Abstract
Objectives
Transport hub is an important part of urban comprehensive transportation system. Traffic-related air pollution can reach high level because of difficulty of diffusion and increase of emission in transport hub. However, whether exposure in this semi-closed traffic micro-environment causes acute changes in pulmonary function of commuters still needs to be explored.
Methods
Forty young healthy adults participated in this randomized, crossover study. Each participant underwent 2 h exposure in a designated transport hub and, on a separate occasion, in an appointed park. Personal exposures to fine particulate matter (PM2.5), black carbon (BC) and carbon monoxide (CO) were measured. Forced expiratory volume in 1 s (FEV1) and peak expiratory flow (PEF) were assessed pre-, during and post-exposure. Mixed linear models were used to analyze the pulmonary effects of traffic-related air pollutants.
Results
Participants had significantly higher exposures to PM2.5, BC and CO in the transport hub than in the park. Exposure in transport hub induced significant reductions in FEV1 and PEF compared with the park during exposure 1 and 2 h. The reductions were significant associated with traffic-related air pollutants. For instance, per 10 μg/m3 increment in PM2.5 was associated with −0.15 % (95 % CI −0.28, −0.02 %) reduction in FEV1 during exposure 2 h. However, effects became attenuate after 2 h exposure.
Conclusions
Short-term exposure in transport hub had acute reduction effects on pulmonary function. More attention should be paid to the health effects of exposure in the semi-closed traffic micro-environment.
Electronic supplementary material
The online version of this article (doi:10.1007/s12199-016-0531-5) contains supplementary material, which is available to authorized users.
Keywords: Traffic-related air pollution, Pulmonary function, Young healthy adults, Transport hub, Acute effects
Introduction
Traffic emission is an important source of ambient air pollution in urban area [1]. Numerous studies have shown that chronic exposure to traffic-related air pollution was significantly associated with respiratory mortality and morbidity [2–6]. However, exposure to traffic emission, often includes short-term (within hours) exposure to relatively high levels of traffic-related air pollutants [7].
Pulmonary function is an objective marker for respiratory health and a predictor for cardiopulmonary mortality and morbidity [8]. Acute effects on pulmonary function of exposure to traffic exhaust have been studied in controlled chamber studies [9–15]. However, considering the real-world exposure where mixtures of pollutants are more complex, the results of chamber studies may have some difficulties when applied to the exposure in real-world traffic micro-environment.
Nowadays, researchers began to pay attention to the pulmonary effects of exposure to real-world traffic micro-environment. Although some studies have explored the acute effects of short-term exposure to traffic emission on pulmonary function in real-world settings, the results were inconsistent. Some indicated negative effects [16–19], while others reported no acute influence [20–23]. In addition, some positive associations between traffic exposure during cycling and pulmonary function were found [24, 25].
The inconsistence may due to the different status of health. Healthy adults [18, 19] and patients [16, 17] may response differently to traffic exposure. In addition, various activity levels can influence the commuters’ minute ventilations which lead to different inhaled doses of pollutants. However, the effects of inhaled doses were usually not estimated. Furthermore, we noted that the concentrations of traffic-related air pollutants in these studies were relatively low. For instance, the average concentration of fine particle (PM2.5) in high-traffic route was only 5.1 μg/m3 [21].
Beijing is a megacity with about 21 million residents and more than 5 million vehicles in 2014 [26]. The average concentration of PM2.5 in Beijing was 85.9 μg/m3 in 2014 according to the Beijing Environment State Bulletin [27]. Considering traffic emission is the major source of air pollution in Beijing urban area, the levels of PM2.5 can be higher in traffic micro-environments. Transport hub is an important part of urban comprehensive transportation system in Beijing. Commuters can transfer from bus to subway or other commuting modes conveniently in the hub. However, these transport hubs always have semi-closed structure which hindered the diffusion of traffic emission. Thus the concentrations of traffic-related air pollutants can reach high levels. Although commuters’ exposure in the transport hub is short-term, considering numerous number of commuters and their approximate to traffic emission, this will be an important environmental health problem. However, whether short-term exposure in this semi-closed traffic micro-environment can cause acute effects (within hours) on pulmonary function of commuters still needs to be explored.
To explore acute pulmonary effects of exposure to traffic-related air pollution in semi-closed transport hub, pulmonary function of young healthy adults was followed during and after short-term exposure in a large transport hub in Beijing. Young healthy adults were chosen as to avoid the influence of medication. Furthermore, effects of inhaled doses of traffic-related air pollutants were also estimated in addition to the effects of concentrations.
