Abstract
Dietary fat increases carotenoid bioavailability by facilitating their transfer to the aqueous micellar fraction during digestion. However, the specific effect of both quantity and type of dietary fat required for optimal carotenoid absorption remained unexplored. In the present study, the effect of amount and type of vegetable oils on carotenoid micellarization from carrot, spinach, drumstick leaves and papaya using in vitro digestion/Caco-2 cell model have been assessed. Although, dietary fat (0.5–10% w/w) significantly increased the micellarization of carotenoids from all the test foods, the extent of increase was determined by the food matrix (papaya > drumstick = spinach > carrot) and polarity of carotenoids (lutein > β-carotene = α-carotene > lycopene). Among the dietary fats tested the carotenoid micellarization was twofold to threefold higher with dietary fat rich in unsaturated fatty acids (olive oil = soybean oil = sunflower oil) compared to saturated fatty acids (peanut oil = palm oil > coconut oil). Intestinal cell uptake of lutein exceeded that of β-carotene from micellar fraction of spinach leaves digested with various oils. However, cellular uptake of β-carotene is depended on the carotenoid content in micellar fraction rather than the type of fat used. Together these results suggest that food matrix, polarity of carotenoids and type of dietary fat determines the extent of carotenoid micellarization from vegetables and fruits.
Keywords: Carotenoids, Micellarization, Bioaccessibility, Dietary fat, Caco-2 cells
Introduction
Vitamin A deficiency continues to be a significant public health problem in developing countries including India (WHO report 2009; Laxmaiah et al. 2012). Studies in pre-school and school children reported very high prevalence of sub-clinical vitamin A deficiency (defined as <20 µg/dL serum retinol) in India (Sivakumar et al. 2006; Laxmaiah et al. 2012). The pro-vitamin A carotenoids from plant foods are the major source of vitamin A in populations subsisting on vegetarian diet. The recommended dietary allowances (RDA) of vitamin A for Indians is 600 µg/day in adult man which translates to 4800 µg/day of β-carotene, considering the conversion ratio of 1:8 (RDA for Indians, 2010). About 1/3rd of the population in India do not meet these recommended levels and data on time trends of dietary intakes also shows no increase in intakes over the last decade (Ramachandran 2009), consistent with observed high prevalence of deficiency. Therefore, dietary diversification, food fortification, biofortification and genetically modified (GM) varieties of staple foods enriched in pro vitamin A carotenoids are being considered as potential food based strategies to control vitamin A deficiency. However, enhancing the bioavailability of carotenoids during routine culinary practice or food processing is perhaps one of the critical factors in improving the vitamin A status.
Carotenoid bioavailability is influenced by numerous factors such as food matrix, quantity and composition of carotenoids and food preparation. For instance bioavailability of carotenoids from vegetables was found to be more when it is consumed with dietary fat (Unlu et al. 2005; Brown et al. 2004; Jayarajan et al. 1980). The postprandial appearance of β-carotene in triacylglycerol-rich lipoprotein fraction was higher with beef-tallow (rich in saturated fat) compared to sunflower oil (rich in unsaturated fat) in women (Hu et al. 2000). Further, carotenoid absorption was higher with olive oil, rich in monounsaturated fat, compared to corn oil, rich in polyunsaturated fatty acids (Clark et al. 2000). In contrast, the quantity of fat also appears to exert major influence on carotenoid bioavailability, apart from the source of lipid, from mixed vegetable salads in human subjects (Goltz et al. 2012). Together, these results unequivocally suggest that both the quantity and composition of dietary fat influence the carotenoid bioavailability. Simulated in vitro digestion followed by measuring the transfer of carotenes to the aqueous micellar fraction is a widely accepted model to measure their bioavailability (Failla et al. 2008; Garrett et al. 1999). The data obtained using this model is well correlated with that of studies in humans (Reboul et al. 2006). The microscopic studies have reported unique morphology of carotene pigments in different types of foods, which may also have a bearing on bioavailability (Schweiggert et al. 2012, 2014). Therefore, understanding the effect of both quantity and type of dietary fat on bioavailability of carotenoids from individual foods, in the context of varied food matrix, carotene pigment ultrastructure and carotenoid composition merits attention.
In this study we have investigated the effect of quantity and type of fat on the efficiency of carotenoid micellarization from carrot, spinach, drumstick leaves and papaya using simulated in vitro digestion model. Further, we attempted to compare the effect of food matrix and polarity of carotenes on the efficiency of micellarization. Finally, we have also studied the effect of type of dietary fat on intestinal uptake of carotenoids in enterocyte like differentiated Caco-2 cells. The results demonstrate that carotenoid micellarization is determined by their pigment structure, degree of polarity and dietary fat.
Materials and methods
Materials
Carotenoid standards were procured from CaroteNature (Basel, Switzerland). Fetal bovine serum was obtained from Hi-Media (Mumbai, India). Trypsin, l-glutamine, non-essential amino acids, antibiotic and antimycotic mix were obtained from Invitrogen (CA, USA). HPLC grade methanol, dichloromethane were procured from Fisher Scientific (Mumbai, India). Dulbecco’s minimal essential medium (DMEM), porcine pepsin, pancreatin, porcine bile extract, butylated hydroxytoluene and all other reagents were obtained from Sigma Chemical Co. (Bangalore, India).
