have undergone a model of SCI into the spinal cord of uninjured mice. This led to hind limb paralysis and neuropathology that was complement and Fc-receptor dependent (Ankeny et al., 2009).
Furthermore, in the absence of stroke, the production of anti-NF antibodies is correlated with cognitive dysfunction in an animal model. Rats immunized with a neurofilament protein extracted from the electric fish Torpedo perform significantly worse than controls in a T-maze alternation test, indicating a deficit in short term memory (Chapman et al., 1991). Immunohistochemistry revealed binding of antibodies to the perikarya and neurites of neurons in the septum and hippocampus of the NF immunized rats, with no such staining evident in adjuvant immunized control rats. This suggests that the immunized rats developed antibodies specific for the Torpedo fish NF immunogen that cross-reacted with rat brain neurofilaments.
However, because NF is an intracellular protein rather that an ion channel, receptor, or synaptic protein, the production of anti-NF antibodies may not be the direct cause of cognitive dysfunction in immunized rats. Instead it could be T cells specific for NF that are the direct cause of cognitive dysfunction due to their ability to be activated by peptides derived from intracellular proteins presented by MHC molecules on the cell surface. In this paradigm, the production of anti-NF antibodies could be more of an indicator that an antigen-specific immune reaction has taken place rather than an actual effector mechanism of cognitive impairment. Experiments that definitively test whether stroke induced autoantibody production leads to cognitive impairment still need to be performed.
The strongest evidence that B cell mediated responses can contribute to post-stroke cognitive impairment comes from our work in mice. We recently reported that B-lymphocyte infiltration into the stroke lesion, an example of which is shown in Figure 2, and antibody diffusion into the surrounding neuropil, occurs in the months after stroke in mouse models. Furthermore, in a mouse model of delayed cognitive impairment after stroke, we found that B-lymphocytes were required to produce the delayed onset of cognitive impairment (Doyle et al., 2015). Specifically, mice developed chronic inflammatory responses in the stroke core within 7 weeks of the stroke that included plasma cells present in the stroke core and isotype-switched antibodies (IgG and IgA) in the hippocampus adjacent to the stroke core. In correlation, memory impairment appeared between 1 and 7 weeks after stroke, and hippocampal long-term potentiation (LTP), which was normal 1 week after stroke, was then progressively impaired at 7 and 12 weeks after stroke. Demonstrating that the impairment in LTP was more than just a correlation with antibody accumulation in the hippocampus, MuMT mice devoid of B-lymphocytes did not develop delayed cognitive impairment or LTP deficits at 7 weeks after stroke, and wildtype mice treated with a B-lymphocyte-depleting antibody 5 days after stroke also did not develop a delayed cognitive deficit (Doyle et al., 2015).
Figure 2. B Lymphocytes accumulate in the stroke core in the weeks after stroke.
Fluorescent immunostaining for B220 (red) and CD11c (green) demonstrate that B cells are present in the lesion 7 weeks following stroke in C57BL/6 mice, and are organized into clusters surrounded by cells expressing CD11c. CD11c is a marker found on most dendritic cells and some plasma cells. B cell clusters commonly form at sites of chronic inflammation, and their presence is associated with poor outcome in multiple sclerosis, traumatic brain injury, rheumatoid arthritis and spinal cord injury (Ankeny and Popovich, 2010; Carragher et al., 2008). Scale bar, 20µm.
A chimeric version of the monoclonal anti-CD20 antibody we used, Rituximab, is FDA-approved as a B cell-depleting drug for rheumatoid arthritis, CD20+ non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, granulomatosis with polyangiitis, and microscopic polyangiitis. The ablation of B-lymphocytes following stroke could be a promising avenue for preventing B cells from impairing recovery and contributing to post-stroke dementia.
To determine how relevant this might be to human post-stroke dementia we evaluated B-lymphocyte responses in human post-mortem brain tissue from the Rush Religious Orders Study and Rush Memory and Aging Project (Doyle et al., 2015). We selected autopsy specimens with gross infarcts and no pathologic diagnosis of Alzheimer’s Disease or other neuropathological disorder. Control specimens came from matched subjects without stroke or dementia. We immunostained sections containing ischemic infarcts with CD20 to detect B cells and found that there were significantly more B-lymphocytes/cm2 in the infarct core and in the area adjacent to the infarct core in about two-thirds of cases than in brain tissue from controls. IgG immunostaining revealed that antibodies are present in the brain in stroke survivors to a greater extent than in controls. Five of the 21 stroke subjects exhibited cellular patterns of IgG immunostaining, whereas control brains had little to no immunostaining of the neuropil. The cellular patterns of immunostaining varied among stroke subjects, which may be indicative of autoantibodies binding to a diverse array of CNS antigens.