Materials and methods
Study subjects and design
This study was designed as a randomized, crossover study. We recruited 40 young healthy college students from Peking University Health Science Center as the study subjects. Before volunteers were included in the study, they should answer a health screening questionnaire. The inclusion criteria were as follows: no history of smoking; free of respiratory diseases; no medication that might affect pulmonary function; no other chronic diseases; no allergic factors and body mass index (BMI) ≤ 30 kg/m2. About the allergic factors, if the volunteers has diagnosed or self-reported diseases related to allergic factors, including allergic rhinitis, allergic asthma, allergic conjunctivitis, allergic dermatitis and so on, they were politely excluded in the study. Sixty-five college students responded to our recruitment advertisement in the campus bulletin board, and 40 of them met the inclusion criteria and were willing to participate in the study after the study protocols had been explained.
In this study, each participant stayed for 2 h in a designated transport hub, and in an appointed park for 2 h in another exposure scenario. The designated transport hub is the biggest traffic transfer center which named “Dongzhimen Transport Hub” in Beijing, commuters can transfer from bus to subway or airport express in this hub. The buses which powered by diesel in the hub are at idling status most of the time in order waiting for commuters to get on and off. The traffic flow is about 600 buses per hour. The appointed park is traffic-free with an area of 1.7 hectare.
Equal numbers of participants were randomly assigned to each exposure sequence with at least a week interval. Participants were driven for approximately 10 min to the exposure sites from the university campus in a gasoline-powered car. The exposure during travel is comparable in the two exposure scenarios. During the exposure scenarios, exercises were avoided and the participants were at rest status. Pulmonary functions of participants were measured on arrival and hourly during each session, and post-exposure 5, 7, 9 and 22 h when the participants back to campus. Personal exposure to traffic-related air pollution was measured during the 2 h of exposure. The study was carried out in weekdays between May 2012 and October 2012, sandstorm and rainy days were avoided.
The Institutional Review Board of Peking University Health Science Center approved the study, and informed consent was obtained from all individual participants in the study.
Assessments of traffic-related air pollutants
We measured the concentrations of traffic-related air pollutants including PM2.5, black carbon (BC) and carbon monoxide (CO) throughout each exposure session. A specially designed backpack with monitoring instruments was placed beside the participants to measure their personal exposure as described in our previous published paper [28].
Concentrations of PM2.5 were measured by a real-time portable aerosol monitor (Side-Pak, Model AM 510; TSI, Shoreview, MN, USA). Meanwhile, concentrations of BC and CO were measured by a portable, battery-powered micro-Aethalometer (microAeth Model AE51; Magee Scientific, Berkeley, CA, USA) and T15n-enhanced CO instrument (Langan Products, San Franciso, CA, USA), respectively. The measurement interval was set as 1 min. The filters of microAeth Model AE51were changed each measuring day. Considering the influence of meteorological factors, temperature and relative humidity were also measured using HOBO Pro V2 Temp/RH (Onset, Pocasset, MA, USA) in the study.
The instruments were calibrated routinely during the study period before used in the exposure session. Data of measurements were extracted using the corresponding software of each instrument.
Measurements of pulmonary function
Forced expiratory volume in 1 s (FEV1) and peak expiratory flow (PEF) and of each study subjects were measured by an electronic spirometer (Model 2110; Vitalograph Ltd., Buckingham, UK) in the study. Before the formal measurements, trained technicians instructed the study subjects to use the spirometer. A training period for each study subject lasted for about 1 week before the formal study started until the participants were familiar with the FEV1/PEF measuring technique to ensure the quality of the measurements. The applied procedures by the technicians conformed with the 2005 ATS/ERS procedures, and the instruments fulfilled the technical criteria of ATS/ERS.
Spirometric measurements were made before exposure, hourly during each exposure session, and at 5–22 h post-exposure. Each time point included two blows which were automatically recorded into the electronic spirometer with a time point annotation. Data of FEV1/PEF in the electronic spirometer were extracted using the Software for FEV1/PEF Diary (Version 2.03). If the participants made a good test, the extract data annotation will indicate “yes”, otherwise indicate “no”. If the participants had no good test on a specific occasion, they rested for a few minutes, and performed again. The better reading of the blows which presented the pulmonary function of the study subjects more accurately were selected and used in the final data analysis. During the study period, a symptom diary was also used to record any onset of respiratory symptoms (such as cough, wheeze, phlegm, chest tightness, throat irritation or shortness of breath) of the participants.
In total, 560 pulmonary function measurements were taken in the study period. Considering one participant’s FEV1/PEF measurement at post-exposure 5 h, two participants’ FEV1/PEF measurements at post-exposure 7, 9 and 22 h in the transport hub were shown to be not good test as the data extracted from the FEV1/PEF Diary Software indicated, so these data were excluded. Final analysis included 553 measurements in the transport hub exposure occasion and 560 measurements in the park exposure occasion.
