Abstract
The present study was undertaken to evaluate antimicrobial and antioxidant effect of essential oils on the quality of fresh (raw, ready to cook) chicken sausages. Several preliminary trials were carried out to optimize the level of four essential oils viz., clove oil, holybasil oil, thyme oil and cassia oil and these essential oils were incorporated at 0.25, 0.125, 0.25 and 0.125%, respectively in fresh chicken sausages. Quality evaluation and detailed storage stability studies were carried out for fresh chicken sausages for 20 days at refrigeration temperature (4 ± 1 °C). Refrigerated storage studies revealed that TBARS of control was significantly higher than treatment products whereas, total phenolics and DPPH activity was significantly lower in control. Among treatments, clove oil products had significantly lower TBARS but higher total phenolic content and DPPH activity followed by cassia oil, thyme oil and holybasil oil products. Microbial count of essential oil incorporated products were significantly lower than control and remained well below the permissible limit of fresh meat products (log107 cfu/g). Cassia oil products were observed with better anti-microbial characteristics than clove oil products at 0.25% level of incorporation, whereas, thyme oil products were better than holy basil oil products at 0.125% level. Storage studies revealed that clove oil (0.25%), holy basil oil (0.125%), cassia oil (0.25%) and thyme oil (0.125%) incorporated aerobically packaged and refrigerated fresh chicken sausages had approx. 4–5, 2–3, 5–6 and 2–3 days longer shelf life than control, respectively.
Keywords: Fresh chicken sausage, Essential oils, Storage stability, Anti-oxidant activity, Sensory evaluation, Shelf life
Introduction
Poultry meat has become a very popular food commodity around the world and its consumption has continuously increased over the last 3–4 decades. Some of the reasons for the popularity are the relatively low cost of production, low fat content and high nutritional value of poultry meat. Presently, chicken meat has become the highest contributor (35%) to total meat production in India and occupies important components of Indian non-vegetarian diet. Sausages are one of the oldest processed meat products and have been famous in many areas since long times. Emulsion based sausages are more relished in developing countries whereas, in developed countries, fresh sausages are more popular than emulsion based sausages. Fresh sausage is a sausage ‘‘made from selected cuts of fresh meat (not cooked or cured) and stored at refrigeration or freezing temperature prior to being consumed” (Liu et al. 2009). Italian sausages, bratwurst, bockwurst and thuringer (fresh), are some common examples of fresh sausages. Their maximum storage life is normally three days at +4 °C or below and if the product is deep-frozen at −18 °C, the storage life can be extended up to three months. Processed chicken products consumption has also dramatically increased over the last decades viz, patties, sausages, nuggets, meat balls etc. Since, poultry meat products possess many desirable nutritional characteristics, such as low lipid content and high polyunsaturated fatty acid content (Nkukwana et al. 2014), as a result prolonging the shelf life of perishable chicken products is one of the main concern for the poultry industry.
Lipid oxidation is one of the main causes of chemical degradation in food. The process of food (fat) oxidation produces a rancid flavour and decreases the sensory scores and nutritive value of the products, thus making them unacceptable to consumers (Belitz H et al. 2009). To inhibit or reduce oxidative deterioration, effective antioxidants are incorporated in such products. Antioxidant application is the simplest technique for reducing lipid oxidation. Anti-oxidants are organic molecules of either synthetic or natural origin which are capable of scavenging the active forms of oxygen involved in the progression of oxidation reaction (Qwele et al. 2013). Synthetic antioxidants, such as BHA, BHT and propyl gallate (PG), have been utilized in various foods to reduce the oxidation process. Consumers reject the incorporation of synthetic compounds in their food because of the potential harmful consequence of these compounds. Thus, there is need of identification of novel and natural source of antioxidants that would serve as alternatives to the synthetic compounds. Application of natural anti-oxidants is believed to be essential to inhibit or delay the lipid oxidation reaction in meat and meat products (Falowo et al. 2014). Researchers have directed their attention in recent years towards extracts from herbs and spices which have been used traditionally for centuries to prolong the shelf life and improve the palatability of foods. Several studies have been conducted during last decades regarding utilization of essential oils as natural compounds with distinguished properties (antioxidant and antimicrobial activity).
Essential oils (EOs) are aromatic and volatile oily extracts obtained from medicinal and aromatic plant materials, including flowers, buds, roots, bark, and leaves (Hyldgaard et al. 2012) by means of expression, fermentation, extraction or steam distillation. These are one of the best alternatives for synthetic anti-oxidants, owing to their strong antimicrobial activities. Essential oils are classified as GRAS by the USFDA. However, before their use as “natural preservatives”, there if requirement of careful investigation so that when added to the food product, the latter appeals sensorially acceptable to the consumer.
Cassia (Cinnamomum cassia) is important not only as a food spice but it also possess medicinal properties viz, antimicrobial, anti-tumorigenic, anti-inflammatory and anti-diabetic (Verspohl et al. 2005). It is obtained by steam distillation from the stem bark of C. cassia. The main constituents of thyme include thymol, carvacrol and flavonoids (Burt et al. 2005). Thymol (active phenolic in thyme essential oil) disrupts vesicles and cell membranes, and impairs ergosterol biosynthesis thus, consequently affects cell membrane integrity (Ahmad et al. 2011). The major component of clove oil is usually considered to be eugenol, with âcaryophyllene and eugenyl acetate, being present although in lower concentrations (Alma et al. 2007). Eugenol treatment alters cell membrane and cell wall structures of proliferating resulting in the release of cellular content (Bennis et al. 2004). Methyl chavicol, methyl cinnamate, methyl eugenol, citral, and linalool are generally the main chemotypes in basil Holy basil (Ocimum sanctum) is known as tulsi and is reported to possess antibacterial and insecticidal properties (Carovic-Stanko et al. 2010). Considering the fact that there is a need for research regarding the use of natural additives or alternative methods in order to extend/prolong shelf life and/or improve food safety, the present study is carried out to evaluate antimicrobial and antioxidant effect of essential oils on the quality of fresh chicken sausages.
Materials and methods
Raw materials
Chicken meat and casings
Dressed spent hens were procured from Central Avian Research Institute (CARI), Izatnagar within 4 h of slaughter. These carcasses were brought to abattoir of LPT Division, IVRI and manually deboned and trimming of tendons, separable connective tissue and body fat was also done under hygienic conditions. Chicken meat obtained was packaged in clean low density polyethylene bags (200 gauge) and kept for conditioning in a refrigerator at 4 °C for about 24 h. Thereafter, the samples were transferred and kept in deep freezer (Blue Star, FS345, Denmark) for storage at −18 ± 2 °C till further use. Cellulose casings (C17 × 84ft.) were purchased from Euromate Food Tech. Pvt. Ltd., New Delhi.
Essential oils and non meat ingredients
Food grade essential oils were purchased from reputed commercial suppliers. Refined salt (Tata Salt, Tata Chemicals Ltd., Mumbai) was purchased from local market of Bareilly. Food additives incorporated in the formulations were of food grade quality and procured from reputed firms i.e., sodium nitrite (Merck Specialities Pvt. Ltd., Mumbai), sodium tripolyphosphate (Central Drug House Pvt. Ltd., (CDH) New Delhi).
Chemicals
All the chemicals and reagents for laboratory analysis of chicken sausages were of analytical grade and procured from standard firms viz, Qualigens, GS chemicals, Sigma, CDH and Merck. The culture media and their additives used in the study were procured from Himedia® Laboratories, Mumbai.
Packaging materials
Linear low density polyethylene films (200 gauge) used for aerobic packaging of the products were purchased from local market of Bareilly.
Processing of fresh chicken sausages
Fresh chicken sausages were prepared by mixing various ingredients in a mixer (Table 1).
Table 1.