Finally, we evaluated serum antibodies in subjects from a prospectively enrolled cohort of stroke patients who were subjected to cognitive testing with a Mini-Mental State Exam (MMSE) at 30, 90, 180, and 365 days after stroke (Becker et al., 2016). Using 30-day scores as a post-stroke baseline, we found that of 58 subjects, 17% had a decline of 2 points or more while the other 83% were stable or improved. Anti-myelin basic protein (MBP) antibody titers predicted cognitive decline in univariate analysis and were the only independent predictor of cognitive decline in multivariate analysis. MBP was tested because of its very high protein expression in brain. In this small group, we did not detect an association between post-stroke cognitive decline and autoantibody titers against a second brain antigen, proteolipid protein. Larger studies with more detailed cognitive testing and longer follow up periods will be required to determine whether serum and/or CSF autoantibodies are predictive and causative in post-stroke cognitive decline and dementia.
6.) Regulatory B-lymphocytes are beneficial in acute stroke
Regulatory B cells represent only 0.5–0.7% of CD19 positive B cells isolated from unimmunized WT mice, but they are an important subset that secrete high levels of IL-10 (Chen et al., 2012). Although adaptive B-lymphocyte immune responses to stroke require time to develop and appear to be an impediment to recovery, there is evidence that immediate regulatory B-lymphocyte responses to stroke may be beneficial. In the first 48 hours following stroke B cell deficient mice have larger infarct volumes, higher mortality and more severe functional deficits compared to wild-type mice in a model of intraluminal filament middle cerebral artery occlusion (MCAO). They also have increased numbers of activated T-cells, macrophages, microglia, and neutrophils in the area of the stroke (Ren et al., 2011). These effects can be reversed in B cell deficient mice that are restored with wildtype B cells, but not in B cell deficient mice restored with IL-10 deficient B cells. This implicates IL-10 producing regulatory B cells as a cell type that can restrict ischemic damage during the initial inflammatory cascade. Regulatory B cells also limit inflammation in animal models of MS via the production of IL-10 (Matsushita et al., 2010; Wolf et al., 1996).
More progress needs to be made in the characterization of regulatory B cells in mice and humans. They have not yet been examined in relation to cognition after stroke. In addition, the cell surface markers and transcription factors specific to regulatory B cells have yet to be fully defined. There are no common markers that define both human and mouse regulatory B cells, efficient IL-10 production is their only accepted distinguishing feature (Yang et al., 2013). Also it is unknown if the activation of regulatory B cell responses following stroke is antigen dependent. Apoptotic cells and toll receptor (TLR) signaling have been shown to be sufficient to initiate IL-10 production in naive B cells (Yang et al., 2013). However, to enable further IL-10-production after an initial burst, B cells require CD40 and the BCR to be engaged. This leads to amplification of IL-10-producing B cells (Yang et al., 2013). The fact that B cells are capable of limiting ischemic injury as early as two days following stroke suggests that their protective effect in stroke is initially antigen independent. This is in contrast to the pathogenic role of B-lymphocytes in the weeks following stroke that appears to be antigen dependent.
7.) Summary
It is possible that an autoimmune B lymphocyte mediated process, triggered by stroke, may be one of the mechanisms that play a role in the development of post-stroke cognitive decline. This hypothesis is based on both animal and human studies. In a mouse stroke model, mice with B lymphocyte infiltrates in their infarct cores develop late cognitive decline, and blocking the B cell response using a mouse analog of rituximab, an FDA-approved anti-CD20 antibody, prevents this cognitive decline (Doyle et al., 2015). Also, B cell infiltrates, similar to those seen in mice, exist in the infarct cores of stroke patients and autoantibodies against brain antigens are reported in the serum of some patients with vascular dementia after stroke. This includes antibodies against neurofilament, GFAP, S100B, and the white matter antigens MBP and PLP (Bornstein et al., 2001; Mecocci et al., 1995; Shibata et al., 2012). Immunoglobulins are also present in the CSF of about 25% of stroke survivors in the chronic phase of stroke (Pruss et al., 2012b; Rostrom and Link, 1981; Tsementzis et al., 1986), and in a small study anti-MBP antibody titers were associated with cognitive decline in the year after stroke (Becker et al., 2016). Antibody in the CNS could be harmful due to multiple mechanisms including antibody mediated cellular toxicity, fixation of complement, inhibition of signal transduction, or direct induction of apoptosis (Cheson and Leonard, 2008). Thus while it is likely that many autoantibodies are neutral or even beneficial to recovery, the evidence is growing that autoantibodies can interfere with neuronal function and could mediate cognitive impairment after stroke.
Finally, it is important to emphasize that it is unlikely that B lymphocytes act alone in the pathogenesis of immune mediated post-stroke cognitive decline. Innate immune cells, T cells, and even neuronal responses likely also play a role.
Highlights.