Vegetables and fruit processing
Carrots, spinach, drumstick leaves and papaya are the most commonly consumed carotenoid rich vegetables and fruit, respectively in India and therefore, selected for the study. Orange carrots (Daucus carota), tender leaves of spinach (Spinacia oleracea) and drumstick leaves (Moringa oleifera) and fully ripened papaya (Carica papaya, local variety Surya) were purchased fresh from the local market. The vegetables and fruits were cleaned with milli-Q water twice and pat-dried on a blotting paper. The vegetables were cut into small pieces, microwaved for 3 min at maximum power to simulate cooking, cooled and pureed in a kitchen blender. The papaya fruit pulp was prepared and used directly for the in vitro digestion without cooking as it is generally consumed raw. The vegetable puree or fruit pulp were used immediately for in vitro digestion.
Vegetable oils and fatty acid composition
Vegetable oils were procured locally. The fatty acid composition of the oils was analyzed as fatty acid methyl esters by gas chromatography using an SP-2330 capillary column (30 m X 0·25 mm; Supelco, Bellefonte, PA, USA) as described earlier (Ghafoorunissa et al. 1995).
Simulated in vitro digestion
The simulated gastric and small intestinal phases of digestion were carried out as described previously (Pullakhandam and Failla 2007; Garrett et al. 1999). The digestion reactions (in 50 mL screw cap tubes) contained either 2.0 g of pureed vegetables or fruit pulp in the absence and presence of dietary fat (0–10%, w/w), which was added prior to the initiation of gastric phase of digestion. Briefly, 2 g of food homogenate was mixed with 35 mL of saline, and pH of the contents was adjusted to 2 with 2 M HCl followed by addition of 2 mL porcine pepsin stock solution (40 mg/mL in 100 mM HCl). The final volumes were made to 40 mL with saline, blanketed with nitrogen gas and incubated submersed in a 37 °C shaking water bath for a period of 1 h. At the end of gastric digestion, the pH was increased to 6 with 1 M NaHCO3, followed by addition of 3 mL bile extract (60 mg porcine bile extract/mL in 100 mM NaHCO3) and 2 mL of pancreatin (10 mg/mL)-lipase (10 mg/mL) stock solution. The pH of all the samples was adjusted to 6.5 with 1 M NaOH, and the final volumes were made to 50 mL. The samples were then blanketed with nitrogen and incubated as above for 2 h. At the end of digestion, an aliquot of the product (referred as “digesta”) was centrifuged (5810R, Eppendorf, Germany) at 20,000 g at 4 °C for 60 min to separate the aqueous fraction containing mixed micelles. The aqueous fraction was filtered through 0.2 µ surfactant free cellulose acetate membrane (Corning, NY 1831, USA) to remove microcrystalline carotenoid aggregates, if any and microbial contamination. The filtrate is referred as the “micellar fraction”. Aliquots of digesta and micellar fractions were stored at −20 °C under a blanket of nitrogen and analyzed within 2–3 days.
Caco-2 cell culture
Caco-2 cells were obtained from the National Centre for Cell Sciences (NCCS, Pune, India), and grown as described previously (Palika et al. 2013). Briefly, cells, between passages 28–35, were seeded at a density of 50,000 cells/cm2 in 6-well plates and cultured in complete DMEM with 10% fetal bovine serum (FBS, supplemented with 1% NEAA, 0.4 mM glutamine and 1% antibiotic– antimycotic solution) and maintained at 37 °C in an incubator with a 5% CO2/95% air atmosphere at constant humidity.
Carotenoid uptake
Carotenoid uptake from the micellar fraction generated during in vitro digestion of spinach in the absence and presence of 2.5% dietary fat was studied in differentiated Caco-2 cells, at 12–13 days post-confluence as described previously (Pullakhandam and Failla 2007). Briefly, aliquots of micellar fraction obtained after intestinal digestion were immediately diluted in DMEM (1:4) and fed to the differentiated Caco-2 cells for 3 h. At the end of incubation, the spent medium was removed; monolayers were washed once with ice-cold phosphate-buffered saline (PBS, pH 7.2,) containing 0.5% bovine serum albumin to remove residual carotenoids adhering to the cell surface and twice with PBS only. At the end, cells were scraped into 1 mL PBS, and stored under nitrogen at −20 °C for a maximum of 2–3 days before analysis.
Extraction of carotenoids
Total carotenoids were extracted as described previously (Pullakhandam and Failla 2007). Briefly, the vegetable puree or fruit pulp (2 g) was mixed with methanol and saponified. The carotenoids were then extracted with petroleum ether:acetone (2:1 vol/vol), dried under nitrogen and analyzed by HPLC as described below. The frozen samples of digesta (2 mL), micellar fraction (3 mL), cell pellets (sonicated in 1 mL PBS for 30 s.) were thawed and extracted thrice with an equal volume of petroleum ether:acetone (2:1 vol/vol). The extracts were combined and dried under nitrogen at 37 °C. The residue was solubilized in methanol/dichloromethane (80:20 v/v) and analyzed immediately. Using apo-8 carotenal as a recovery standard, extraction efficiency was routinely 95–105%.