Composition of fresh chicken sausages
| Ingredients | Percentage (%) |
|---|---|
| Lean meat | 92 |
| Ice flakes | 6 |
| Sodium chloride | 1.6 |
| STPP | 0.4 |
| Sodium nitrite (ppm) | 150 |
The frozen meat was partially thawed at refrigeration temperature (4 ± 1 °C) for about 16–18 h and cut into intact pieces. It was divided into different batches (500 g each) for control and treatments. Individual essential oils were applied on the surface of thawed meat pieces (sterile distilled water was used for control samples). Then, the meat samples were cut into small pieces with the help of autoclaved knife. Pre-weighed quantity of chicken meat, salt and sodium tripolyphosphate was added and mixing was carried out for about 2 min in a mixer. It was mixed again for 2 min after the addition of ice flakes containing dissolved nitrite. Meat mix was then stuffed into 20 mm casings using manual sausage filler and linked at about 12 cm intervals. Sausages were aerobically packaged for further analysis and kept at refrigeration temperature (4 ± 1 °C). Before sensory evaluation, the sausages were cooked in pressure free steam for 45 min. A desired core temperature (72 °C) of cooking was maintained during cooking and temperature was monitored using a probe thermometer (digital thermometer, WT-1, China). The sausages were cooled to room temperature and peeled off the casings (Fig. 1).
Fig. 1.
Flow diagram for preparation of fresh chicken sausages
Detailed study
Frozen chicken meat (1000 g packaged individually) were unpacked and assigned randomly to four different treatments viz, clove oil (0.25%), holy basil oil (0.125%), cassia oil (0.25%) and thyme oil (0.125%). The fresh sausages were then processed as explained earlier, then aerobically packaged in LDPE films and stored at refrigeration temperature (4 ± 1 °C). Physico-chemical qualities viz, pH, TBARS value, moisture content, anti-oxidant activity (total phenolics, DPPH radical scavenging activity), sensory attributes (cooked product) and microbiological characteristics (total plate count, yeast and mould count, psychrophilic count and Coliforms count) were assessed on 0, 3, 5, 10, 15 and 20 days in aerobically packaged products.
Physico-chemical analysis
Yield (in percent)
Yield of chicken sausages was calculated on percent basis based on weight of sausages before cooking which is given below:
pH
pH of chicken sausages was determined as per the procedure of Troutt et al. (1992). 10 g of sample was blended with 50 ml of distilled water for 1 min using an Ultra Turrax tissue homogenizer (Model IKA®T 18, Janke and Kenkel, IKA Labor Technik, Germany). The pH of the homogenate was recorded by immersing a combined electrode of a digital pH meter (Model CP 901, Century Instrument Ltd. India).
Thiobarbituric acid reacting substances (TBARS) number
The TBARS value of chicken sausages was determined as per the method described by Tarladgis et al. (1960). Optical density was recorded using spectrophotometer (Model: Beckman DU 640, USA) at 538 nm. The O.D. was multiplied by the factor 7.8 and TBARS value was expressed as mg malonaldehyde/kg of sample.
Total phenolics
Preparation of samples for estimating total phenolics was done according to the method described by Naveena et al. (2008) and total phenolic content in fresh chicken sausages were quantified using the Folin–Ciocalteu colorimetric method as described by Makkar (2000). Absorbance of the resulting blue colour was measured using spectrophotometer (Model: Beckman DU 640, USA) at 760 nm against blank after incubation for 40 min at room temperature.
DPPH radical scavenging activity
Measurements with the DPPH assay were taken using a method used by Tepe et al. (2005). 0.1 g of the samples and 5 ml of 0.004% DPPH in methanol were added to a test tube. The samples were subjected to homogenization for 30 s using Ultra Turrax tissue homogenizer (Model IKAT® 18, IKA Labor Technik, Germany). The samples were kept at room temperature for 30 min with constant mixing. Absorbance was measured using spectrophotometer (Model: Beckman DU 640, USA) at 517 nm. Methanol was used as a blank and measurements were expressed as absorbance. Decrease in absorbance indicated increased antioxidant activity level of the essential oils.
Microbiological analysis
All the microbiological parameters were determined by following standard methods of APHA (2001).
Preparation of serial dilutions
Laminar flow chamber (Model: YS1-188, Yarco Sales, Pvt. Ltd., New Delhi) was pre-sterilized by ultra-violet radiation for 15 min before use. About 10 g of sample was weighed aseptically and transferred to a sterile plastic container containing 90 ml of sterile 0.1% peptone water (Hi-Media®). The sample was homogenized for 2 min using Ultra Turrax tissue homogenizer (Model IKA®T 18, Janke and Kenkel, IKA Labor Technik, Germany) under aseptic condition. Serial dilutions of 10−1, 10−2, 10−3 and so on, were made as per requirement.
Total plate count, Psychrophilic count, Yeast and mould count and coliform count
Plate Count Agar (Hi Media, Mumbai) for total plate count (TPC) & psychrophilic count (PC), potato dextrose agar for yeast and mould count (YMC) and violet red bile agar for coliform count was prepared for total plate count and psychrophilic count, yeast and mould count and coliform count respectively, as per manufacturer’s instructions. The plates were incubated at 35 ± 2 °C for 24 h for checking sterility. 100 µl from each dilution was poured into sterile petriplates and spread gently with the help of sterile L-spreader. The plates were incubated at 35 ± 2 °C for 48 h in case of total plate count & coliform count, at 4–7 °C for 7 days in case of psychrophilic count and at 25 °C for 5 days in case of yeast and mould count. Plates showing 30–300 colonies were counted. The number of colonies was multiplied by the reciprocal of the dilution and expressed as log10 cfu/g.
Sensory evaluation
Sensory attributes for chicken sausages were evaluated using an eight point descriptive scale (Keeton 1983) where 8 score was given for an excellent product and 1 was for extremely poor product. The sensory panelists consisted of scientists and post graduate students of Livestock Products Technology Division of IVRI. The panelists were briefed about the nature of the experiments without disclosing the identity of the samples. The samples were served warm (40–60 °C) by pre-heating the samples in microwave oven (LG®, Model MC-7148 MS, 1200 W microwave power, India) for 2 min and sensory evaluation was conducted around 3.30–4 pm. Panelists were requested to evaluate using sensory evaluation proforma for different attributes such as general appearance, flavour, binding, texture, juiciness and overall acceptability of the product. Plain water was provided to rinse the mouth between tasting of each sample.
Statistical analysis
Samples were taken in duplicates for all the parameters except sensory attributes, where each sample was evaluated by seven sensory panellists.
Three trials were conducted for each experiment, total being six observations for anti-oxidant and microbiological parameters and 21 observations for sensory evaluation (n = 6 and n = 21) for consistency of the results. The data generated from various trials under experiments were pooled and analyzed by statistical method of one way-ANOVA, two way-ANOVA and Mean ± S.E using Statistical Package for the Social Sciences (SPSS) software package developed as per the procedure of Snedecor and Cochran (1989). Duncan’s multiple range test and critical difference were determined at the 5% significance level. Statistically analysed data was tabulated and interpreted.
Results and discussion
Physico-chemical characteristics
pH
During storage, pH followed an increasing trend throughout the storage period in all the samples, although the rate of increase was slower in case of EO treated products (Table 2). Control product was observed with significant increase (P < 0.05) in pH at each interval of storage whereas, treatment products showed significant increase (P < 0.05) in pH from day 10 onwards. It was also found that control had significantly higher (P < 0.05) pH value than EO treated products from day 3 onwards. Among treatment products, non-significant difference (P > 0.05) was observed in pH values up to day 15, however cassia oil products had significantly lower (P < 0.05) pH than clove oil incorporated products on day 20 of storage. During storage, increase in pH might be attributed to the microbial metabolites and endoprotease activity (Mokhtar et al. 2014). As stated by Gill (1983), bacteria after utilizing glucose, acts on amino acids which were released during protein breakdown and thus ammonia accumulates, which is a product of amino acid degradation and thus, pH rises. Similar results have also been reported by Mokhtar et al. (2014) in fresh beef patties during storage period of 9 days and 15 days, respectively. Lower pH in treatment products as compared to control suggested that active components of EOs such as phenols, terpenes, p-cymene, terpenoids might have acted effectively to inhibit or reduce the development of spoilage micro-organisms (Wojciak et al. 2011). Similar results were also obtained by Naveena et al. (2008) in BHT incorporated raw chicken patties.
Table 2.
