Abstract
Roots of Rhodiola imbricata Edgew from Indian trans-Himalayan cold desert known for their nutritional and medicinal attributes were evaluated for the dietary amino acids, fatty acids and mineral composition. Nine essential and twelve non-essential amino acids were quantified. The contents ranged between 91.33 and 1640.67 µg/g. Histidine (1434.33 µg/g), lysine (1329.33 µg/g) and threonine (1015.67 μg/g) were dominant essential amino acids, while glycine (1640.67 µg/g), proline (1263.67 µg/g), alanine (1142.33 µg/g), cystine HCL (1136.33 μg/g) and nor leucine (1038.67 μg/g) were major non essential amino acids. The total lipid was found to be rich source of saturated fatty acids such as capric acid (19.91%), caproic acid (10.87%), palmitic acid (9.42%), lignoceric acid (6.16%) and behenic acid (5.71%), which together constituted 52% of the lipid content. Linoleic acid (15.06%), oleic acid (12.38%), arachidonic acid (8.38%), linolelaidic acid (6.11%) and docosadienoic acid (5.99%) were prominent unsaturated fatty acids (UFAs). Mono unsaturated fatty acids (MUFAs) and poly unsaturated fatty acids (PUFAs) were 35.64% and 12.33% of the lipid content respectively. Calcium (11034.17 mg/kg), potassium (2143.25 mg/kg), iron (1441.17 mg/kg), magnesium (581.99 mg/kg), phosphorous (376.72 mg/kg) and sodium (109.75 mg/kg) were detected as the major dietary minerals.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2469-4) contains supplementary material, which is available to authorized users.
Keywords: Rhodiola imbricata root, Amino acids, Fatty acids, Minerals, Phytofoods
Introduction
The Indian trans-Himalayan cold desert region is a great repository of natural botanical resources having diverse utility towards development of phytofoods, health promoting natural products and other medicinal and therapeutic formulations (Ballabh et al. 2007; Dhar 2013; Tayade 2015). Rhodiola imbricata Edgew. (Family: Crassulaceae) is a trans-Himalayan high altitude medicinal herb widely used in the traditional system of medicine. It is also famous as Golden Root or Himalayan stone crop and locally known as Shrolo marpo in Ladakh. In India, it is mainly distributed at an altitude of 14,000–18,500 ft above mean sea level (AMSL) in the rocky slopes, wet places and higher passes of Ladakh region of trans-Himalayan cold desert (Tayade 2015). It is a source of various secondary metabolites like alkaloids, phenylpropanoids, phenylethanol derivatives, flavonoids, terpenoids, phenolic acids etc. with promising pharmacological properties and health promoting effects (Kumar et al. 2012). Using the root of this plant, our institute has developed a number of phytoproducts having high nutritive attributes and antioxidant properties (Ballabh et al. 2007; Chaurasia et al. 2011; Dhar et al. 2012; Dhar et al. 2013a, b). Recently, Choudhary et al. (2015) isolated and characterized phenolic compounds from this plant root with antioxidant and anticancer potential. Avasthi et al. (2016) also performed the bioassay guided screening and characterization of secondary metabolites of R. imbricata collected from Himalayan high altitude region of Ladakh.
In our continuous efforts to delineate the antioxidant potential, vitamin content and composition of bioactive phytochemotypes in R. imbricata root, we reported the secondary metabolite profile of R. imbricata (Tayade et al. 2013a, b, c). However, the primary metabolites such as amino acids, fatty acids and mineral profile of this plant root have not been studied before. Amino acids, fatty acids and minerals derived from plant sources are also known as important dietary components for human and animal nutrition. Therefore, estimation of these nutritional attributes is essential for phytofood development. We report qualitative and quantitative analysis of amino acids, fatty acids and dietary minerals in R. imbricata root using pre column phenylisothiocyanate (PITC) derivatization following reverse phase-high performance liquid chromatography (RP-HPLC), fatty acid methyl esters (FAMEs) derivatization with gas chromatography coupled with flame ionization detector (GC-FID) and inductively coupled plasma optical emission spectrometry (ICP-OES) analysis.