Statistical analysis
Descriptive data for exposure variables and pulmonary function were calculated first. The data were presented as means (±SD) for normally distributed variables and median with percentile 25 and percentile 75 for other distributed variables.
Wilcoxon test was used to compare the differences in exposure variables. To control the heterogeneity in pulmonary function levels among different study subjects, FEV1 and PEF data were transformed to percentage changes (%), by subtracting the baseline value of the subject pre-exposure from absolute values during and post-exposure at different time points, than dividing the residuals by the baseline value, and finally multiplying the outcomes by 100 %. Comparisons of pulmonary function deviations were performed with the paired samples t test.
Inhaled doses of traffic-related air pollutants were calculated by multiplying concentrations of the pollutants, participants’ minute ventilation, and duration of exposure, then dividing by body surface area (BSA) to correct for differences in airway epithelial surface area. The value of BSA was calculated using the equation according to the Ref. [29].
According to the classification of activity levels, staying in the transport hub or park is a light intensity activity. Minute ventilations of men and women were 9.6 × 10−3 and 7.7 × 10−3 m3/min respectively at light intensity activity level as the Exposure Factors Handbook of Chinese Population indicated [30].
Mixed linear models were used to analyze effects of concentrations and inhaled doses of traffic-related air pollution on changes in lung function during and after exposure compared with before. In the models, we regressed the percent change from baseline in the lung function against pollutant concentrations or inhaled doses using the mixed data of the transport hub and the park. The mixed linear models included a random intercept for each subject to account for correlations in repeated measurements and a spatial-power covariance structure was used to model correlations between the unequally spaced repeated measurements. Potential confounders were adjusted in the models, including age, sex, BMI, day of week, time of measurement, site, temperature and relative humidity. We assessed the effects of PM2.5, BC, and CO, respectively. Subsequently, we used two-pollutant models, in which two of the three pollutants were analyzed simultaneously.
Final results were reported as percent changes in pulmonary function with 95 % confidence intervals (CIs) for per incremental increases in traffic-related air pollutants.
All statistical analyses were performed by SAS software for Windows (version 9.2; SAS Institute, Cary, NC, USA) and the level of significance was defined as P < 0.05.
Results
Descriptive statistics of air pollution and pulmonary function
Characteristics of the 40 study subjects were presented in Table 1. Nobody used respiratory medication in the study. All of them participated in the two exposure scenarios successfully.
Table 1.
Descriptive characteristics of the subjects (N = 40)
| Characteristica | |
|---|---|
| Age (years) | 24.4 (19, 32) |
| BMI (kg/m2) | 20.9 (18.1, 27.0) |
| Sex, no. (%) | |
| Men | 17 (42.5 %) |
| Women | 23 (57.5 %) |
aMean (min, max) presented unless otherwise specified
Participants had significantly higher exposures to PM2.5, BC and CO in the transport hub than in the park as shown in Table 2. Especially for BC, the average concentration of it was about five times higher in the transfer hub compared with that in the park. The three pollutants measured in the study were highly correlated with Spearman’s rank-correlation coefficients as follows: 0.87 for the correlation of PM2.5 with BC, 0.87 for the correlation of PM2.5 with CO, and 0.81 for the correlation of BC with CO.
Table 2.
Exposure measurements in the transport hub and the park
| Exposure variable | Transport hub | Park | P value | ||||
|---|---|---|---|---|---|---|---|
| Median | P25 | P75 | Median | P25 | P75 | ||
| PM2.5 (μg/m3) | 162.10 | 107.20 | 210.25 | 53.00 | 13.00 | 67.60 | 0.01* |
| BC (μg/m3) | 17.89 | 11.67 | 23.52 | 3.52 | 1.11 | 5.71 | 0.01* |
| CO (ppm) | 2.52 | 1.84 | 2.99 | 1.08 | 0.54 | 1.53 | 0.01* |
| Temp (°C) | 29.33 | 26.27 | 30.39 | 28.39 | 25.66 | 28.40 | 0.51 |
| RH (%) | 53.29 | 46.56 | 58.12 | 39.21 | 29.71 | 52.94 | 0.01* |
P25 percentile 25, P75 percentile 75
* P < 0.05
BC was the carbonaceous constituent of the particulate matter, and it’s usually considered as elemental carbon. Generally, the particle size of BC is smaller than 1 μm. In traffic micro-environment, it mainly comes from the incomplete combustion of fossil fuels especially diesel, and was used as an indicator of traffic particles [31]. We calculated ratios of BC/PM2.5 in different micro-environments. The ratio of BC/PM2.5 in the transport hub was 0.11, which was much higher than the value of 0.06 in the park. This indicated more serious particle pollution from the traffic sources in the transport hub.