Effect of optimum level of different essential oils on physico-chemical characteristics of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C) (Mean ± S.E.)*
| Parameter | Refrigerated storage period (days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 10 | Day 15 | Day 20 | |
| pH | ||||||
| Control | 6.12e ± 0.02 | 6.21d1 ± 0.01d1 | 6.31c1 ± 0.03c1 | 6.42b1 ± 0.02b1 | 6.56a1 ± 0.04a1 | NE |
| Clove oil | 6.10b ± 0.01bc | 6.16b12 ± 0.02b12 | 6.25b2 ± 0.01b2 | 6.37b12 ± 0.04b12 | 6.44ab4 ± 0.03ab4 | 6.56a2 ± 0.02a2 |
| Holy basil oil | 6.14b ± 0.01bc | 6.18b12 ± 0.02b12 | 6.26b2 ± 0.02b2 | 6.35b2 ± 0.04b2 | 6.43a4 ± 0.03a4 | NE |
| Cassia oil | 6.11b ± 0.02bc | 6.16b12 ± 0.06bc12 | 6.27b12 ± 0.04b12 | 6.36b12 ± 0.06b12 | 6.46ab34 ± 0.03ab34 | 6.59a1 ± 0.02a1 |
| Thyme oil | 6.13b ± 0.07bc | 6.17b12 ± 0.05bc12 | 6.27b12 ± 0.06b12 | 6.38b12 ± 0.02b12 | 6.49a23 ± 0.04a23 | NE |
| TBARS (mg malonadehyde/kg) | ||||||
| Control | 0.21e1 ± 0.02e1 | 0.44d1 ± 0.04d1 | 0.74c1 ± 0.04c1 | 1.12b1 ± 0.06b1 | 1.61a1 ± 0.04a1 | NE |
| Clove oil | 0.16f23 ± 0.03f23 | 0.25e23 ± 0.01e23 | 0.37d34 ± 0.05d34 | 0.60c34 ± 0.02c34 | 0.83b4 ± 0.04b4 | 1.53a12 ± 0.03a12 |
| Holy basil oil | 0.18e12 ± 0.03e12 | 0.30d2 ± 0.03d2 | 0.54c2 ± 0.05c2 | 0.83b2 ± 0.04b2 | 1.18 ± 0.04a2 | NE |
| Cassia oil | 0.16d23 ± 0.02d23 | 0.26d23 ± 0.08d23 | 0.39cd34 ± 0.07 cd34 | 0.64bc45 ± 0.03bc45 | 0.86b4 ± 0.06b4 | 1.62a1 ± 0.03a1 |
| Thyme oil | 0.15e23 ± .07e23 | 0.24d23 ± 0.05d23 | 0.45c3 ± 0.03c3 | 0.72b3 ± 0.05b3 | 1.04a3 ± 0.02a3 | NE |
| Moisture content (%) | ||||||
| Control | 71.42 ± 0.43 | 71.65 ± 0.81 | 71.29 ± 1.14 | 70.84 ± 0.96 | 70.49 ± 0.96 | NE |
| Clove oil | 71.83a ± 0.91a | 72.10a ± 0.56a | 71.68a ± 0.86a | 71.47a ± 1.16a | 71.17ab ± 0.97ab | 70.71b ± 0.67b |
| Holy basil oil | 71.64 ± 0.84 | 71.97 ± 1.01 | 71.51 ± 0.72 | 71.16 ± 0.88 | 70.79 ± 0.88 | NE |
| Cassia oil | 71.59a ± 0.76a | 71.85a ± 0.89a | 71.52a ± 0.62a | 71.13a ± 0.93a | 70.74ab ± 0.84ab | 70.45b ± 0.48b |
| Thyme oil | 71.43 ± 0.88 | 71.70 ± 0.62 | 71.37 ± 0.41 | 70.98 ± 1.06 | 70.54 ± 0.84 | NE |
| Cooking yield (%) | ||||||
| Control | 81.78 ± 0.67 | 81.67 ± 0.51 | 81.38 ± 0.93 | 80.83 ± 0.70 | NE | – |
| Clove oil | 81.94 ± 0.42 | 81.89 ± 0.63 | 81.67 ± 0.43 | 81.52 ± 0.40 | 81.26 ± 0.37 | – |
| Holy basil oil | 81.06 ± 0.72 | 81.01 ± 0.91 | 80.93 ± 0.84 | 80.79 ± 0.65 | NE | – |
| Cassia oil | 82.32 ± 0.47 | 82.28 ± 0.87 | 82.19 ± 0.84 | 82.02 ± 0.81 | 80.91 ± 0.52 | – |
| Thyme oil | 80.29 ± 0.60 | 80.23 ± 0.66 | 80.12 ± 0.67 | 80.04 ± 0.71 | NE | – |
n = 6
NE not estimated, TBARS Thiobarbituric acid reacting substances
* Mean ± S.E. bearing different superscripts row wise (alphabet) and column wise (numeral) differ significantly (P < 0.05)
TBARS
Significant increase (P < 0.05) in TBARS values of all the products was observed with increase in storage days, however rate of increase was comparatively slower in case of treatment products indicating more oxidative stability of treatment products (Table 2). During entire storage, significant increase (P < 0.05) in TBARS was observed in control, clove oil, holy basil oil and thyme oil products at each interval of storage period, however cassia oil products showed significant increase (P < 0.05) from day 5 onwards. Results also indicated that control had significantly higher (P < 0.05) TBARS than all treatment products throughout storage period. Among treatments, significant difference (P < 0.05) in TBARS was observed from day 5 onwards, the value being significantly lower (P < 0.05) for clove oil products. Among treatment products, clove oil products showed least increase in TBARS throughout storage period followed by cassia oil products. The general decreasing TBARS was in the order holy basil oil > thyme oil > clove oil = cassia oil products.
During storage, increase in TBARS of products could be due to increased oxidation of lipids and volatile metabolites production in the presence of oxygen during aerobic storage (Sharma et al. 2014). Reduction of TBARS in treatment products might be attributed to polyphenols present in EOs that have anti-oxidant effects (Jayawardana et al. 2015). Phenolic compunds (secondary metabolites in plants) are capable of inhibiting oxidation while they get oxidised in the process (Falowo et al. 2014). They delay or inhibit oxidation mainly by two processes: either by acting as electron donor to terminate oxidation cycle (Allen and Cornforth 2010) or by removing free radical initiators (Antolovich et al. 2002). These results are in good agreement with Georgantelis et al. (2007) who observed lower TBARS values for fresh pork sausage treated with rosemary extract and chitosan. Addition of rosemary and carnosine to beef patties also resulted in significant reduction (P < 0.05) in TBARS during storage (Sanchez-Escalante et al. 2001). The difference in TBARS value among treatments might be attributed due to difference in composition of various EOs which resulted in different anti-oxidant behaviour of EOs. However, it is clear that fresh chicken sausages with clove oil and cassia oil exhibited low and acceptable TBARS values i.e. less than 0.9 mg MDA/kg meat. Estevez et al. (2007) also reported that natural anti-oxidant (Sage and rosemary essential oils in their study) were more effective protetctors against oxidation in liver pate as they inhibited the generation of malonaldehyde than BHT.
Moisture content
During storage period, gradual non-significant (P > 0.05) increase in moisture content was observed in all the samples up to day 3, however after day 3 of storage, moisture content decreased in all samples up to end of storage period (Table 2). Significant decrease (P < 0.05) was noticed in moisture content of clove oil and cassia oil products from day 15 onwards. Results also revealed that there was no significant difference (P > 0.05) in moisture content between control and treatment products. Non-significant difference (P > 0.05) was also observed among treatment products. The mean moisture content obtained in the present study was in accordance with Serpen et al. (2012). Non-significant increase in moisture content of products up to day 3 of storage might be due to the effect of salt in increasing binding of water to protein molecules (Sampaio et al. 2012). However, the gradual decrease in moisture content of the products after day 3 of storage could be due to evaporative loss through packaging material during aerobic packaging and oxidative damage to the sarcoplasm. From the above results, it can be concluded that EO incorporation in meat products had no effect on moisture content of samples throughout the storage period. Similar results have been reported by Siewe et al. (2015) in raw beef patties incorporated with S. aromaticum and C. citrates essential oil.
Product yield
Gradual non-significant (P > 0.05) decrease was noticed in product yields of all fresh chicken sausages during storage period (Table 2). There was no significant difference (P > 0.05) between product yields of control and treatment products throughout storage period. Among treatments, no significant difference (P > 0.05) was found in product yields. Decrease in product yields of products with increase in storage period might be related to decrease in moisture content of product. Naveena et al. (2008) had also reported no significant difference (P > 0.05) in yields between control, pomegranate rind powder extract and vitamin C incorporated chicken patties. Among treatments, variation in product yields of products might be attributed to the differences in various essential oil structures that affect polyphenol-protein binding structures (Naveena et al. 2008).