HPLC analysis of carotenoids
HPLC analysis of carotenoids was carried out as described previously (Mashurabad et al. 2016). Briefly, carotenoids were analyzed using Agilent 1100 HPLC system (Agilent, Model 1100, Puala Alto, CA, USA) equipped with an auto-sampler, and UV–Vis detection system controlled by Chemstation software. Either 50 or 100 µL aliquots of reconstituted carotenoid extracts from digesta or filtered aqueous fraction, respectively were injected and fractionated on a reverse-phase column (ODS, C-18, 250X4.6 mm, 5 µm, Thermo) with methanol: dichloromethane (80:20, v:v) at a flow rate of 1 mL/min. The flow was monitored at 450 nm for a period of 30 min. Carotenoids were identified and quantified by comparing retention times and peak areas with authentic standards.
Statistics
The percent (%) micellarization of carotenoids in the filtered aqueous fraction was computed considering the total carotenoid content in digesta as 100%. Data were analyzed using SPSS version 11.0 (SPSS, Chicago, IL). Descriptive statistics including mean and SD were calculated for the efficiency of micellarization (%) of carotenoids from digested foods. Means were compared using one-way analysis of variance (ANOVA) followed by least significant differences test. Linear regression analysis and Pearson’s coefficients were calculated to compare the relationship between β-carotene content in the micellar fraction to that of its intestinal cell uptake. All experiments were conducted in triplicate and each experiment was repeated twice to provide six independent observations. Differences were considered significant at P < 0.05.
Results
Carotenoids content of vegetables and fruits and fatty acid composition of dietary fat
The carotenoid content of test foods is given in Table 1. The β-carotene content of carrot was highest followed by drumstick leaves, spinach and papaya. Lutein content of drumstick leaves was 1.5 times higher compared to spinach. α-carotene was detectable only in carrot while lycopene was detected only in papaya. The fatty acid composition of different vegetable oils is given in Table 2. The unsaturated fatty acid content was higher in sunflower oil, olive oil and soybean compared to peanut > palm > coconut oil. The medium chain fatty acid content (<C14) was higher in coconut oil compared to all other oils. However, soybean alone contained linolenic acid (C18:3, 6.6%) and peanut oil contained docosanoic acid (C22:0, 4%), while the oleic acid (C18:1, 78%) content was higher in olive oil.
Table 1.
Carotenoid content (mean ± SD) of vegetables and fruit
| Name of sample | µg/100 g fresh weight | |||
|---|---|---|---|---|
| β-carotene | α-carotene | Lutein | Lycopene | |
| Carrot | 4423 ± 42 | 2123 ± 28 | – | – |
| Spinach | 2605 ± 51 | – | 3890 ± 65 | – |
| Drumstick | 3200 ± 65 | – | 5360 ± 79 | – |
| Papaya | 1050 ± 42 | – | – | 76.7 ± 4.6 |
Table 2.
Fatty acid composition of vegetable oils (g/100 g)
| Fatty acids | Olive | Soybean | Sunflower | Peanut | Palm | Coconut |
|---|---|---|---|---|---|---|
| Capric acid C10:0 | – | – | – | – | – | 3.50 |
| Lauric acid C12:0 | – | – | – | – | 0.26 | 47.30 |
| Myristic acid C14:0 | – | 0.08 | 0.08 | 0.06 | 1.13 | 24.00 |
| Myristoleic acid C14:1 | – | – | – | – | – | – |
| Palmitic acid C16:0 | 10.80 | 11.00 | 6.83 | 14.10 | 40.30 | 11.10 |
| Palmitoleic acid C16:1 | 0.73 | – | – | – | – | – |
| Stearic acid C18:0 | 3.30 | 5.67 | 3.50 | 3.95 | 4.20 | 3.70 |
| Oleic acid C18:1 | 78.6 | 21.80 | 28.14 | 42.70 | 42.90 | 8.00 |
| Linoleic acid C18:2 | 6.44 | 54.70 | 61.10 | 35.10 | 11.10 | 2.20 |
| Linolenic acid C18:3 | – | 6.58 | – | – | – | – |
| Docosanoic acid C22:0 | – | – | – | 3.99 | – | – |
Effect of dietary fat content on micellarization of carotenoids from carrot, spinach, drumstick and papaya
Addition of 0.5–5% olive oil increased the micellarization of lutein, β-carotene, α-carotene and lycopene from carrot, drumstick leaves, spinach, and papaya (Table 3). However, lutein micellarization from spinach and drumstick leaves was higher with 1% olive oil, and remained similar thereafter. The extent of increase in carotenoid micellarization remained similar after 5% dietary fat, indicating saturation. Therefore, all the subsequent experiments were performed with 2.5% dietary fat.
Table 3.