As Table 3 indicated both FEV1 and PEF showed similar decreasing trends during exposure in the transport hub, and the reductions gradually became smaller post-exposure. The trends of change in pulmonary function during and post-exposure were smaller in the park.
Table 3.
Pulmonary function measures of the study subjects during and post-exposure in transport hub and park
| Time points | FEV1 (L) | PEF (L/min) | ||||||
|---|---|---|---|---|---|---|---|---|
| Transport hub | Park | Transport hub | Park | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| Pre-exposure | 40 | 3.16 ± 0.68 | 40 | 3.13 ± 0.64 | 40 | 451.03 ± 130.55 | 40 | 448.55 ± 120.49 |
| During exposure 1 h | 40 | 3.05 ± 0.64 | 40 | 3.12 ± 0.64 | 40 | 430.68 ± 126.26 | 40 | 439.98 ± 120.82 |
| During exposure 2 h | 40 | 3.02 ± 0.65 | 40 | 3.08 ± 0.65 | 40 | 427.15 ± 127.27 | 40 | 437.76 ± 122.20 |
| Post-exposure 5 h | 39 | 3.10 ± 0.61 | 40 | 3.09 ± 0.62 | 39 | 438.78 ± 121.29 | 40 | 440.57 ± 120.33 |
| Post-exposure 7 h | 38 | 3.09 ± 0.65 | 40 | 3.10 ± 0.64 | 38 | 443.68 ± 119.36 | 40 | 447.65 ± 125.15 |
| Post-exposure 9 h | 38 | 3.12 ± 0.65 | 40 | 3.09 ± 0.67 | 38 | 437.39 ± 116.07 | 40 | 439.50 ± 120.93 |
| Post-exposure 22 h | 38 | 3.09 ± 0.63 | 40 | 3.11 ± 0.68 | 38 | 436.42 ± 120.24 | 40 | 438.32 ± 124.40 |
SD standard deviation
Fewer than 2 % study subjects reported the onset of respiratory symptoms after exposure. Because of the small proportions, these differences were not further analyzed.
Changes in pulmonary function
Reductions in FEV1 and PEF were calculated as percentage deviations from the baseline. Mean FEV1 was lower during exposure 1 h in both sites. The subsequent decrement was greater in transport hub, with a maximal decline during exposure 2 h (−4.43 % in the transport hub vs. −1.59 % in the park). The differences between sites were significant during exposure 1 and 2 h, however, the differences became smaller and had no significance post-exposure 5–22 h (Table 4).
Table 4.
Comparisons of percent changes in FEV1 during and post-exposure in the transport hub and the park
| Time points | Transport hub | Park | P value | ||
|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | ||
| During exposure 1 h | −3.48 | −4.43, −2.53 | −0.32 | −1.28, 0.64 | 0.01* |
| During exposure 2 h | −4.43 | −5.47, −3.39 | −1.59 | −2.68, −0.50 | 0.04* |
| Post-exposure 5 h | −2.53 | −3.93, −1.13 | −2.28 | −3.90, −0.66 | 0.13 |
| Post-exposure 7 h | −2.22 | −3.56, −0.88 | −1.96 | −3.68, −0.24 | 0.37 |
| Post-exposure 9 h | −1.11 | −2.41, 0.21 | −1.28 | −2.85, 0.29 | 0.67 |
| Post-exposure 22 h | −2.21 | −4.25, −0.17 | −1.64 | −3.55, 0.27 | 0.21 |
95 % CI 95 % confidence intervals
* P < 0.05
The pattern for the percent changes in PEF deviation was similar, with a maximal drop during exposure 2 h (−5.29 % in the transport hub vs. −2.41 % in the park, P < 0.05). The differences in changes in PEF between the two sites were not significant between post-exposure 5–22 h (Table 5).
Table 5.