Anti-oxidant activity
Total phenolics
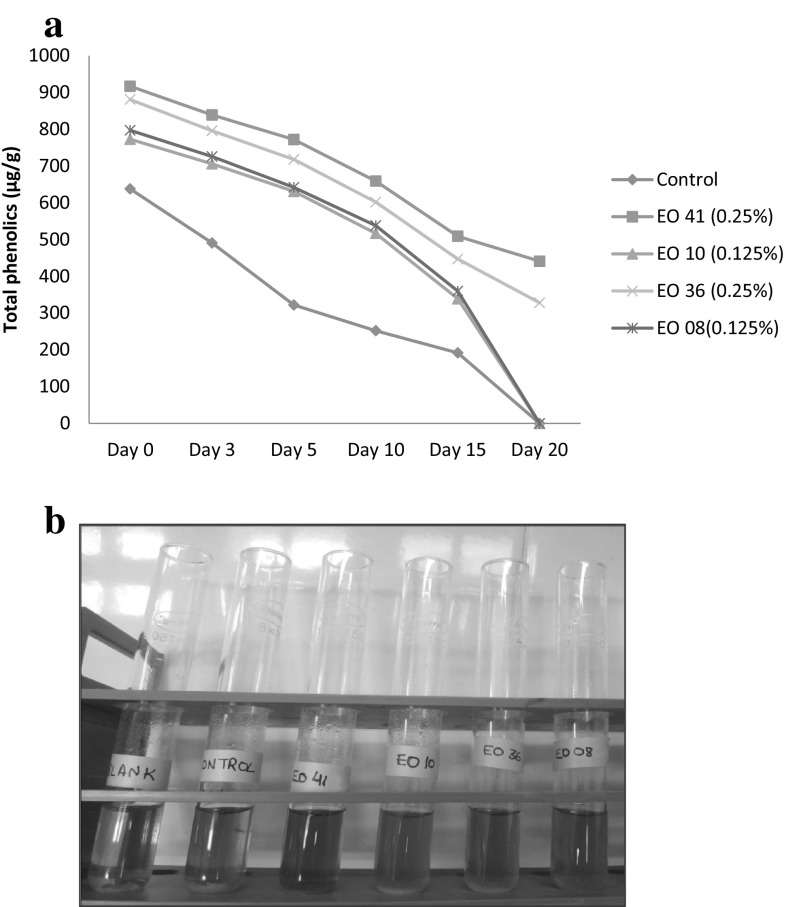
With advancement in storage period, phenolic content decreased significantly (P < 0.05) in all fresh chicken sausage products at each interval of storage period (Table 3). However, the rate of decrease of total phenolics content was slower in treatment products as compared to control indicating that treatment products retained significantly higher (P < 0.05) total phenolics content up to end of storage period. In addition to this, it was also observed that control had significantly lower (P < 0.05) total phenolics content than treatment products throughout storage period (Fig. 2). Significant difference (P < 0.05) in total phenolics content was also observed among treatment products. Clove oil products had significantly higher (P < 0.05) total phenolics content followed by cassia oil products throughout the storage period. Holy basil oil and thyme oil products had comparable total phenolics content on day 0 and day 10 of storage. However, the general decreasing order of total phenolic content among treatments was clove oil > cassia oil > thyme oil > holy basil oil.
Table 3.
Effect of optimum level of different essential oils on anti-oxidant activity of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C) (Mean ± S.E.)*
| Parameter | Refrigerated storage period (days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 10 | Day 15 | Day 20 | |
| Total phenolics (µg/g) | ||||||
| Control | 638a5 ± 3.23 | 491b5 ± 1.41 | 322c5 ± 4.62 | 252d4 ± 5.26 | 192e5 ± 3.03 | NE |
| Clove oil | 917a1 ± 2.13 | 839b1 ± 3.52 | 772c1 ± 2.74 | 659d1 ± 4.00 | 509e1 ± 3.67 | 441f1 ± 2.07 |
| Holy basil oil | 773a34 ± 3.08 | 706b4 ± 4.19 | 631c4 ± 1.95 | 517d3 ± 2.94 | 339e4 ± 2.92 | Ne |
| Cassia oil | 881a2 ± 4.73 | 796b2 ± 2.30 | 718c2 ± 3.13 | 602d2 ± 1.87 | 447e2 ± 4.03 | 328f2 ± 5.14 |
| Thyme oil | 797a3 ± 4.67 | 726b3 ± 3.16 | 642c3 ± 3.43 | 538d3 ± 3.37 | 360e3 ± 5.14 | NE |
| DPPH (% inhibition) free radical scavenging activity | ||||||
| Control | 24.32a4 ± 0.93 | 20.97b3 ± 0.49 | 16.87c4 ± 0.82 | 14.12d4 ± 1.04 | 11.67e5 ± 0.47 | NE |
| Clove oil | 29.76c1 ± 0.78 | 31.76b1 ± 0.91 | 34.34a1 ± 0.59 | 28.34c1 ± 0.78 | 23.89d1 ± 0.28 | 17.79e1 ± 1.17 |
| Holy basil oil | 25.05a3 ± 1.11 | 24.87a2 ± 0.53 | 23.17b3 ± 0.68 | 20.03c3 ± 0.53 | 13.54d34 ± 0.92 | NE |
| Cassia oil | 27.78c2 ± 0.89 | 30.02ab1 ± 1.26 | 33.89a1 ± 0.97 | 25.78d2 ± 0.61 | 21.78e2 ± 0.74 | 14.13f2 ± 0.88 |
| Thyme oil | 24.68c34 ± 0.74 | 26.39b2 ± 0.74 | 28.34a2 ± 0.51 | 21.41d3 ± 0.50 | 14.97e3 ± 0.66 | NE |
n = 6
NE not estimated, DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging
* Mean ± S.E. bearing different superscripts row wise (alphabet) and column wise (numeral) differ significantly (P < 0.05)
Fig. 2.
Effect of optimum level of different essential oils (EOs) on total phenolic content of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C). a Graph representing the trend of total phenolics during storage of fresh chicken sausages. b Test-tubes containing samples of fresh chicken sausages (DAY 0). Dark green colour represents more total phenolic content in the sample (EO 41 = Clove essential oil; EO 10 = Holy basil essential oil; EO36 = Cassia essential oil and EO 08 = Thyme essential oil)
During storage, slower rate of decrease in total phenolic content of treatment products as compared to control could be due to the presence of bioactive compounds in essential oils with antioxidant activities which might interfere with propagation reactions (Russo et al. 2000) besides inhibiting the enzymatic systems involved in initiation reactions, or they could act as hydrogen donators, free radical scavengers, metallic ion chelators or as substrate of radicals such as superoxide or hydroxyl. Higher total phenolic content in treatment products might be due to the fact that essential oils are composed of various phenolic constituents such as phenolic acids, phenolic diterpenes, flavonoids and volatile oils, which are responsible for their antioxidant properties and the main effect is caused by phenolic –OH groups (Falowo et al. 2014). This also establishes a good relationship between TBARS and total phenolics content. Similar results have also been reported by Devatkal et al. (2011) in raw chicken patties added with know and pomegranate fruit by products, respectively. Moyo et al. (2011) found 2.02% of total polyphenols in the composition of Moringa oleifera leaves. Qwele et al. (2013) had also reported that the meat from goat fed with Moringa oleifera leaves had a higher concentration of total phenolics content (mainly tannin content) than the meat of goat fed control diet, thus implying the antioxidant potential of these leaves in meat sample. However, variation in antioxidant activity among treatments might be ascribed to their different phenolic composition.