Materials and methods
Chemicals and reagents
HPLC grade acetonitrile, acetyl-chloride, 2-propanol, n-hexane, ethanol, methanol, sodium acetate trihydrate and glacial acetic acid were procured from Merck (Merck KGaA, Darmstadt, Germany). Triethylamine (TEA), phenylisothiocyanate (PITC) and amino acid standards, sodium hydrogen phosphate (Na2HPO4) and phosphoric acid (H3PO4) were obtained from Sigma–Aldrich (St. Louis, MO, USA). The FAMEs standard mixture (Supelco 37-Component FAME Mix, 47885-U, Supelco, Bellefonte, PA, USA) was used. Industrial grade nitrogen was used (Sigma Gases & Services, Delhi, India). Rest of the chemicals and solvents were of analytical grade. High purity water prepared with Milli-R/Q water purification system (Millipore, Bedford, MA, USA) was used for chemical analysis.
Plant materials
R. imbricata roots were collected from the trans-Himalayan region (Chang-La Top, Changthag valley, altitude 17,500 ft AMSL, latitude 34°2′49.81″N, longitude 77°55′49.78″E) of India in the month of October 2011 after the period of senescence. For plant material collection, all the necessary permits were acquired from the concerned authority Dr. B. Balaji (IFS), Divisional Forest Officer, Leh Forest Division, Jammu & Kashmir, India. The root samples were carefully washed, cut into small pieces and shade dried at room temperature for 15 days. Then, it was finely powdered and used for extraction. The matured root of R. imbricata having optimum bioactivities were collected after the period of senescence (Tayade et al. 2013a, b).
Amino acid analysis by RP-HPLC
The amino acid content of the root sample was determined using RP-HPLC with pre-column PITC derivatization according to our recent report with minor modifications (Dhar et al. 2013b).
Individual 21 amino acid standards were derivatized with PITC to a final concentration of 40 µg/ml. This solution was filtered and stored at −20 °C for further analysis. Acid hydrolysis was performed for extraction of amino acids from plant root (AOAC, 1990). Aliquots of prepared plant extract and working amino acid standard solutions were concentrated and dried under vacuum (37 °C, 20 mmHg), following addition of coupling reagent (methanol/water/TEA, 2:2:1, v/v). The solution was mixed and dried immediately under vacuum. Thereafter, the PITC reagent (methanol/TEA/water/PITC, 7:1:1:1, v/v) was mixed properly and kept at room temperature for 20 min prior to drying in vacuum.
Chromatographic analysis was performed by injecting 20 µl of the sample and standard in RP-HPLC system. Separation of amino acids was performed on reverse phase C-18 column (5 µm, 150 × 4.6 mm) (Pickering Laboratories, Inc., Mountain-View, California, USA) The chromagraphic conditions have been depicted in Table 1. Windows® 98 2000 Data Station and CLASS-VP™ Version 6.13 software was used for data acquisition and analysis.
Table 1.
Gradient program employed for the separation of PITC derivatized amino acidsa
| Run timeb (min) | Flow rate (ml/min) | % Buffer Ac | % Buffer B (60% acetonitrile in water) |
|---|---|---|---|
| 0 | 1 | 100 | 0 |
| 0.1 | 1 | 95 | 5 |
| 5 | 1 | 90 | 10 |
| 14 | 1 | 90 | 10 |
| 25 | 1 | 60 | 40 |
| 30 | 1 | 50 | 50 |
| 35 | 1 | 40 | 60 |
| 40 | 1 | 10 | 90 |
| 52 | 1 | 10 | 90 |
| 62 | 1 | 95 | 5 |
| 65 | 1 | 100 | 0 |
a Column temperature was maintained at 39 °C
b Run time was 62 min with 3 min column regeneration time
c Sodium acetate buffer [19 g of sodium acetate trihydrate was dissolved in 1 l of HPLC grade water. To this 0.5 ml of TEA was added and the contents were mixed properly. The pH of the solution was attuned to 6.4 with glacial acetic acid. To the filtrate (940 ml) acetonitrile (60 ml) was added, mixed and filtered through a 0.22 µm Millipore membrane]
Fatty acid analysis by GC-FID
Fatty acids were analysed by GC-FID following FAMEs derivatization similar to the method described in previous work (Dhar et al. 2013b). Fat was extracted into ether, methylated to FAMEs using BF3 in methanol, 14% (w/w). FAMEs were then measured quantitatively by a GC-4000A system (East & West Analytical Instruments, Beijing, China) coupled with flame ionization detector, split/split-less mode injector (5 ml/min), capillary column (HP-88, 100 m × 0.25 mm × 0.20 µm film, Agilents Technologies Ltd., Santa Clara, CA, USA) and A5000 Chromatogram Data Processing Workstation Software, Version 1.6 (AOAC, 2002).