Effect of olive content on efficiency of carotenoid micellarization from vegetables and fruit
| Vegetable/Fruit | % olive oil | % micellarization | |||
|---|---|---|---|---|---|
| Lutein | β-carotene | α-carotene | Lycopene | ||
| Carrot | 0 | – | 4.8 ± 0.9a | 4.6 ± 0.8a | – |
| 1 | – | 13.2 ± 1.2b | 12.6 ± 1.8b | – | |
| 2.5 | – | 22.6 ± 2.6c | 24 ± 2.2c | – | |
| 5 | – | 23.2 ± 2.9c | 26 ± 2.7c | – | |
| 10 | – | 26.2 ± 3.9c | 28 ± 2.2c | – | |
| Spinach | 0 | 22 ± 2.7a | 7.2 ± 0.2a | – | – |
| 1 | 59 ± 2.2b | 13 ± 1.4b | – | – | |
| 2.5 | 62 ± 2.6b | 34 ± 2.6c | – | – | |
| 5 | 67 ± 2.9b | 36 ± 3.1c | – | – | |
| 10 | 62 ± 2.2b | 32 ± 3.1c | – | – | |
| Drumstick | 0 | 27 ± 1.7a | 5.2 ± 0.8a | – | – |
| 1 | 61 ± 2.6b | 16 ± 1.5b | – | – | |
| 2.5 | 61 ± 2.7b | 31 ± 1.6c | – | – | |
| 5 | 62 ± 1.9b | 32 ± 1.9c | – | – | |
| 10 | 59 ± 3.8b | 29 ± 2.5c | – | – | |
| Papaya | 0 | – | 8.2 ± 0.6a | – | 3.4 ± 0.2a |
| 1 | – | 21.5 ± 1.7b | – | 6.7 ± 0.8b | |
| 2.5 | – | 45 ± 2.8c | – | 16.9 ± 0.8c | |
| 5 | – | 47 ± 1.9c | – | 22.1 ± 1.4d | |
| 10 | – | 48 ± 2.7c | – | 19.2 ± 2.1d | |
The percent efficiency of micellarization was computed considering the carotenoid content in test food as 100%. Data are mean ± SD for 6 observations generated in two independent experiments. Means without a common superscript in a column and within a test food differ significantly (p < 0.05).–refers to not determined
Effect of food matrix and carotenoid polarity on micellarization
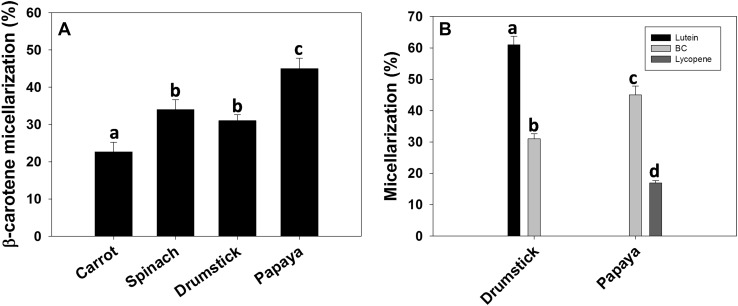
Extent of β-carotene micellarization was dependent on the food matrix in the order of papaya > spinach = drumstick leaves > carrot (Fig. 1a). Among the carotenoids, the micellarization of lutein was higher from drumstick leaves compared to β-carotene while it was higher from papaya compared to lycopene (Fig. 1b).
Fig. 1.
Effect of carotene distribution and polarity on efficiency of carotenoid micellarization: A β-carotene micellarization was compared among test foods to understand the effect of food matrix on micellarization. B The lutein, BC and lycopene micellarization was compared amongst drumstick leaves and papaya (both contain carotenoids in fat globules) to understand the effect of polarity of carotenes with that of efficiency of micellarization. The bars represent mean ± SD of 6 observations, and the bars with different superscripts differ significantly (p < 0.05)
Effect of type of vegetable oil on micellarization carotenoids from vegetables and fruits
The micellarization of carotenoids increased due to addition of all types of vegetables oils (2.5% w/w) from carrot (BC and AC), spinach (Lutein, BC), drumstick leaves (Lutein, BC) and papaya (BC and lycopene) (Table 4). However, the extent of increase in micellarization of carotenes was higher with oils rich in unsaturated fatty acids (olive = soybean = sunflower) compared to oils rich in saturated fat (peanut = palm > coconut), with the exception of lutein which remained independent of the type of dietary fat. Interestingly, micellarization of all the carotenoids was lowest with coconut oil, rich in saturated, but medium chain fatty acids.
Table 4.