DPPH free radical scavenging activity
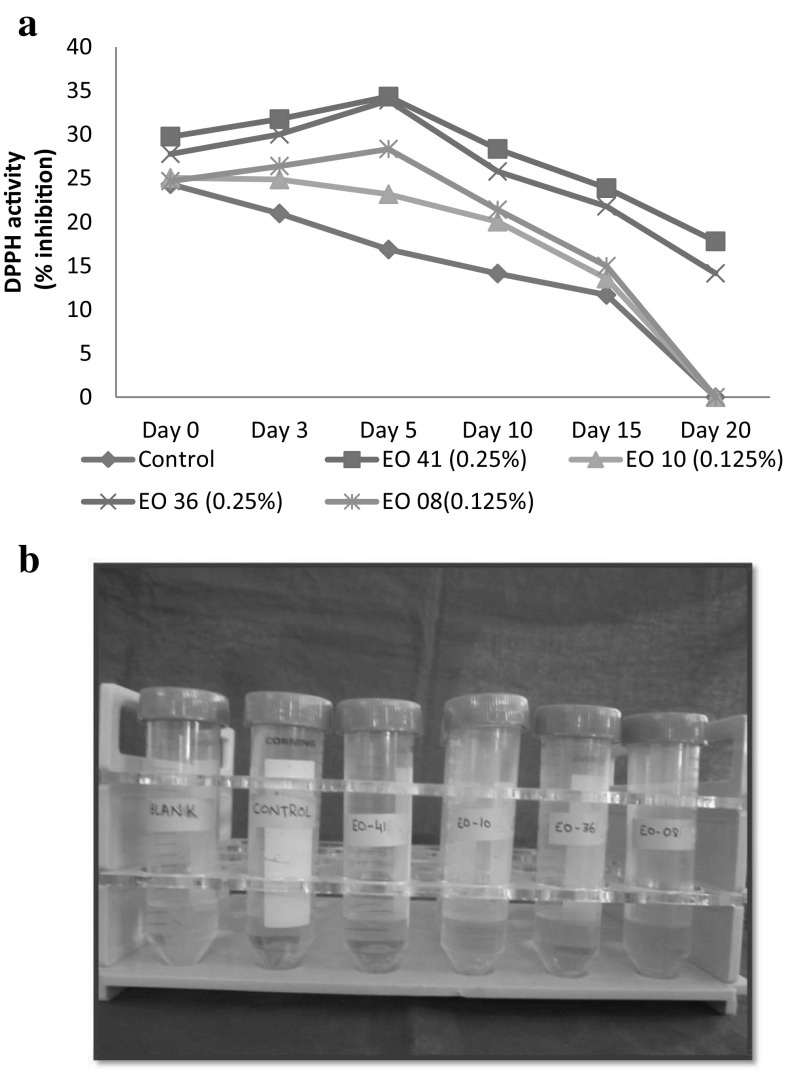
As the storage period progressed, DPPH activity significantly decreased (P < 0.05) in control and holy basil oil products at each interval of storage period, however clove oil, cassia oil and thyme oil products exhibited significant increase (P < 0.05) in DPPH activity up to day 5 of storage and then decreased significantly (P < 0.05) in all the products (Table 3). Clove oil products showed minimum decrease in DPPH activity followed by cassia oil products during storage period. It was also observed that significantly higher (P < 0.05) DPPH activity was present in treatment products than control throughout storage period. DPPH activity varied significantly (P < 0.05) among treatment products and it was found to be significantly higher (P < 0.05) in clove oil products indicating that it was better inhibitor of free radicals formation. However, clove oil and cassia oil products had comparable DPPH activity on day 3 and day 5 (Fig. 3). There was no significant difference (P > 0.05) in DPPH activity between holy basil oil and thyme oil products during entire storage period. However, the general decreasing order of DPPH activity was clove oil > cassia oil > thyme oil > holy basil oil products.
Fig. 3.
Effect of optimum level of different essential oils on DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C). a Graph representing the trend of DPPH free radical scavenging activity during storage of fresh chicken sausages. b Test-tubes containing samples of fresh chicken sausages (DAY 0). (EO 41 = Clove essential oil; EO 10 = Holy basil essential oil; EO36 = Cassia essential oil and EO 08 = Thyme essential oil)
During storage period, initial increase in DPPH activity of treatment products up to day 5 of storage period might be due to less free radical formation and increased EOs efficiency however, after 5 days, effectiveness of oil might have been reduced due to factors such as high pH, increased oxidation in products and thus, could not inhibit free radicals formed at high percentage. Similar trend of DPPH activity has been reported by Gulcin et al. (2012). Higher DPPH activity in treatment products might be attributed to the presence of various antioxidants (phenolic acids, flavonoids, phenolic diterpenes and monoterpenes) in essential oil which were able to reduce the stable free radical DPPH to non radical form DPPH-H (Gulcin et al. 2012). Qwele et al. (2013) also revealed that there was significant scavenging potential of meat from goats fed with Moringa oleifera leaves as compared to control and high scavenging potential of meat was attributed to its high hydrogen donating ability.
Microbiological characteristics
Total plate count
During storage, significant increase (P < 0.05) in TPC was observed in both control and treatment products at each interval of storage period (Table 4). However, treatment products showed slower rate of increase in TPC than control. It was also revealed that there was significantly higher (P < 0.05) TPC in control than treatment products throughout storage period. Among treatments, no significant difference (P > 0.05) was noticed between clove oil and cassia oil products up to day 3 of storage. There was non-significant difference (P > 0.05) between TPC of holy basil oil and thyme oil products throughout the storage period. Holy basil oil and thyme oil products were observed with significantly higher (P < 0.05) TPC than clove oil and cassia oil products due to lower level of essential oils used in the former products. Among four treatments, cassia oil products showed significantly lower (P < 0.05) microbial load at the end of storage period indicating that this oil had strongest antibacterial activity among treatments. The general decreasing order of TPC was holy basil oil > thyme oil > clove oil > cassia oil.
Table 4.
Effect of optimum level of different essential oils on microbiological characteristics of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C) (Mean ± S.E.)*
| Parameter | Refrigerated storage period (days) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 10 | Day 15 | Day 20 | |
| Total plate count (log10 cfu/g) | ||||||
| Control | 3.21e1 ± 0.09e1 | 4.11d1 ± 0.02d1 | 4.52c1 ± 0.04c1 | 5.63b1 ± 0.03b1 | 6.91a1 ± 0.06a1 | NE |
| Clove oil | 2.60f23 ± 0.15f23 | 2.88e3 ± 0.09e3 | 3.11d3 ± 0.08d3 | 3.73c3 ± 0.07c3 | 4.49b3 ± 0.12b3 | 5.21a1 ± 0.15a1 |
| Holy basil oil | 2.91e12 ± 0.06e12 | 3.26d2 ± 0.09d2 | 3.88c2 ± 0.07c2 | 4.81b2 ± 0.08b2 | 5.26a2 ± 0.08a2 | NE |
| Cassia oil | 2.49f3 ± 0.11f3 | 2.72e3 ± 0.14e3 | 2.96d4 ± 0.04d4 | 3.57c3 ± 0.12c3 | 4.19b4 ± 0.06b4 | 4.84a2 ± 0.09a2 |
| Thyme oil | 2.82e23 ± 0.18e23 | 3.12d2 ± 0.19d2 | 3.81c2 ± 0.06c2 | 4.73b2 ± 0.07b2 | 5.19a2 ± 0.04a2 | NE |
| Psychrophilic count (log10 cfu/g) | ||||||
| Control | ND | 1.09c ± 0.11c | 1.22bc ± 0.14bc | 1.48b ± 0.19b | 1.78a1 ± 0.15a1 | NE |
| Clove oil | ND | ND | ND | ND | 1.2823 ± 0.0923 | 1.41 ± 0.11 |
| Holy basil oil | ND | ND | 1.06c ± 0.09c | 1.21b ± 0.10b | 1.41a12 ± 0.07a12 | NE |
| Cassia oil | ND | ND | ND | ND | 1.16b4 ± 0.08b4 | 1.30a ± 0.06a |
| Thyme oil | ND | ND | ND | 1.17b ± 0.14b | 1.32a12 ± 0.07a12 | NE |
| Yeast and mould count (log10 cfu/g) | ||||||
| Control | ND | 1.26c ± 0.07c | 1.44b ± 0.13b | 1.61b ± 0.17b | 1.89a1 ± 0.09a1 | NE |
| Clove oil | ND | ND | ND | ND | 1.173 ± 0.083 | 1.30 ± 0.09 |
| Holy basil oil | ND | 1.09c ± 0.10c | 1.18c ± 0.09c | 1.29b ± 0.14b | 1.38a2 ± 0.15a2 | NE |
| Cassia oil | ND | ND | ND | ND | 1.103 ± 0.183 | 1.24 ± 0.15 |
| Thyme oil | ND | ND | 1.06b ± 0.12b | 1.18a ± 0.14a | 1.27a2 ± 0.21a2 | NE |
| Coliforms count (log10 cfu/g) | ||||||
| Control | ND | ND | 1.12a ± 0.09a | 1.30b ± 0.15b | 1.63c1 ± 0.06c1 | NE |
| Clove oil | ND | ND | ND | ND | ND | 1.24 ± 0.15 |
| Holy basil oil | ND | ND | ND | 1.16 ± 0.10 | 1.282 ± 0.062 | NE |
| Cassia oil | ND | ND | ND | ND | ND | 1.16 ± 0.07 |
| Thyme oil | ND | ND | ND | ND | 1.222 ± 0.082 | NE |
n = 6
ND not detected, NE not estimated, cfu colony forming units
* Mean ± S.E. bearing different superscripts row wise (alphabet) and column wise (numeral) differ significantly (P < 0.05)
Increase in TPC of treatment products with advancement in storage time could be attributed to the reduced effect of essential oils against rapid rate of increase in number of microorganisms during aerobic packaging. Increase in total plate count with increase in storage period has also been reported by Sachindra et al. (2005), Syne et al. (2013). However, even after 20 days of storage, TPC were well below the permissible limit of log107 cfu/g for fresh meat products (Pothakos et al. 2012). Lower TPC observed in treatment products than control could be attributed to anti-microbial constituents present in essential oils (Hyldgaard et al. 2012). Anti-microbial activity of essential oils has been consistently linked to its phenolic constituents such as carvacrol, eugenol, and thymol. Hydroxyl groups in phenolic compounds are mainly responsible for their antimicrobial activity. Citral (0.5–3% w/v) has also been shown to be effective against S. typhimurium in fish cubes with reduction in the final population of up to 1.5 log cfu/g (Kim et al. 1995). The difference in antibacterial activity among treatment products at same level of incorporation might be due to difference in the chemical composition of essential oils (Siewe et al. 2015).