Determination of minerals
The root sample was digested by hot block method with QBlock equipment (Questron Technologies Corp., Mississauga, Germany) (Dhar et al. 2013b). The digestion program was set for plant sample and reagents were mixed according to the manufacturer’s directions. The mineral elements viz. calcium, chromium, copper, cobalt, iron, magnesium, manganese, molybdenum, nickel, phosphorous, potassium, sodium, and zinc were quantified by ICP-OES (Varian, VISTA-MPX, CCD Simultaneous ICP-OES, United States).
Results and discussion
The dearth of information regarding the amino acid composition, fatty acid profile and mineral content of R. imbricata root which could play a vital role not only for the bio-activity and pharmacological properties but also provide new insights into the physiological adaptation aspects of the plant in the severe stressful environment of trans-Himalaya, motivated us towards the analysis of amino acid composition, fatty acid profiling and mineral content analysis in this plant root.
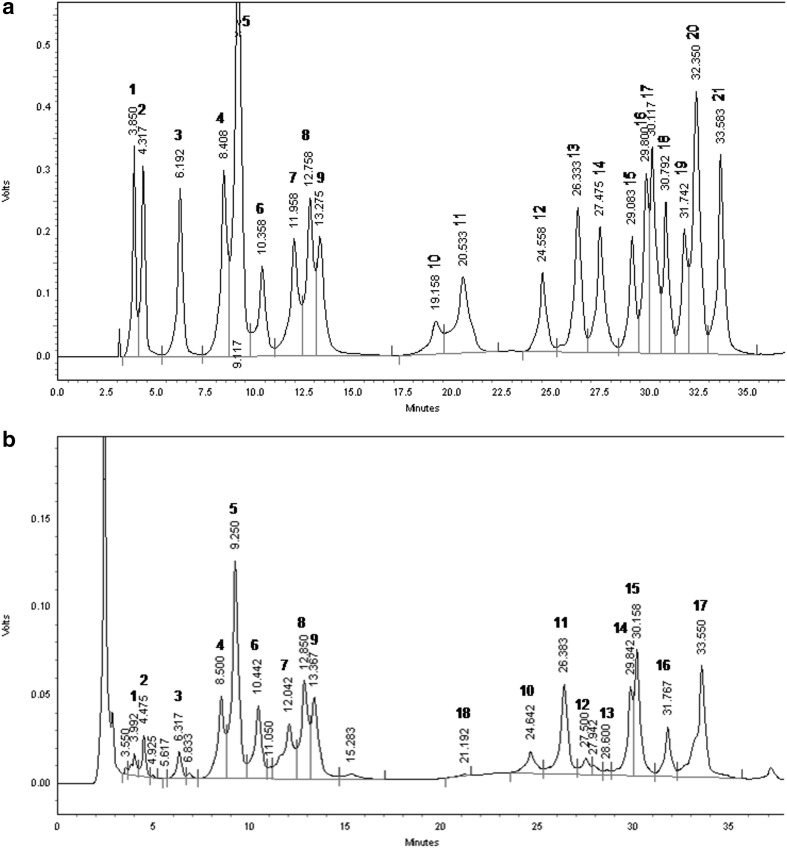
Amino acid content in R. imbricata root determined by RP-HPLC has been depicted in Table 2. The peaks were identified by comparing the retention time of amino acid standards and those in the sample (Fig. 1). Quantitation was performed using the external standard method by preparing calibration curves derived from linear regression analysis (Statistica 5.1, StatSoft, Tulsa, OK). There were 21 amino acids including 9 essential and 12 non-essential amino acids determined in the root sample of R. imbricata (Table 2). The content ranged between 91.33 ± 7.77 and 1640.67 ± 11.85 µg/g. Gly (1640.67 µg/g) was found to exhibit the highest content followed by His (1434.33 µg/g), Lys (1329.33 µg/g), Pro (1263.67 µg/g), Ala (1142.33 µg/g), Cys HCL (1136.33 µg/g), and Nor Leu (1038.67 µg/g). His and Lys were found to be the dominant contributors of essential amino acids, while the major non-essential amino acids were Gly, Pro, Ala, Cys HCL and Leu. L-2-amino-n-butyric acid, Trp and Orn were not detected. Val was found to be in the below detection limit (BDL) range and hence its content was not quantified. In agricultural and food sciences, precise identification of edible plants and cataloguing of food products and nutraceutical botanicals have become important issues with the ever increasing alertness in the consumers (Donno et al. 2016). Free amino acids correspond to a constant fraction of numerous natural foods, and on occasion, their quantity has been exploited for differentiation rationales (Maro et al. 2011; Bharate and Bharate 2014). The recent advances in amino acid profiling techniques provide a good alternative approach by converting enantiomers to diastereomers through chemical reactions with chiral derivatizing reagents, and by this strategy chromatographic separation can be easily achieved (Chandrul and Srivastava 2010). Pre-column derivatization and separation of amino acids using RP-HPLC is a well versed method for its cost-effectiveness, simplicity, sensitivity and speed of separation (Ilisz et al. 2012). We utilized the Pico-Tag technique with PITC derivatizing reagent that has the advantage of being highly perceptive and proficient of detecting nanogram (ng) quantities of amino acids (Dhar et al. 2013b). Plants subjected to different environmental and physiological stresses can accumulate amino acids in their system that play pivotal role in combating the stress. Amino acids are osmolyte that can regulate ion transport, stomatal opening, activation of phytohormones and growth