Comparisons of percent changes in PEF during and post-exposure in the transport hub and the park
| Time points | Transport hub | Park | P value | ||
|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | ||
| During exposure 1 h | −4.51 | −5.66, −3.36 | −1.91 | −3.01, −0.81 | 0.03* |
| During exposure 2 h | −5.29 | −6.44, −3.87 | −2.41 | −3.84, −0.98 | 0.02* |
| Post-exposure 5 h | −2.42 | −4.17, −0.67 | −2.08 | −3.62, −0.54 | 0.08 |
| Post-exposure 7 h | −1.63 | −3.59, 0.33 | −1.76 | −3.72, 0.20 | 0.32 |
| Post-exposure 9 h | −3.02 | −5.03, −1.02 | −2.87 | −4.50, −1.24 | 0.24 |
| Post-exposure 22 h | −3.23 | −5.23, −1.23 | −2.82 | −4.73, −0.91 | 0.36 |
95 % CI 95 % confidence intervals
* P < 0.05
Estimated effects of traffic-related air pollutants on pulmonary function
Effects of concentrations and inhaled doses of traffic-related air pollutants were both estimated.
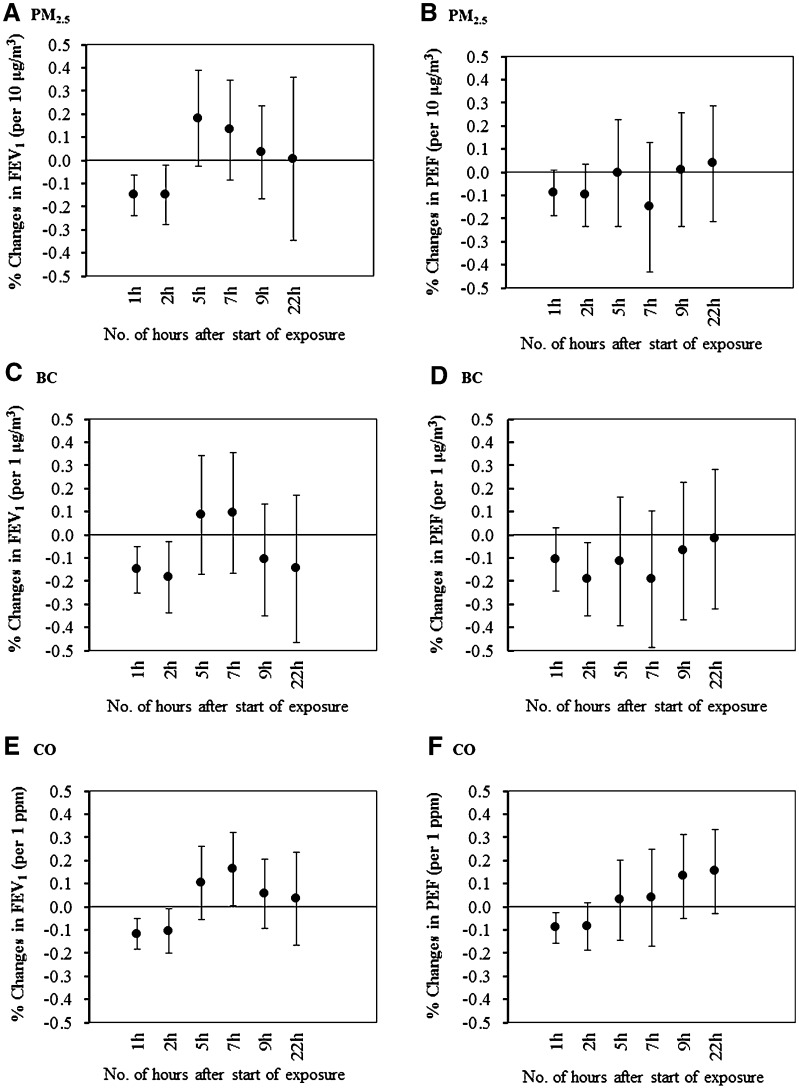
Reductions in FEV1 were associated with PM2.5 concentration during exposure 1 and 2 h (P < 0.05), as Fig. 1 showed that FEV1 decreased by −0.15 % (95 % CI −0.28, −0.02 %) per 10 μg/m3 increment in PM2.5 during exposure 2 h. However, the effects became attenuate and had no significance post-exposure 5–22 h. The reductions were also consistently associated with BC during exposure. For instance, per 1 μg/m3 increment in BC was associated with −0.18 % (95 % CI −0.34, −0.03 %) reduction in FEV1 during exposure 2 h. The reductions were also associated, although less consistently with CO. The pattern for changes in PEF was that BC was significantly associated with PEF during exposure 2 h and CO during exposure 1 h similarly, while the strength was relatively small compared with FEV1 (Fig. 1). After adjustment for co-pollutants in the two-pollutant models, the effects of black carbon remained the most consistent (Table S1 and Table S2 in supplementary material).
Fig. 1.