Psychrophilic count
With advancement in storage period, PC increased significantly (P < 0.05) in all the products except clove oil products which exhibited non-significant increase (P > 0.05) in PC throughout the storage period (Table 5). Results also indicated that control had significantly higher (P < 0.05) PC than all treatment products on day 15 of storage. Among treatments, significant difference (P < 0.05) in PC was observed only on day 15 of storage. Cassia oil products showed significantly lower (P < 0.05) count followed by clove oil, thyme oil and holy basil oil products. However, thyme oil and holy basil oil products had non-significant difference (P > 0.05) in PC throughout the storage period.
Table 5.
Effect of optimum level of different essential oils on sensory attributes of aerobically packaged fresh chicken sausages during refrigerated storage (4 ± 1 °C) (Mean ± S.E.)*
| Treatments | Refrigerated storage period (days) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 10 | Day 15 | |
| General appearance | |||||
| Control | 7.28a ± 0.08a | 7.24a ± 0.09a | 7.21a ± 0.09a | 7.06b ± 0.06b | -spoiled- |
| Clove oil | 7.30a ± 0.07a | 7.22a ± 0.08a | 7.13b ± 0.08b | 6.94bc ± 0.08bc | 6.67c ± 0.07c |
| Holy basil oil | 7.29a ± 0.06a | 7.24a ± 0.08a | 7.17ab ± 0.07ab | 6.98b ± 0.08b | -spoiled |
| Cassia oil | 7.28a ± 0.06a | 7.22a ± 0.07a | 7.16a ± 0.10a | 6.95bc ± 0.07bc | 6.74c ± 0.04c |
| Thyme oil | 7.31a ± 0.07a | 7.26a ± 0.06a | 7.17ab ± 0.07ab | 6.96b ± 0.08b | -spoiled- |
| Flavour | |||||
| Control | 7.12a1 ± 0.06a1 | 7.08ab1 ± 0.08ab1 | 6.99bc ± 0.08bc | 6.78c1 ± 0.08c1 | -spoiled- |
| Clove oil | 6.94a2 ± 0.10a2 | 6.89a12 ± 0.08a12 | 6.81ab ± 0.07ab | 6.63bc2 ± 0.09bc2 | 6.46c ± 0.08c |
| Holy basil oil | 6.98a12 ± 0.06a12 | 6.88a12 ± 0.09a12 | 6.76ab ± 0.08ab | 6.59b2 ± 0.07b2 | -spoiled- |
| Cassia oil | 7.04a12 ± 0.07a12 | 6.97a1 ± 0.07a1 | 6.90a ± 0.09a | 6.81a1 ± 0.09a1 | 6.54b ± 0.08b |
| Thyme oil | 6.92a2 ± 0.06a2 | 6.86a12 ± 0.08a12 | 6.82a ± 0.09a | 6.60b2 ± 0.07b2 | -spoiled- |
| Binding | |||||
| Control | 7.32a ± 0.05a | 7.26a ± 0.06a | 7.19ab ± 0.04ab | 7.02b ± 0.09b | -spoiled- |
| Clove oil | 7.34a ± 0.07a | 7.25ab ± 0.07ab | 7.19b ± 0.08b | 7.04c ± 0.05c | 6.76d ± 0.12d |
| Holy basil oil | 7.28a ± 0.06a | 7.21a ± 0.07a | 7.13 ± 0.06ab | 6.91b ± 0.08b | -spoiled- |
| Cassia oil | 7.30a ± 0.07a | 7.24a ± 0.08a | 7.18ab ± 0.08ab | 7.07b ± 0.07b | 6.82c ± 0.11c |
| Thyme oil | 7.30a ± 0.08a | 7.26a ± 0.08a | 7.16ab ± 0.07ab | 6.98b ± 0.09b | -spoiled- |
| Texture | |||||
| Control | 7.31a ± 0.04a | 7.21a ± 0.07a | 7.12a ± 0.06a | 6.88b ± 0.09b | -spoiled- |
| Clove oil | 7.28a ± 0.06a | 7.21a ± 0.06a | 7.15ab ± 0.08ab | 6.95bc ± 0.09bc | 6.69c ± 0.10c |
| Holy basil oil | 7.29a ± 0.07a | 7.19a ± 0.07a | 7.12a ± 0.08a | 6.90b ± 0.10b | -spoiled- |
| Cassia oil | 7.31a ± 0.06a | 7.22a ± 0.05a | 7.16ab ± 0.08ab | 6.92b ± 0.05b | 6.72c ± 0.08c |
| Thyme oil | 7.30a ± 0.07a | 7.22a ± 0.09a | 7.14a ± 0.05a | 6.92b ± 0.08b | -spoiled- |
| Juiciness | |||||
| Control | 7.29a ± 0.04a | 7.23a ± 0.06a | 7.12a ± 0.06a | 6.94b ± 0.08b | -spoiled- |
| Clove oil | 7.26a ± 0.07a | 7.21ab ± 0.06ab | 7.15a ± 0.07a | 7.01b ± 0.07b | 6.79c ± 0.09c |
| Holy basil oil | 7.27a ± 0.05a | 7.21ab ± 0.06ab | 7.13ab ± 0.06ab | 6.98b ± 0.07b | -spoiled- |
| Cassia oil | 7.32a ± 0.06a | 7.26a ± 0.05a | 7.17a ± 0.04a | 7.06ab ± 0.08ab | 6.81b ± 0.12b |
| Thyme oil | 7.28a ± 0.07a | 7.24a ± 0.08a | 7.15ab ± 0.06ab | 7.02b ± 0.06b | -spoiled- |
| Overall acceptability | |||||
| Control | 7.12a1 ± 0.04a1 | 7.02ab1 ± 0.07ab1 | 6.90bc ± 0.05bc | 6.71c ± 0.06c | -spoiled- |
| Clove oil | 6.91a12 ± 0.09a12 | 6.82a12 ± 0.07a12 | 6.78a ± 0.07a | 6.64b ± 0.06b | 6.53b ± 0.08b |
| Holy basil oil | 6.97a1 ± 0.05a1 | 6.90a1 ± 0.07a1 | 6.82ab ± 0.08ab | 6.59b ± 0.08b | -spoiled- |
| Cassia oil | 6.94a12 ± 0.07a12 | 6.81ab12 ± 0.08ab12 | 6.73ab ± 0.08ab | 6.68b ± 0.09b | 6.59c ± 0.06c |
| Thyme oil | 6.89a12 ± 0.05a12 | 6.78ab12 ± 0.09ab12 | 6.71ab ± 0.10ab | 6.61b ± 0.06b | -spoiled- |
n = 21
* Mean ± S.E. bearing different superscripts row wise (alphabet) and column wise (numeral) differ significantly (P < 0.05)
Absence of psychrophilic bacteria in the products during initial periods of storage might be attributed to retardation of log phase as a result of reduced metabolic rate due to sudden change in the physical environment. During storage period, treatment products showed lower incremental pattern in PC as compared to control. Similar results have been reported by Badee et al. (2013) in chicken meat added with marjoram essential oil. In all the products, PC remained well below the permissible limit of 105−6 cfu/g in fresh meat (Kamel 2013). Lower count observed in treatment products than control could be attributed to anti-microbial constituents (phenolic compounds) present in essential oils (Hyldgaard et al. 2012). Among treatments, difference in antimicrobial activity might be due to difference in composition and mode of action of essential oils in food matrix.