substances, chelating effect on micronutrients and play vital role in detoxification of heavy metals. They are also responsible for the synthesis and functional properties of specific enzymes, gene expression, and redox-homeostasis (Rai 2002). Most importantly, in higher plants the amino acids serve as precursors for secondary metabolism (Zhao et al. 1998). Thus, the amino acids are directly related to plant stress physiology and have diverse preventive and recovery effects. In general, the major amino acids are present in elevated concentrations and typically associated with the primary carbon metabolism and nitrogen assimilation. Whereas, minor amino acids that comprise essential amino acids of human diet is generally less abundant (Noctor et al. 2002). The present analysis revealed a higher concentration of essential amino acids especially His and Lys than non-essential amino acids in R. imbricata root. Research investigations on the availability of amino acids in different Rhodiola sp. are very limited. In the last decade only, six types of Rhodiola L. from Xingjiang region of China were studied to estimate the amino acids in the root and rootstalk and they were reported to contain 8–18 amino acids including 3–7 essential type. Out of the six Rhodiola sp. grown in Xingjiang region, Rhodiola rosea L. is licensed in the traditional medicinal system of China as to herbal drug owing to the presence of most classes of amino acids (Ruan et al. 2001). These results also revealed that R. imbricata will be an excellent source of the natural amino acids that are useful to human consumption. This is the first ever study on the amino acid profiling of R. imbricata root that would help research efforts on metabolomics.
Table 2.
Content, type of amino acid, retention time (RT) and peak area as quantified by RP-HPLC (n = 3)
| Peak no. | Amino acid | Abbreviation | Type | RT (min) | Peak area | Content (µg/g) |
|---|---|---|---|---|---|---|
| 1. | l-Arginine | Arg | Non essential | 3.992 | 216,927 | 214.67 ± 7.09 |
| 2. | l-Aspartic Acid | Asp | Non essential | 4.475 | 324,956 | 434.67 ± 8.74 |
| 3. | l-Glutamic Acid | Glu | Non essential | 6.317 | 273,840 | 320.67 ± 7.77 |
| 4. | l-Serine | Ser | Non essential | 8.500 | 1,210,077 | 839.67 ± 10.97 |
| 5. | l-Glycine | Gly | Non essential | 9.250 | 3,064,675 | 1640.67 ± 11.85 |
| 6. | l-Histidine | His | Essential | 10.442 | 1,154,401 | 1434.33 ± 10.02 |
| 7. | l-Threonine | Thr | Essential | 12.042 | 1,232,241 | 1015.67 ± 8.02 |
| 8. | l-Alanine | Ala | Non essential | 12.850 | 1,517,814 | 1142.33 ± 11.02 |
| 9. | l-Proline | Pro | Non essential | 13.367 | 1,269,920 | 1263.67 ± 10.50 |
| 10. | l-Methionine | Met | Essential | 24.642 | 434,764 | 736.67 ± 8.02 |
| 11. | L-Cystine HCL | Cys HCl | Non essential | 26.383 | 1,450,843 | 1136.33 ± 11.72 |
| 12. | L-Cystine | Cys | Non essential | 27.500 | 267,474 | 239.33 ± 8.39 |
| 13. | l-Isoleucine | Ile | Essential | 28.600 | 66,781 | 91.33 ± 7.77 |
| 14. | l-Leucine | Leu | Essential | 29.842 | 1,084,489 | 928.67 ± 10.79 |
| 15. | L-Nor Leucine | Nor Leu | Non essential | 30.158 | 1,569,530 | 1038.67 ± 10.21 |
| 16. | l-Phenylalanine | Phe | Essential | 31.767 | 689,754 | 855.33 ± 9.02 |
| 17. | l-Lysine | Lys | Essential | 33.550 | 2,147,649 | 1329.33 ± 11.55 |
| 18. | L-2-amino-n-butyric acid | Abu | Non essential | ND | ND | ND |
| 19. | l-Valine | Val | Essential | 21.192 | 27,197 | BDL |
| 20. | l-Tryptophan | Trp | Essential | ND | ND | ND |
| 21. | l-Ornithine | Orn | Non essential | ND | ND | ND |
ND not detectable; BDL below detection limit
Fig. 1.