Stroke can generate autoreactive immune responses.
B-lymphocyte responses to stroke cause delayed cognitive impairment in normal mice.
Autoantibodies are associated with cognitive decline after stroke in humans.
Autoimmune responses to brain antigens may be a cause of post-stroke dementia.
Acknowledgments
Funding: This work was supported by the National Institutes of Health, grant numbers R21NS078571 (Buckwalter) and R00NR013593 (Doyle).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Popovich PG. B cells and autoantibodies: complex roles in CNS injury. Trends Immunol. 2010;31:332–338. doi: 10.1016/j.it.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058–1067. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia : clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- Barry H, Byrne S, Barrett E, Murphy KC, Cotter DR. Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull. 2015;39:19–23. doi: 10.1192/pb.bp.113.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Tanzi P, Zierath D, Buckwalter MS. Antibodies to myelin basic protein are associated with cognitive decline after stroke. Journal of neuroimmunology. 2016;9–11:295–296. doi: 10.1016/j.jneuroim.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejot Y, Aboa-Eboule C, Durier J, Rouaud O, Jacquin A, Ponavoy E, Richard D, Moreau T, Giroud M. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke. 2011;42:607–612. doi: 10.1161/STROKEAHA.110.595553. [DOI] [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Brimberg L, Mader S, Fujieda Y, Arinuma Y, Kowal C, Volpe BT, Diamond B. Antibodies as Mediators of Brain Pathology. Trends Immunol. 2015;36:709–724. doi: 10.1016/j.it.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Alroy G, Weiss Z, Faigon M, Feldon J, Michaelson DM. Anti-neuronal antibodies similar to those found in Alzheimer’s disease induce memory dysfunction in rats. Neuroscience. 1991;40:297–305. doi: 10.1016/0306-4522(91)90121-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metabolic brain disease. 2012;27:487–493. doi: 10.1007/s11011-012-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. The New England journal of medicine. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clinical chemistry. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nature reviews. Immunology. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond B, Volpe BT. A model for lupus brain disease. Immunol Rev. 2012;248:56–67. doi: 10.1111/j.1600-065X.2012.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Jian Z, Stary CM, Yi W, Xiong X. Molecular Pathogenesis of Anti-NMDAR Encephalitis. Biomed Res Int. 2015;2015:643409. doi: 10.1155/2015/643409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS. B-Lymphocyte-Mediated Delayed Cognitive Impairment following Stroke. J Neurosci. 2015;35:2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Pul R, Bach JP, Stangel M, Dodel R. Pathogenic and physiological autoantibodies in the central nervous system. Immunol Rev. 2012;248:68–86. doi: 10.1111/j.1600-065X.2012.01128.x. [DOI] [PubMed] [Google Scholar]
- Ha JC, Richman DP. Myasthenia gravis and related disorders: Pathology and molecular pathogenesis. Biochimica et biophysica acta. 2015;1852:651–657. doi: 10.1016/j.bbadis.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Hulsbrink R, Hashemolhosseini S. Lambert-Eaton myasthenic syndrome -diagnosis, pathogenesis and therapy. Clin Neurophysiol. 2014;125:2328–2336. doi: 10.1016/j.clinph.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Janardhan V, Wolf PA, Kase CS, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW. Anticardiolipin antibodies and risk of ischemic stroke and transient ischemic attack: the Framingham cohort and offspring study. Stroke. 2004;35:736–741. doi: 10.1161/01.STR.0000117575.48205.2D. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Symes W, Little KC, Sun P, Wen D, Qiao L, Young D, During MJ, Barber PA. Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size. Stroke. 2013;44:2212–2219. doi: 10.1161/STROKEAHA.113.001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Akatsu H, Iizuka H, Morimoto C. Could serum antibody to poly(ADP-ribose) and/or histone H1 be marker for senile dementia of Alzheimer type? Annals of the New York Academy of Sciences. 2007;1109:338–344. doi: 10.1196/annals.1398.040. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nature reviews. Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EC, Acharya NK, Han M, Zavareh SB, Sedeyn JC, Venkataraman V, Nagele RG. Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood-brain barrier breakdown. Brain research. 2010;1345:221–232. doi: 10.1016/j.brainres.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Ekman R, Parnetti L, Senin U. Antihistone and anti-dsDNA autoantibodies in Alzheimer’s disease and vascular dementia. Biological psychiatry. 1993;34:380–385. doi: 10.1016/0006-3223(93)90182-d. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Parnetti L, Romano G, Scarelli A, Chionne F, Cecchetti R, Polidori MC, Palumbo B, Cherubini A, Senin U. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer’s disease and vascular dementia. Journal of neuroimmunology. 1995;57:165–170. doi: 10.1016/0165-5728(94)00180-v. [DOI] [PubMed] [Google Scholar]