Yeast and Mould count
During storage period, significant increase (P < 0.05) in YMC was observed for control and holy basil oil products from day 3 and day 5 onwards, respectively (Table 4). However, cassia oil and clove oil products were observed with non-significant increase (P > 0.05) in count throughout the storage period. Results also indicated that there was no significant difference (P > 0.05) in YMC between control and treatment products throughout the storage period except on day 15. Control had significantly higher (P < 0.05) yeast and mould count (YMC) than treatment products on day 15 of storage. Among treatments, significant difference (P < 0.05) was observed in YMC only on day 15 of storage. Cassia oil and clove oil products had significantly lower (P < 0.05) YMC than thyme oil and holy basil products. The general decreasing order of TPC was holy basil oil > thyme oil > clove oil > cassia oil products.
The absence of YMC in control and treatment products during initial periods of storage might be due to unfavourable conditions for their growth such as high pH, lower temperature and higher water activity of product. Lower YMC in treatment products as compared to control proves the antifungal activity of essential oil constituents in meat products (Rao et al. 2010). Thymol (active phenolic in thyme essential oil) disruptes vesicles and cell membranes; inhibits biosynthesis of ergosterol in Candida strains, which in turn disrupts integrity of cell membrane (Ahmad et al. 2011).
Coliform count
With increase in storage period, coliform count increased significantly (P < 0.05) in control at each interval of storage period, however no significant increase (P > 0.05) was observed in treatment products throughout the storage period (Table 4). Critical difference analysis indicated that control had significantly higher (P < 0.05) count than treatment products on day 15 of storage. However among treatments, no significant difference (P > 0.05) was observed throughout the storage period. Absence of coliforms in initial period of storage in treatment products might be due to anti-microbial action of EOs present in products, however, effectiveness of EOs might have decreased against rapid increase in number of coliform with passage of time.
Sensory scores
During storage period, significant decrease (P < 0.05) in general appearance scores of all products was observed from day 5 onwards (Table 5). However, there was no significant difference (P > 0.05) in general appearance scores between control and treatment products. No significant difference (P > 0.05) was observed even among treatments. Decreased appearance of the products could be due to some surface dehydration in aerobic packaging (Sharma et al. 2015). With progressive storage, significant decrease (P < 0.05) in flavour scores of control and treatment products was observed from day 3 and day 5 onwards, respectively. Control had significantly higher (P < 0.05) flavour scores than clove oil and thyme oil products on day 0 and day 10 of storage, however no significant difference (P > 0.05) was observed between flavour scores of control and cassia oil products throughout the storage period. Among treatments, there was no significant difference (P > 0.05) throughout storage period, however cassia oil products was observed with non-significantly higher (P > 0.05) flavour scores than other treatment products followed by clove oil products. The decrease in flavour scores might be due to an increase in TBA value and free fatty acids in meat products (Tarladgis et al. 1960) under aerobic conditions. Decrease in flavour scores of chicken patties incorporated with 130 ppm of oleoresin as compared to control had also been reported by Naveena et al. (2008).
With advancement in storage period, binding and texture scores showed significant decrease (P < 0.05) in both control and treatment products from day 5 onwards, however cassia oil products exhibited significant decrease (P < 0.05) in binding scores from day 10 onwards. Binding scores showed no significant difference (P > 0.05) between control and treatment products throughout the storage period. No significant difference (P > 0.05) was observed even among treatments. Among treatments, clove oil and cassia oil products had non-significantly higher (P > 0.05) scores than other treatments from day 5 onwards. Decrease in binding scores of products could be attributed to breakdown of meat proteins due to higher microbial counts on these days.
During storage, significant decrease (P < 0.05) in juiciness scores was observed for both control and treatment products from day 5 onwards, however cassia oil products exhibited significant decrease (P < 0.05) from day 10 onwards. Critical difference analysis indicated that juiciness scores did not vary significantly (P > 0.05) between control and treatment products throughout the storage period. Among treatments, there was no significant difference (P > 0.05) in juiciness scores however, cassia oil products were observed with non-significantly higher (P > 0.05) scores than other treatments during entire storage period. Decrease in juiciness of products with increasing storage period could be attributed to loss of moisture during aerobic storage. Decrease in sensory scores of products incorporated with EOs had also been reported by Siewe et al. (2015).
Significant decrease (P < 0.05) in overall acceptability scores of control and holy basil oil, thyme oil and clove oil products were observed from day 3 to day 5 of storage, respectively. However, there was no significant decrease (P > 0.05) in scores of cassia oil products up to day 10 of storage, and thereafter it increased significantly (P < 0.05) up to end of storage period. Critical difference analysis indicated that no significant difference (P > 0.05) was observed in overall acceptability scores between control and treatment products; however control had non-significantly higher (P < 0.05) scores than treatments throughout the storage period. Among treatments, there was no significant difference in overall acceptability scores. However, holy basil oil product was observed with marginally higher scores than other treatments up to day 5 of storage, thereafter cassia oil products had non-significantly higher (P > 0.05) overall acceptability scores followed by clove oil products. A decrease in overall acceptability scores was expected because of decrease in scores of other attributes with the advancement of storage period. The decrease in overall acceptability scores of the products during storage has been reported by Zargar et al. (2014) in chicken sausages.
Conclusion
During refrigerated storage of aerobically packaged fresh chicken sausages for 20 days, it was found that pH and TBARS increased with increase in storage period for both control and treatment products, however increase was least in case of clove oil products. Total phenolics content and DPPH activity decreased significantly during storage and clove oil products were observed with significantly higher values for both the parameters which indicated that clove oil had better oxidative stability among treatments. Microbial counts were well below the permissible limit of fresh meat products and cassia oil products were observed with significantly lower microbial count followed by clove oil products throughout the storage period. All sensory attributes decreased significantly after 5 days of storage period in control and treatment products. Among treatments, holy basil oil and cassia oil remained quite acceptable up to 10th and 15th day of storage, respectively than other two treatments. Thus, it can be concluded that out of four essential oils evaluated, use of cassia oil at 0.25% level is recommended in fresh chicken sausages.