Effect of type of vegetable oil (2.5%) on micellarization of carotenoids
| Vegetable/Fruit | Carotene | Olive | Soybean | Sunflower | Peanut | Palm | Coconut |
|---|---|---|---|---|---|---|---|
| Carrot | BC | 22.6 ± 2.2a | 24 ± 2.8a | 22.6 ± 2.2a | 16 ± 2.8b | 15 ± 2.1b | 7.8 ± 0.8c |
| AC | 23.2 ± 2.9a | 26 ± 2.7a | 24.6 ± 2.6a | 14 ± 1.8b | 13 ± 2.5b | 7.2 ± 0.8c | |
| Spinach | LuT | 66 ± 4.6a | 67 ± 5.6a | 68 ± 5.9a | 66 ± 5.8a | 68 ± 6.2a | 42 ± 4.7b |
| BC | 34 ± 2.6a | 35 ± 2.9a | 32 ± 1.2a | 26 ± 0.8b | 24 ± 0.6b | 11 ± 0.2c | |
| Drumstick | Lut | 61 ± 2.7a | 61 ± 3.6a | 64 ± 4.9a | 62 ± 2.8a | 62 ± 7.2a | 48 ± 3.7b |
| BC | 31 ± 0.6a | 30 ± 0.8a | 29 ± 0.5a | 22 ± 0.6b | 21 ± 1.1b | 8.1 ± 0.8c | |
| Papaya | BC | 45 ± 1.8a | 49 ± 2.1a | 43 ± 1.5a | 31 ± 2.1b | 29 ± 1.4b | 12 ± 0.6c |
| Lyco | 16.9 ± 0.8a | 17.2 ± 0.7a | 15.8 ± 0.6a | 10.6 ± 0.5b | 10.6 ± 0.8b | 8.4 ± 0.2c |
The percent efficiency of micellarization was computed considering the carotenoid content in test food as 100%. Data are mean ± SD for 6 observations generated in two independent experiments. Means without a common superscript in a row differ significantly (p < 0.05)
Effect of dietary fat on intestinal cell uptake of β-carotene and lutein from spinach
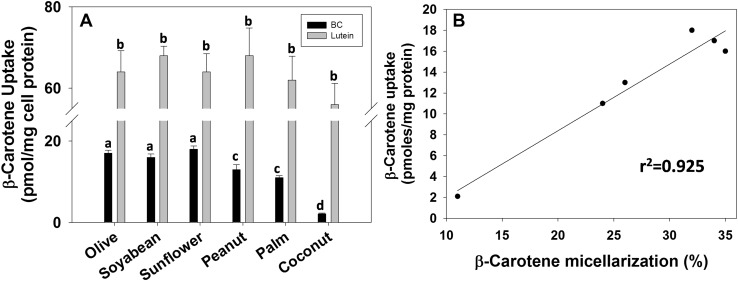
Cellular accumulation of lutein from the micellar fraction of spinach was higher compared to β-carotene either in the absence or presence of dietary fat (Fig. 2A). The uptake of lutein was increased with the addition of dietary fat, but it remained independent of the type of dietary fat used. In contrast, β-carotene uptake was dependent on the type of dietary fat used, akin to its micellarization. Further, a positive relationship was observed between β-carotene content in the micellar fraction with that of its cellular accumulation as evidenced by Pearson's correlation (R = 0.975 and p < 0.001) and regression analysis (r2 = 0.925, Fig. 2B).
Fig. 2.
Effect of type of dietary fat on β-carotene uptake in intestinal cells: the aqueous micellar fraction obtained after digestion of spinach puree with 2.5% dietary fat from indicated vegetable oils was fed to the differentiated Caco-2 cells for a period 3 h. The cellular β-carotene content was estimated as described in methods (A). The micellarization of BC was plotted against its cellular uptake, and the line represents the linear regression (B). The bars represent mean ± SD of 6 observations, and the bars with different superscripts differ significantly (p < 0.05)
Discussion
Micellarization is a prerequisite for intestinal absorption of dietary carotenoids, which in turn is influenced by food matrix, physicochemical properties of carotenoids and dietary fat. Our results demonstrate that addition of dietary fat (0–10%) increases the micellarization of lutein from spinach (22–62%) and drumstick leaves (27–59%), β- carotene from carrot (4.8–26.2%), spinach (7.2–32%), drumstick leaves (5.2–29%) and papaya (8.2–48%), α-carotene (4.6–28%) from carrot, and lycopene from papaya (3.4–19.2%). Further, the micellarization of carotenes increased dose dependently from 0 to 2.5% dietary fat followed by saturation, while the saturation of lutein micellarization required only 1% dietary fat. The β-carotene micellarization varied among different test foods in the order of papaya > spinach = drumstick > carrot. The efficiency of micellarization decreased in the order of lutein > β-carotene = α-carotene > lycopene independent of the amount of dietary fat. Although lutein micellarization increased with dietary fat, it remained independent of the type of dietary fat. Interestingly, micellarization of other carotenoids was dependent on the type of dietary fat in the order of olive = soybean = sunflower > peanut = palm > coconut oil. These results suggest that food matrix, carotenoid polarity and dietary fat determine the extent of carotenoid micellarization and therefore, bioavailability from plant foods.
The carotenoid composition and content of test foods was in agreement with previous reports (Veda et al. 2007; Bhaskarachary et al. 1995; Chitchumroonchokchai et al. 2004; Pullakhandam and Failla 2007). The minor differences observed in carotene content among test foods are likely due to natural seasonal and cultivar variations. The fatty acid composition of vegetable oils was also consistent with published results (Johnson and Saikia 2009; Failla et al. 2014). Co-consumption of dietary fat is known to increase the carotenoid absorption in humans (Jayarajan et al. 1980; Brown et al. 2004; Unlu et al. 2005; Goltz et al. 2012). Previously, we and others have demonstrated that inclusion of fat during simulated in vitro digestion increases micellarization, explaining the higher absorption observed in humans (Pullakhandam and Failla 2007; Chitchumroonchokchai et al. 2004). It was postulated that dietary fat mediates the transfer of carotenes from lipid droplets to the aqueous micellar fraction and thereby facilitates their micellarization (Borel et al. 1996; Rich et al. 2003). In the absence of dietary fat, micellarization of lutein exceeded that of other carotenoids. Addition of 0.5–10% dietary fat increased the micellarization of all the carotenoids compared to its absence, and is in agreement with that of previous observations with mixed salads (Failla et al. 2014). However, increments in micellarization were higher between 0 and 2.5% dietary fat compared to 2.5–10%, which could be due to saturation or limiting amounts of bile salts and other reagents present.