Percent changes and 95 % CI in FEV1 and PEF associated with per incremental increases in traffic-related air pollutants’ concentrations using the mixed data of the transport hub and the park. (Changes in the FEV1 and PEF were shown according to incremental changes for PM2.5 (a, b), BC (c, d), CO (e, f), Per incremental increases for each pollutant were PM2.5: 10 μg/m3; BC, 1 μg/m3; CO 1 ppm; Time points for abbreviation: 1, 2 h means during exposure 1 and 2 h, respectively; 5, 7, 9, 22 h means post-exposure 5, 7, 9 and 22 h, respectively)
Estimated inhaled doses were mostly significant associated with pulmonary function changes during exposure 1 and 2 h, while the effects became weaken post-exposure as indicated in Table 6.
Table 6.
Percent changes in FEV1 and PEF associated with per incremental increases in inhaled doses
| Time points | FEV1 (%) | PEF (%) | ||||
|---|---|---|---|---|---|---|
| PM2.5 | BC | CO | PM2.5 | BC | CO | |
| During exposure 1 h | −0.11 (−0.21, −0.01)* | −0.14 (−0.25, −0.02)* | −0.07 (−0.13, −0.01)* | −0.08 (−0.18, 0.02) | −0.15 (−0.27, −0.03)* | −0.06 (−0.12, 0.01) |
| During exposure 2 h | −0.23 (−0.36, −0.10)* | −0.23 (−0.38, −0.08)* | −0.16 (−0.24, −0.07)* | −0.14 (−0.28, −0.01)* | −0.23 (−0.38, −0.08)* | −0.12 (−0.21, −0.03)* |
| Post-exposure 5 h | 0.13 (−0.02, 0.30) | 0.07 (−0.13, 0.26) | 0.07 (−0.04, 0.17) | −0.01 (−0.18, 0.17) | −0.09 (−0.30, 0.12) | 0.07 (−0.04, 0.17) |
| Post-exposure 7 h | 0.10 (−0.07, 0.27) | 0.07 (−0.12, 0.27) | 0.11 (−0.02, 0.23) | −0.11 (−0.32, 0.10) | −0.15 (−0.40, 0.11) | 0.03 (−0.11, 0.17) |
| Post-exposure 9 h | 0.03 (−0.13, 0.18) | −0.08 (−0.26, 0.10) | 0.04 (−0.06, 0.14) | 0.01 (−0.18, 0.19) | −0.05 (−0.28, 0.17) | 0.09 (−0.03, 0.21) |
| Post-exposure 22 h | 0.01 (−0.20, 0.21) | −0.11 (−0.35, 0.13) | 0.02 (−0.11, 0.16) | 0.03 (−0.16, 0.22) | −0.01 (−0.24, 0.22) | 0.10 (−0.02, 0.22) |
Percent changes and 95 % CIs were both presented. Increases inhaled doses for each pollutant were PM2.5: per 10 μg/m2; BC, per 1 μg/m2; CO, per 0.1 mg/m2
* P < 0.05
Discussion
In our study, traffic-related air pollutants were at much higher levels in the transport hub compared with concentrations in commuting modes or traffic micro-environment in other countries [21, 25]. The traffic-related air pollution problem including PM2.5 pollution is serious in Beijing. Although the pollution tendency has gradually become better these years with the great effort of implementation of pollution prevention and control measures, air pollution control in Beijing is still a difficult task in a long term. Our study provided evidence of the acute pulmonary effects of traffic-related air pollution in Beijing.
We found that stay for 2 h in the transport hub where the buses were powered by diesel resulted in significant reduction in pulmonary function. Changes were significant greater than those provoked by staying at the park. PM2.5, BC and CO were significantly associated with FEV1 during exposure 1 and 2 h, but not post-exposure 5–22 h. Associations between traffic-related air pollutants with PEF showed similar trends, however, the strength was relatively small compared with FEV1. Although the changes were small, considering commuters always transfer in the transport hub, and PEF and FEV1 are indicators of big airway function in the respiratory system, exposure to high levels of traffic-related air pollutants in this semi-closed traffic micro-environment in Beijing may arguably make this an important risk factor for their respiratory health.
Acute respiratory effects of exposure to diesel exhaust have also been studied in controlled chamber studies [9–15]. Most studies generally did not found effects on lung function parameters in relation to short-term air pollution chamber exposure [9–14]. Compared with the chamber studies, our study explored the pulmonary effects in the natural environment, where more complex traffic-related air pollutants existed, this may lead to the discrepancies between the findings of the chamber studies and our own. The results of our study may be more suitable when translated to real-world exposure.