References
- Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan L, Manzoor N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis. 2011;30:41–50. doi: 10.1007/s10096-010-1050-8. [DOI] [PubMed] [Google Scholar]
- Allen K, Cornforth D. Comparison of spice-derived antioxidants and metal chelators on fresh beef color stability. Meat Sci. 2010;85:613–619. doi: 10.1016/j.meatsci.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Alma MH, Ertas M, Nitz S, Kollmannsberger H. Chemical composition and content of essential oil from the bud of cultivated Turkish clove (Yzygium Aromaticum L) Bio Res. 2007;2(2):265–269. [Google Scholar]
- Antolovich M, Prenzler P, Patsalides E. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- APHA . Compendium of method for microbiological examination of food. 4. Washington: Speck M.L. American Public Health Association; 2001. [Google Scholar]
- Badee A, Moawad R, ElNoketi M, Gouda M. Antioxidant and antimicrobial activities of marjoram (Origanum majorana L,) essential oil. J Appl Sci Res. 2013;9:1193–1201. [Google Scholar]
- Belitz HD, Grosch W, Schieberle P. Coffee, tea, cocoa. In: Belitz HD, Grosch W, Schieberle P, editors. Food Chemistry. 4. Berlin: Springer; 2009. pp. 938–970. [Google Scholar]
- Bennis S, Chami F, Chami N, Bouchikhi T, Remmal A. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Lett Appl Microbiol. 2004;38:454–458. doi: 10.1111/j.1472-765X.2004.01511.x. [DOI] [PubMed] [Google Scholar]
- Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJ. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157: H7 by addition of food stabilizers. J Food Prot. 2005;68:919–926. doi: 10.4315/0362-028X-68.5.919. [DOI] [PubMed] [Google Scholar]
- Carović-Stanko K, Orlić S, Politeo O, Strikić F, Kolak I, Milos M, Satovic Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010;119:196–201. doi: 10.1016/j.foodchem.2009.06.010. [DOI] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. The effect of salt extract of kinnow and pomegranate fruit by-products on colour and oxidative stability of raw chicken patties during refrigerated storage. J Food Sci Technol. 2011;48:472–477. doi: 10.1007/s13197-011-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M, Ramrez R, Ventanas S, Cava R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pate. LWT. 2007;40:58–65. doi: 10.1016/j.lwt.2005.07.010. [DOI] [Google Scholar]
- Falowo AB, Fayemi PO, Muchenje O. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: a review. Food Res Int. 2014;64:171–181. doi: 10.1016/j.foodres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA. Effect of rosemary extract chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 C. Meat Sci. 2007;76:172–181. doi: 10.1016/j.meatsci.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Gill C. Meat spoilage and evaluation of the potential storage life of fresh meat. J Food Prot. 1983;46:444–452. doi: 10.4315/0362-028X-46.5.444. [DOI] [PubMed] [Google Scholar]
- Gulcin İ, Elmastaş M, Aboul-Enein HY. Antioxidant activity of clove oil–A powerful antioxidant source. Arab J Chem. 2012;5:489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action synergies and interactions with food matrix components. Front Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Sci Technol. 2015;64:1204–1208. doi: 10.1016/j.lwt.2015.07.028. [DOI] [Google Scholar]
- Kamel SM. The use of some herbs for improving the refrigerated storage stability of minced camel meat. Scientific J Microbiol. 2013;2:95–103. [Google Scholar]
- Keeton JT. Effects of fat and NaCl/phosphate levels on the chemical and sensory properties of pork patties. J Food Sci. 1983;48:878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x. [DOI] [Google Scholar]
- Kim JM, Marshall MR, Cornell JA, Iii JFP, Wei CI. Antibacterial activity of carvacrol citral and geraniol against salmonella typhimurium in culture medium and on fish cubes. J Food Sci. 1995;60:1364–1368. doi: 10.1111/j.1365-2621.1995.tb04592.x. [DOI] [Google Scholar]
- Liu DC, Tsau RT, Lin YC, Jan SS, Tan FJ. Effect of various levels of rosemary or Chinese mahogany on the quality of fresh chicken sausage during refrigerated storage. Food Chem. 2009;117:106–113. doi: 10.1016/j.foodchem.2009.03.083. [DOI] [Google Scholar]
- Makkar H P S (2000) Quantification of tannins in tree foliage: A laboratory manual for the FAO/IAEA Co-ordinated Research Project on ‘Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on Tanniniferous Tree Foliage’, FAO/IAEA Working Document IAEA Vienna Austria, pp 1–6
- Mokhtar SM, Youssef KM, Morsy NE. The effects of natural antioxidants on colour lipid stability and sensory evaluation of fresh beef patties stored at 4 °C. J Agroaliment Process Technol. 2014;20(3):282–292. [Google Scholar]
- Moyo B, Masika PJ, Hugo A, Muchenje V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African. J Biotechnol. 2011;10(60):12925–12933. [Google Scholar]
- Naveena BM, Sen A, Vaithiyanathan S, Babji Y, Kondaiah N. Comparative efficacy of pomegranate juice pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008;80:1304–1308. doi: 10.1016/j.meatsci.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Nkukwana TT, Muchenje V, Masika PJ, Hoffman LC, Dzama K, Descalzo AM. Fatty acid composition and oxidative stability of breast meat from broiler chickens supplemented with Moringa oleifera leaf meal over a period of refrigeration. Food Chem. 2014;142:255–261. doi: 10.1016/j.foodchem.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Pothakos V, Samapundo S, Devlieghere F. Total mesophilic counts underestimate in many cases the contamination levels of psychrotrophic lactic acid bacteria (LAB) in chilled-stored food products at the end of their shelf-life. Food Microbiol. 2012;32:437–443. doi: 10.1016/j.fm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Qwele K, Muchenje V, Oyedemi SO, Moyo B, Masika PJ. Chemical composition, fatty acid contents and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013;93:455–462. doi: 10.1016/j.meatsci.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Rao A, Zhang Y, Muend S, Rao R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob Agents Chemother. 2010;54:5062–5069. doi: 10.1128/AAC.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, Barcellona M, Vanella A. Bioflavonoids as antiradicals antioxidants and DNA cleavage protectors. Cell Biol Toxicol. 2000;16:91–98. doi: 10.1023/A:1007685909018. [DOI] [PubMed] [Google Scholar]
- Sachindra N, Sakhare P, Yashoda K, Rao DN. Microbial profile of buffalo sausage during processing and storage. Food Control. 2005;16:31–35. doi: 10.1016/j.foodcont.2003.11.002. [DOI] [Google Scholar]
- Sampaio G, Saldanha T, Soares R, Torres E. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012;135:1383–1390. doi: 10.1016/j.foodchem.2012.05.103. [DOI] [PubMed] [Google Scholar]
- Sanchez-Escalante A, Djenane D, Torrescano G, Beltrán JA, Roncalés P. The effects of ascorbic acid taurine carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001;58:421–429. doi: 10.1016/S0309-1740(01)00045-6. [DOI] [PubMed] [Google Scholar]
- Serpen A, Gökmen V, Fogliano V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012;90:60–65. doi: 10.1016/j.meatsci.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Sharma H, Sharma BD, Mendiratta S, Talukder S, Ramasamy G. Efficacy of flaxseed flour as bind enhancing agent on the quality of extended restructured mutton chops. Asian-Australas J Anim Sci. 2014;27:247. doi: 10.5713/ajas.2013.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Sharma BD, Talukder S, Giriprasad R. Utilization of gum tragacanth as bind enhancing agent on the quality of extended restructured mutton chops. J Food Sci Technol. 2015;52(3):1626–1633. doi: 10.1007/s13197-013-1172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe FB, Mbougueng PD, Tatsadjieu LN, Noumo TN, Mbofung CM. The potential application of syzygium aromaticum and Cymbopogon citratus essential oils as natural preservatives of beef patties. Food Nutr Sci. 2015;6:374. doi: 10.4236/fns.2015.63038. [DOI] [Google Scholar]
- Snedecor G, Cochran W. Statistical methods. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- Syne SM, Ramsubhag A, Adesiyun AA. Microbiological hazard analysis of ready-to-eat meats processed at a food plant in Trinidad West Indies. Infect Ecol Epidemiol. 2013 doi: 10.3402/iee.v3i0.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarladgis BG, Watts BM, Younathan MT, Dugan L., Jr A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Tepe B, Sokmen M, Akpulat HA, Sokmen A. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005;92:89–92. doi: 10.1016/j.foodchem.2004.07.016. [DOI] [Google Scholar]
- Troutt ES, Hunt MC, Johnson DE, Claus JR, Kastner CL, Kropf DH. Characteristics of low-fat ground beef containing texture-modifying ingredients. J Food Sci. 1992;57:19–24. doi: 10.1111/j.1365-2621.1992.tb05415.x. [DOI] [Google Scholar]
- Verspohl EJ, Bauer K, Neddermann E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res. 2005;19:203–206. doi: 10.1002/ptr.1643. [DOI] [PubMed] [Google Scholar]
- Wojciak KM, Dolatowski ZJ, Okoń A. The effect of water plant extracts addition on the oxidative stability of meat products. Acta Sci Pol Technol Aliment. 2011;10:175–188. [Google Scholar]
- Zargar FA, Kumar S, Bhat ZF, Kumar P. Effect of pumpkin on the quality characteristics and storage quality of aerobically packaged chicken sausages. SpringerPlus. 2014;3:39. doi: 10.1186/2193-1801-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]