The carotene pigments have been reported to exist as either crystalline (carrot) or in fat globule like structures in green leaves (Schweiggert et al. 2012, 2014; Xia et al. 2015). Among the test foods the micellarization of β-carotene was higher from papaya > spinach = drumstick > carrot. In agreement with these results, better accessibility of carotenoids from papaya and mango (consisting of fat globule like carotene pigments) compared to carrot and tomato (crystalline carotenoids) have been reported (Schweiggert et al. 2012, 2014). The higher micellarization of carotenoids from papaya and green leaves could be explained by better partitioning of carotenoids from lipid droplets into aqueous phase compared to crystalline carotenoids in carrot. Among the carotenoids, lutein micellarization was higher compared to β-carotene = α-carotene > lycopene. In agreement with these results the absorption of lutein was found to be 5 times higher compared to β-carotene in humans (Van het Hof et al. 1999). Further, micellarization of lycopene was also found to be lower compared to other carotenoids (Huo et al. 2007; Failla et al. 2014). It was postulated that polar carotenoids such as lutein reside at the surface of the lipid droplet and can be transferred directly to the aqueous phase while non-polar carotenoids reside inside the lipid droplet and needs dietary fat for their transfer to the aqueous phase (Borel et al. 1996; Rich et al. 2003). Higher micellarization of lutein (even at lower dietary fat) compared to β-carotene in green leaves, and lower micellarization of lycopene compared to β-carotene from papaya, lends further support to the above notion. Together, these results imply that the efficiency of carotenoid micellarization decreases with increased hydrophobicity, and is independent of the type of food matrix. However, β-carotene micellarization was higher from papaya compared to spinach and drumstick leaves. It has been reported that lutein interferes with the transfer of β-carotene from oil emulsions to mixed micelles in vitro (Tyssandier et al. 2001). Therefore, higher lutein content in the latter might inhibit the β-carotene micellarization from green leaves. Nevertheless, the β-carotene micellarization remained similar from spinach and drumstick leaves, despite the latter containing twofold higher lutein levels. It is therefore likely that other food matrix components might influence the carotene micellarization.
The relative micellarization of carotenoids decreased in the order of olive = soybean = sunflower > peanut = palm > coconut oil independent of the food matrix. These results are similar to the observations with spinach and mixed salad digested with long chain unsaturated fatty acids (Nagao et al. 2013; O’Connell et al. 2008; Failla et al. 2014). For instance, the micellarization of β-carotene from spinach was found to be higher with unsaturated fatty acids compared to saturated fat (Nagao et al. 2013). In this study oleic acid (monounsaturated) better facilitated the micellarization compared to polyunsaturated fatty acids (linoleic acid and α-linolenic acid) from spinach supplemented with 0.1% fat. Further, studies in human subjects also reported higher absorption of carotenoids when supplemented with fat rich in monounsaturated fat (Clark et al. 2000; Goltz et al. 2012). Despite the fact that monounsaturated fatty acid content of olive oil is twofold higher compared to soy and sunflower, the extent of micellarization with these fat sources remained similar in the present study, which could be due variations in amount of dietary fat and source of lipids (fatty acylglycerols vs oils) used for digestion reactions. In contrast, carotenoid micellarization from a mixed vegetable salad was found to be slightly but significantly higher from soybean oil compared to olive oil (Failla et al. 2014). However, in the present study the effect of these two oils remained similar, which could be due to differences in food matrix used for the digestion reactions. The lowest micellarization of carotenoids, including lutein with coconut oil could be explained by its higher content of medium chain fatty acids. The primary role of dietary fat is to facilitate the release of carotenoids from food matrix and their subsequent micellarization (Borel et al. 1996; Rich et al. 2003). The short chain fatty acids with low relative hydrophobicity may not efficiently facilitate the release of carotenoids from plant pigments leading to their poor micellarization. Consistent with this notion, dietary triacylglycerols with long-chain rather than medium-chain fatty acids reported to enhance the micellarization and absorption of β-carotene (Nagao et al. 2013; Huo et al. 2007; Borel et al. 1998).
The intestinal absorption of carotenoids from mixed micelles is mediated by various proteins including SR-B1 and NPC1L1 (Mashurabad et al. 2016; Reboul 2013). The carotene structure and micellar lipid composition might influence the intestinal cell uptake of carotenoids from mixed micelles. The intestinal cell uptake of lutein exceeded that of β-carotene, from micellar fraction of spinach, in differentiated Caco-2 cells, is in agreement with in vitro and human studies (Pullakhandam and Failla 2007; Van het Hof et al. 1999). The uptake of lutein remained independent of type of dietary fat while the uptake of β-carotene was higher with dietary fat rich in unsaturated fat compared to saturated fat, akin to its micellarization. Further, there is a positive relationship between β-carotene uptake and its content in micellar fraction (R2 = 0.925), implying that micellar β-carotene concentration, rather than type of fat determines the intestinal absorption. In agreement with these results dietary fat reported to impact on micellarization but not on intestinal uptake (Huo et al. 2007). Alternatively, preconditioning of intestinal cells with fatty acids reported to increase or decrease the uptake and transcellular transport of carotenoids (Failla et al. 2014; Mashurabad et al. 2016). Therefore, the effects of fatty acids on intestinal carotenoid absorption are likely to be mediated by post-absorptive mechanisms via modulating the lipid transporter expression rather than micellar fat composition per se.