- Melzer N, Budde T, Stork O, Meuth SG. Limbic Encephalitis: Potential Impact of Adaptive Autoimmune Inflammation on Neuronal Circuits of the Amygdala. Front Neurol. 2015;6:171. doi: 10.3389/fneur.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004;108:524–530. doi: 10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M, Janeway C. Janeway’s immunobiology. New York: Garland Science; 2012. [Google Scholar]
- Natarajan A, Hackel BJ, Gambhir SS. A novel engineered anti-CD20 tracer enables early time PET imaging in a humanized transgenic mouse model of B-cell non-Hodgkins lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6820–6829. doi: 10.1158/1078-0432.CCR-13-0626. [DOI] [PubMed] [Google Scholar]
- Ortega SB, Noorbhai I, Poinsatte K, Kong X, Anderson A, Monson NL, Stowe AM. Stroke induces a rapid adaptive autoimmune response to novel neuronal antigens. Discovery medicine. 2015;19:381–392. [PMC free article] [PubMed] [Google Scholar]
- Paige CJ, Wu GE. The B cell repertoire. FASEB J. 1989;3:1818–1824. doi: 10.1096/fasebj.3.7.2497040. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188:2156–2163. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. Journal of neuroscience research. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Probstel AK, Sanderson NS, Derfuss T. B Cells and Autoantibodies in Multiple Sclerosis. International journal of molecular sciences. 2015;16:16576–16592. doi: 10.3390/ijms160716576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Holtje M, Maier N, Gomez A, Buchert R, Harms L, Ahnert-Hilger G, Schmitz D, Terborg C, Kopp U, Klingbeil C, Probst C, Kohler S, Schwab JM, Stoecker W, Dalmau J, Wandinger KP. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012a;78:1743–1753. doi: 10.1212/WNL.0b013e318258300d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Iggena D, Baldinger T, Prinz V, Meisel A, Endres M, Dirnagl U, Schwab JM. Evidence of intrathecal immunoglobulin synthesis in stroke: a cohort study. Archives of neurology. 2012b;69:714–717. doi: 10.1001/archneurol.2011.3252. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostrom B, Link B. Oligoclonal immunoglobulins in cerebrospinal fluid in acute cerebrovascular disease. Neurology. 1981;31:590–596. doi: 10.1212/wnl.31.5.590. [DOI] [PubMed] [Google Scholar]
- Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, Gladstone DJ, Ween J, Snaiderman A, Lanctot KL. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:199–205. doi: 10.1177/0891988710373598. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schroeder K, Herrmann M, Winkler TH. The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity. 2013;46:121–127. doi: 10.3109/08916934.2012.748751. [DOI] [PubMed] [Google Scholar]
- Schuller E, Delasnerie N, Lebon P. DNA and RNA antibodies in serum and CSF of multiple sclerosis and subacute sclerosing panencephalitis patients. Journal of the neurological sciences. 1978;37:31–36. doi: 10.1016/0022-510x(78)90225-3. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain pathology. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D, Cain K, Tanzi P, Zierath D, Becker K. Myelin basic protein autoantibodies, white matter disease and stroke outcome. Journal of neuroimmunology. 2012;252:106–112. doi: 10.1016/j.jneuroim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Huitema AD, van Rijswijk MH, Dinant HJ, Baars JW, Beijnen JH, Vogel WV. CD20 antigen imaging with (1)(2)(4)I-rituximab PET/CT in patients with rheumatoid arthritis. Hum Antibodies. 2011;20:29–35. doi: 10.3233/hab20110239. [DOI] [PubMed] [Google Scholar]
- Tsementzis SA, Chao SW, Hitchcock ER, Gill JS, Beevers DG. Oligoclonal immunoglobulin G in acute subarachnoid hemorrhage and stroke. Neurology. 1986;36:395–397. doi: 10.1212/wnl.36.3.395. [DOI] [PubMed] [Google Scholar]
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–772. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- Weissman JD, Khunteev GA, Heath R, Dambinova SA. NR2 antibodies: risk assessment of transient ischemic attack (TIA)/stroke in patients with history of isolated and multiple cerebrovascular events. Journal of the neurological sciences. 2011;300:97–102. doi: 10.1016/j.jns.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Williamson RA, Burgoon MP, Owens GP, Ghausi O, Leclerc E, Firme L, Carlson S, Corboy J, Parren PW, Sanna PP, Gilden DH, Burton DR. Anti-DNA antibodies are a major component of the intrathecal B cell response in multiple sclerosis. Proc Natl Acad Sci U S A. 2001;98:1793–1798. doi: 10.1073/pnas.031567598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. The Journal of experimental medicine. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cellular & molecular immunology. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekeridou A, Lennon VA. Aquaporin-4 autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2015;2:e110. doi: 10.1212/NXI.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]