RP-HPLC chromatogram of a 21 amino acid standards, b amino acid profile of R. imbricata root. 1 l-Arginine; 2 l-Aspartic Acid; 3 l-Glutamic Acid; 4 l-Serine; 5 Glycine; 6 l-Histidine; 7 l-Threonine; 8 l-Alanine; 9 l-Proline; 10 L-2-amino-n-butyric acid; 11 l-Valine; 12 l-Methionine; 13 L-Cystine HCl; 14 L-Cystine; 15 l-Isoleucine; 16 l-Leucine; 17 L-Nor Leucine; 18 l-Tryptophan; 19 l-Phenylalanine; 20 l-Ornithine; 21 l-Lysine
Fatty acids influence a broad range of cellular processes such as functional ingredients of food and dietary supplements and provide numerous health benefits that include growth promoting effect, nutrition, metabolic functions, and many more (Huang 2007; Abuajah et al. 2015). A variety of techniques have been employed for fatty acid profiling in biological materials. Of these, gas chromatography coupled with flame ionization detection (GC-FID) is a widely used rapid and efficient method for the analysis of complex mixtures of biological samples with compounds of diverse molecular weights (Seppänen-Laakso et al. 2002). Separation, identification and quantification of long-chain fatty acid mixtures by GC-FID have been extensively used to acquire information about the less volatile fatty acids are converted into more volatile derivatives [such as fatty acid methyl esters (FAMEs)] prior to GC analysis. A number of studies were conducted to demonstrate the essential oil composition of Rhodiola sp. (Bai et al. 2005). Research investigation on Rhodiola rosea was also carried out with an objective to determine the phytochemical composition and antioxidant potential (Pooja et al. 2006). However, fatty acid profile of R. imbricata root from Indian trans-Himalayan region has not been investigated till date. In this present report, analysis of fatty acids in R. imbricata root showed the occurrence of 10 fatty acids contributing 99.99% of the total lipid (Table 3). The GC-FID chromatographic separation of 37 FAMEs standards along with fatty acid composition of root sample has been shown in Supplementary Fig. 2 The total lipid was found to be a rich source of SFAs viz. capric acid (19.91%), caproic acid (10.87%), palmitic acid (9.42%), lignoceric acid (6.16%) and behenic acid (5.71%), which together constituted 52% of the total lipid (Table 3). Among the UFAs, linoleic acid (15.06%), oleic acid (12.38%), arachidonic acid (8.38%), linolelaidic acid (6.11%) and docosadienoic acid (5.99%) were prominent. MUFA and PUFA were 35.54% and 12.38% of the total lipid content respectively in the root sample. In general, the nutritional value of fat is evaluated by PUFA/SFA ratio. According to Chang and Huang (1998), MUFA content and ratio of sum of PUFA and MUFA to SFA (PUFA + MUFA/SFA) have potential influence on lipid concentrations in rat plasma and liver. Low MUFA/SFA ratio, low PUFA/SFA ratio, high PUFA/MUFA ratio, and PUFA + MUFA/SFA ratio <2 are the requisite factors for maintenance of low plasma and liver lipid concentration. In the present investigation, MUFA/SFA value was 0.24. In addition, lower PUFA/SFA value (0.68), high PUFA/MUFA value (2.87) was also determined. The estimated value of PUFA + MUFA/SFA was 0.92 due to the occurrence of very low MUFA in R. imbricata root. Studies by previous investigators showed that even though virgin coconut oil contains <90% saturated fats and very low MUFA and PUFA, it has cholesterol and triacylglycerol