In our study, reductions of lung function were significantly associated with traffic-related air pollutants, and BC as an indicator of the carbonaceous constituent of particulate was shown to play an important role. In traffic micro-environment, BC mainly comes from the incomplete combustion of fossil fuels especially diesel, and was used as an indicator of traffic particles [31]. The results of our study were consistent with the growing evidence that the adverse effects of particles on pulmonary functions were attributable to those carbonaceous constituent in particles [17, 32]. Furthermore, high concentrations of BC with drastic variations in our study compared with previous research provided new evidence that exposure to high levels of diesel exhaust in transport hub can cause acute reduction effects on pulmonary function in young healthy adults.
We also estimated effects of inhaled doses on pulmonary function. Given the inhaled dose in our study is based on generic literature values for men and women and not on individual measurements, the small difference could be explained by the influence of the consideration of minute ventilation and body surface area. Minute ventilations are different at various activity levels, so when commuters are at different activity levels, their inhaled doses of traffic-related air pollutants can be varied. And body surface area corrected for differences in airway epithelial surface area. Thus, taking the inhaled doses of the commuters exposed in traffic into consideration is more scientific and reasonable to estimate their actual exposure level.
Our study has several strengths. First, marked different levels of traffic-related air pollution in the transport hub and the park provide a good opportunity to explore whether short-term exposure in traffic micro-environment has acute effects on pulmonary function. Second, each young healthy subject served as their own control-reduced bias from unmeasured factors and medication influence. Furthermore, personal exposure measurements of traffic-related air pollutants minimize the exposure error. However, it is important to note several limitations. Given the strong correlations between the traffic-related air pollutants, it was difficult to differentiate the individual effects of each pollutant. And the effects of more exhausted gas from vehicles such as NOx should be estimated in future studies. In addition, we did not measure indicators reflecting airway inflammation and airway resistance in the study. Although air inflammatory biomarkers had been detected in asthmatic subjects or in some chamber studies, measurements in the healthy adults in traffic micro-environment are still limited. In further researches, these biomarkers should be measured simultaneously with pulmonary function to explore the further mechanisms of traffic-related air pollution’s health effects.
In conclusion, semi-closed structure of the transport hub and idling status of the buses made the levels of traffic-related air pollutants at extremely high levels in the transport hub. Short-term exposure in this traffic micro-environment had acute reduction effects on pulmonary function in young healthy adults. Although the changes were modest in healthy individuals, they may become more substantial in susceptible populations, and such influences have important meaning for public health considering numerous number of commuters transfer in this traffic micro-environment. The results imply more attention should be paid to respiratory health effects of exposure in this important transfer link in urban transportation system.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the support from the National Natural Science Foundation of China (Nos. 81072267 and 81502780), the National High Technology Research and Development Program of China (No. 2012AA062804), Beijing Excellent Doctorial Dissertations Fund (No. 20131000109), Young Talent Support Program of Peking University School of Public Health and Young Scientists Lift Plan from China Association for Science and Technology. In addition, the authors thank all the volunteers who took part in this study and Dr. Lu Ma (Department of Epidemiology and Biostatistics, School of Public Health, Wuhan University, Wuhan, China) for calibrating the spirometer routinely during the study period.
Compliance with ethical standards
Conflict of interest
The authors report no declarations of interest. They did not have a financial relationship with the organization that sponsored the research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.World Health Organization . Health effects of transport-related air pollution. Copenhagen: WHO Regional Office for Europe; 2005. pp. 53–67. [Google Scholar]
- 2.Brunekreef B, Beelen R, Hoek G, et al. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in Netherlands: the NLCS-AIR study. Res Rep Health Eff Inst. 2009;139:5–71. [PubMed] [Google Scholar]
- 3.Dong GH, Zhang P, Sun B, Zhang L, Chen X, Ma N, et al. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration. 2012;84:360–368. doi: 10.1159/000332930. [DOI] [PubMed] [Google Scholar]
- 4.Gruzieva O, Bergström A, Hulchiy O, Kull I, Lind T, Melén E, et al. Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology. 2013;24:54–61. doi: 10.1097/EDE.0b013e318276c1ea. [DOI] [PubMed] [Google Scholar]