The major limitations of the current study are 1. In contrast to previous studies we did not find difference in carotenoid micellarization among olive oil (rich in MUFA) compared to sunflower or soy bean oil (rich in PUFA). 2. We also did not study the carotenoid-esters, isomers or other competing phytochemicals to reliably comment on food matrix effects. 3. The carotenoid pigment ultrastructures are not experimentally verified in the present study.
Conclusion
The results demonstrate that a fairly low amount of dietary fat (1–2.5%) is sufficient to enhance micellarization of dietary carotenoids. Carotenoid micellarization was dependent on the food matrix, the degree of polarity and type of dietary fat used. A general trend of higher micellarization was observed with dietary fat rich in unsaturated fatty acids. However, intestinal uptake of β-carotene was dependent on carotenoid concentration in mixed micelles but not on the type of dietary fat. Therefore, consumption of carotenoid rich foods with a reasonable amount of dietary fat may be advocated to optimize the carotenoid absorption.
Acknowledgements
This work was supported by Indian Council of Medical Research (ICMR), Government of India-(5/9/1076/2012-NUT). PCM and YWJ are supported by a Senior and Junior Research Fellowship, respectively from University Grants Commission, Government of India. We thank Dr. Ahmad Ibrahim, Scientist E, National Institute of Nutrition for fatty acid analysis and critical suggestions.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
References
- Bhaskarachary K, Sankar Rao DS, Deosthale YG, Reddy V. Carotene content of some common and less familiar foods of plant origin. Food Chem. 1995;54(2):189–193. doi: 10.1016/0308-8146(95)00029-I. [DOI] [Google Scholar]
- Borel P, Grolier P, Armand M, Partier A, Lafont H, Lairon D, Azais-Braesco V. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res. 1996;37(2):250–261. [PubMed] [Google Scholar]
- Borel P, Tyssandier V, Mekki N, Grolier P, Rochette Y, Alexandre-Gouabau MC, Lairon D, Azaïs-Braesco V. Chylomicron beta-carotene and retinyl palmitate responses are dramatically diminished when men ingest beta-carotene with medium-chain rather than long-chain triglycerides. J Nutr. 1998;128(8):1361–1367. doi: 10.1093/jn/128.8.1361. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80(2):396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- Chitchumroonchokchai C, Schwartz SJ, Failla ML. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and Caco-2 human intestinal cells. J Nutr. 2004;134(9):2280–2286. doi: 10.1093/jn/134.9.2280. [DOI] [PubMed] [Google Scholar]
- Clark RM, Yao L, She L, Furr HC. A comparison of lycopene and astaxanthin absorption from corn oil and olive oil emulsions. Lipids. 2000;35(7):803–806. doi: 10.1007/s11745-000-0589-8. [DOI] [PubMed] [Google Scholar]
- Failla ML, Huo T, Thakkar SK. In vitro screening of relative bioaccessibility of carotenoids from foods. Asia Pac J Clin Nutr. 2008;17(1):200–203. [PubMed] [Google Scholar]
- Failla ML, Chitchumronchokchai C, Ferruzzi MG, Goltz SR, Campbell WW. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct. 2014;5(6):1101–1112. doi: 10.1039/c3fo60599j. [DOI] [PubMed] [Google Scholar]
- Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion model for estimating the bioavailability of carotenoids from meals. J Agric Food Chem. 1999;47(10):4301–4309. doi: 10.1021/jf9903298. [DOI] [PubMed] [Google Scholar]
- Ghafoorunissa G, Reddy V, Sesikaran B. Palmolein and groundnut oil have comparable effects on blood lipids and platelet aggregation in healthy Indian subjects. Lipids. 1995;30(12):1163–1169. doi: 10.1007/BF02536619. [DOI] [PubMed] [Google Scholar]
- Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res. 2012;56(6):866–877. doi: 10.1002/mnfr.201100687. [DOI] [PubMed] [Google Scholar]
- Hu X, Jandacek RJ, White WS. Intestinal absorption of β-carotene ingested with a meal rich in sunflower oil or beef tallow: postprandial appearance in triglycerol-rich lipoproteins in women. Am J Clin Nutr. 2000;71(50):1170–1180. doi: 10.1093/ajcn/71.5.1170. [DOI] [PubMed] [Google Scholar]
- Huo T, Ferruzzi MG, Schwartz SJ, Failla ML. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J Agric Food Chem. 2007;55(22):8950–8957. doi: 10.1021/jf071687a. [DOI] [PubMed] [Google Scholar]
- ICMR . Nutrient requirements and recommended dietary allowances for Indians: a report of the expert group of the ICMR. Hyderabad: National Institute of Nutrition; 2010. [Google Scholar]
- Jayarajan P, Reddy V, Mohanram M. Effect of dietary fat on absorption of beta carotene from green leafy vegetables in children. Indian J Med Res. 1980;71:53–56. [PubMed] [Google Scholar]
- Johnson S, Saikia N. Fatty acids profile of edible oils and fats in India. New Delhi: Centre for Science and Environment; 2009. [Google Scholar]
- Laxmaiah A, Nair MK, Arlappa N, Raghu P, Balakrishna N, Rao KM, Galreddy C, Kumar S, Ravindranath M, Rao VV, Brahmam GN. Prevalence of ocular signs and subclinical vitamin A deficiency and its determinants among rural pre-school children in India. Public Health Nutr. 2012;15(4):568–577. doi: 10.1017/S136898001100214X. [DOI] [PubMed] [Google Scholar]
- Mashurabad PC, Kondaiah P, Palika R, Ghosh S, Nair MK, Raghu P. Eicosapentaenoic acid inhibits intestinal β-carotene absorption by downregulation of lipid transporter expression via PPAR-α dependent mechanism. Arch Biochem Biophys. 2016;590:118–124. doi: 10.1016/j.abb.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Nagao A, Kotake-Nara E, Hase M. Effects of fats and oils on the bioaccessibility of carotenoids and vitamin E in vegetables. Biosci Biotechnol Biochem. 2013;77(5):1055–1060. doi: 10.1271/bbb.130025. [DOI] [PubMed] [Google Scholar]
- O’Connell O, Ryan L, O’Sullivan L, Aherne-Bruce SA, O’Brien NM. Carotenoid micellarization varies greatly between individual and mixed vegetables with or without the addition of fat or fiber. Int J Vitam Nutr Res. 2008;78:238–246. doi: 10.1024/0300-9831.78.45.238. [DOI] [PubMed] [Google Scholar]
- Palika R, Mashurabad PC, Kilari S, Kasula S, Nair KM, Raghu P. Citric acid mediates the iron absorption from low molecular weight human milk fractions. J Agric Food Chem. 2013;61(46):11151–11157. doi: 10.1021/jf403973j. [DOI] [PubMed] [Google Scholar]
- Pullakhandam R, Failla ML. Micellarization and intestinal cell uptake of beta-carotene and lutein from drumstick (Moringa oleifera) leaves. J Med Food. 2007;10(2):252–257. doi: 10.1089/jmf.2006.250. [DOI] [PubMed] [Google Scholar]
- Ramachandran P. Nutrition transition India 1947–2007. New Delhi: Nutrition Foundation of India; 2009. pp. 278–286. [Google Scholar]
- Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients. 2013;5(9):3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E, Richelle M, Perrot E, Desmoulins-Malezet C, Pirisi V, Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem. 2006;54(23):8749–8755. doi: 10.1021/jf061818s. [DOI] [PubMed] [Google Scholar]
- Rich GT, Faulks AL, Parker ML, Wickham MS, Fillery-Travis A. Solubilization of carotenoids from carrot juice and spinach in lipid phases: I. Modeling the gastric lumen. Lipids. 2003;38(9):933–945. doi: 10.1007/s11745-003-1147-0. [DOI] [PubMed] [Google Scholar]
- Schweiggert RM, Mezger D, Schimpf F, Steingass CB, Carle R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012;135(4):2736–2742. doi: 10.1016/j.foodchem.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Schweiggert RM, Kopec RE, Villalobos-Gutierrez MG, Högel J, Quesada S, Esquivel P, Schwartz SJ, Carle R. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study. Br J Nutr. 2014;111(3):490–498. doi: 10.1017/S0007114513002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar B, Nair KM, Sreeramulu D, Suryanarayana P, Ravinder P, Shatrugna V, Kumar PA, Raghunath M, Vikas RV, Balakrishna N, Kumar PU, Raghuramulu N. Effect of micronutrient supplement on health and nutritional status of schoolchildren: biochemical status. Nutrition. 2006;22(1):S15–S25. doi: 10.1016/j.nut.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Tyssandier V, Lyan B, Borel P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim Biophys Acta. 2001;1533(3):285–292. doi: 10.1016/S1388-1981(01)00163-9. [DOI] [PubMed] [Google Scholar]
- Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135(3):431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- Van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RP, Van Dusseldorp M, Weststrate JA, Hautvast JG. Bioavailability of lutein from vegetables is 5 times higher than that of β-carotene. Am J Clin Nutr. 1999;70(2):261–268. doi: 10.1093/ajcn.70.2.261. [DOI] [PubMed] [Google Scholar]
- Veda S, Platel K, Srinivasan K. Varietal differences in the bioaccessibility of beta-carotene from mango (Mangifera indica) and papaya (Carica papaya) fruits. J Agric Food Chem. 2007;55(19):7931–7935. doi: 10.1021/jf0712604. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Global prevalence of vitamin A deficiency (1995–2005) Geneva: WHO; 2009. [Google Scholar]
- Xia Z, McClements DJ, Xiao H. Influence of physical state of β-carotene (crystallized versus solubilized) on bioaccessibility. J Agric Food Chem. 2015;63(3):990–997. doi: 10.1021/jf504673v. [DOI] [PubMed] [Google Scholar]