- 5.Nirel R, Schiff M, Paltiel O. Respiratory hospitalizations of children and residential exposure to traffic air pollution in Jerusalem. Int J Hyg Environ Heatlh. 2015;218:34–40. doi: 10.1016/j.ijheh.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, et al. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res. 2011;111:1222–1229. doi: 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur S, Nieuwenhuijsen MJ, Colvile RN. Fine particulate matter and carbon monoxide exposure concentrations in urban street transport microenvironment. Atmos Environ. 2007;41:4781–4810. doi: 10.1016/j.atmosenv.2007.02.002. [DOI] [Google Scholar]
- 8.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 9.Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27:359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 10.Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, et al. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162:161–166. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- 11.Nordenhäll C, Pourazar J, Ledin MC, Levin JO, Sandström T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001;17:909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 12.Rudell B, Ledin MC, Hammarström U, Stjernberg N, Lundbäck B, Sandström T. Effects on symptoms and lung function in humans experimentally exposed to diesel exhaust. Occup Environ Med. 1996;53:658–662. doi: 10.1136/oem.53.10.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 14.Stenfors N, Nordenhäll C, Salvi SS, Mudway I, Söderberg M, Blomberg A, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J. 2004;23:82–86. doi: 10.1183/09031936.03.00004603. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Barregard L, Nielsen J, Gudmundsson A, Wierzbicka A, Axmon A, et al. Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part Fibre Toxicol. 2013;10:60. doi: 10.1186/1743-8977-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson BM, Grunewald J, Sköld CM, Lundin A, Sandström T, Eklund A, et al. Limited airway effects in mild asthmatics after exposure to air pollution in a road tunnel. Respir Med. 2010;104:1912–1918. doi: 10.1016/j.rmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 18.Strak M, Janssen NA, Godri KJ, Gosens I, Mudway IS, Cassee FR, et al. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project. Environ Health Perspect. 2012;120:1183–1189. doi: 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuurbier M, Hoek G, Oldenwening M, Meliefste K, van den Hazel P, Brunekreef B. Respiratory effects of commuters’ exposure to air pollution in traffic. Epidemiology. 2011;22:219–227. doi: 10.1097/EDE.0b013e3182093693. [DOI] [PubMed] [Google Scholar]
- 20.Fan ZT, Meng Q, Weisel C, Laumbach R, Ohman-Strickland P, Shalat S, et al. Acute exposure to elevated PM2.5 generated by traffic and cardiopulmonary health effects in healthy older adults. J Expo Sci Environ Epidemiol. 2009;19:525–533. doi: 10.1038/jes.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarjour S, Jerrett M, Westerdahl D, de Nazelle A, Hanning C, Daly L, et al. Cyclist route choice, traffic-related air pollution, and lung function: a scripted exposure study. Environ Health. 2013;12:14. doi: 10.1186/1476-069X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier R, Cascio WE, Ghio AJ, Wild P, Danuser B, Riediker M. Associations of short-term particle and noise exposures with markers of cardiovascular and respiratory health among highway maintenance workers. Environ Health Perspect. 2014;122:726–732. doi: 10.1289/ehp.1307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarnat JA, Golan R, Greenwald R, Raysoni AU, Kewada P, Winquist A, et al. Exposure to traffic pollution, acute inflammation and autonomic response in a panel of car commuters. Environ Res. 2014;133:66–76. doi: 10.1016/j.envres.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strak M, Boogaard H, Meliefste K, Oldenwening M, Zuurbier M, Brunekreef B, et al. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup Environ Med. 2010;67:118–124. doi: 10.1136/oem.2009.046847. [DOI] [PubMed] [Google Scholar]
- 25.Weichenthal S, Kulka R, Dubeau A, Martin C, Wang D, Dales R. Traffic-related air pollution and acute changes in heart rate variability and respiratory function in urban cyclists. Environ Health Perspect. 2011;119:1373–1378. doi: 10.1289/ehp.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beijing Municipal Bureau of Statistics . Beijing statistical yearbook 2014. Beijing: China Statistics Press; 2014. pp. 56–69. [Google Scholar]
- 27.Beijing Municipal Environmental Protection Bureau. Beijing Environment State Bulletin. 2014. http://www.bjepb.gov.cn.
- 28.Huang J, Deng F, Wu S, Lu H, Hao Y, Guo X. The impact of short-term exposure to noise and traffic-related air pollution on heart rate variability in young healthy adults. J Expo Sci Environ Epidemiol. 2013;23:559–564. doi: 10.1038/jes.2013.21. [DOI] [PubMed] [Google Scholar]
- 29.Zuurbier M, Hoek G, Oldenwening M, Meliefste K, Krop E, van den Hazel P, et al. In-traffic air pollution exposure and CC16, blood coagulation, and inflammation markers in healthy adults. Environ Health Perspect. 2011;119:1384–1389. doi: 10.1289/ehp.1003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Environmental Protection of the People’s Republic of China . Exposure factors handbook of Chinese population (adults) Beijing: China Environmental Sciences Press; 2013. pp. 12–44. [Google Scholar]
- 31.World Health Organization . Health effects of black carbon. Copenhagen: WHO Regional Office for Europe; 2012. pp. 23–33. [Google Scholar]
- 32.Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.