lowering properties which may be accredited to small chain fatty acids (Mensink et al. 2003) and polyphenolic antioxidants (Nevin and Rajamohan 2004). In contrast, high MUFA containing diets were also reported to lower cholesterol concentration (Jenkins et al. 2010). It was also reported that the palm oil with elevated saturated fats (50%) is suitable for development of food and food products, soap manufacture and production of bio-diesel (Soh et al. 2006). Therefore, the high yields of lipid from R. imbricata root deserve scientific attention towards its utilization for health promoting medicinal foods, nutraceuticals, dietary botanical supplements, and other natural products. In this investigation, PUFAs were found to be the major contributor of fatty acids which is an important health-promoting nutrient having strong influence in alleviating cardiovascular, inflammatory, and heart diseases, atherosclerosis, autoimmune disorder, diabetes and many other health complains (Finley and Shahidi 2001). Especially, the essential amino acid linoleic acid possesses antioxidant (Peyrat-Maillard et al. 2003), acne reductive, (Latawe et al. 1998) anti-inflammatory, and moisture retentive properties (Darmstadt et al. 2002). Arachidonic acid, oleic acid, linolelaidic acid, and docosadienoic acid were also present in considerable amount in R. imbricata root which have great importance for human health improvement (Harris et al. 2009; Terés et al. 2008). Therefore, further research should be performed on the isolation and biological efficacy evaluation of these bio-active components that may have potential applications towards human health promotion.
Table 3.
Fatty acid composition of R. imbricata root (n = 3)
| Type of fatty acid | IUPAC name | CAS number | Retention time (min) | Peak area | Peak height | Peak width | % Content in total lipid | Content in root (mg/g) |
|---|---|---|---|---|---|---|---|---|
| Saturated fatty acid (SFA) | ||||||||
| Caproic acid (C6:0) | Hexanoic acid | 142-62-1 | 13.58 | 49,613 | 11,702 | 0.247 | 10.87 ± 0.27 | 8.8 ± 0.22 |
| Capric acid (C10:0) | Decanoic acid | 334-48-5 | 22.60 | 90,878 | 13,856 | 0.392 | 19.91 ± 0.49 | 16.2 ± 0.41 |
| Lignoceric acid (C24:0) | Tetracosanoic acid | 557-59-5 | 59.30 | 28,127 | 6418 | 0.742 | 6.16 ± 0.15 | 5.0 ± 0.13 |
| Palmitic acid (C16:0) | Hexadecanoic acid, (9Z)- | 57-10-3 | 40.70 | 42,991 | 9095 | 0.348 | 9.42 ± 0.23 | 7.6 ± 0.19 |
| Behenic acid (C22:0) | Docosanoic acid | 112-85-6 | 55.25 | 26,055 | 955 | 1.164 | 5.71 ± 0.14 | 4.6 ± 0.12 |
| Unsaturated fatty acid (UFA) | ||||||||
| Monounsaturated fatty acid (MUFA) | ||||||||
| Oleic acid (C18:1 n9c) | (9Z)-Octadec-9-enoic acid | 112-80-1 | 47.46 | 56,480 | 11,686 | 0.39 | 12.38 ± 0.31 | 10.0 ± 0.25 |
| Poly unsaturated fatty acid (PUFA) | ||||||||
| Linolelaidic acid (C18:2 n6t) | (9E,12E)-Octadeca-9,12-dienoic acid | 506-21-8 | 48.86 | 27,903 | 4221 | 0.383 | 6.11 ± 0.15 | 5.0 ± 0.14 |
| Linoleic acid (C18:2 n6c) | cis, cis-9,12-Octadecadienoic acid | 60-33-3 | 49.51 | 68,738 | 14,473 | 0.342 | 15.06 ± 0.38 | 12.2 ± 0.31 |
| Arachidonic acid (C20:4 n6) | (5Z,8Z,11Z,14Z)- Eicosatetraenoic acid | 506-32-1 | 57.33 | 38,262 | 2985 | 0.424 | 8.38 ± 0.21 | 6.8 ± 0.17 |
| cis-13,16-Docosadienoic acid (C22:2) | cis-13,16-Docosadienoic acid | 7370-49-2 | 59.05 | 27,314 | 5478 | 0.265 | 5.99 ± 0.15 | 4.9 ± 0.12 |
| ∑ SFA | 52.07 ± 1.28 | |||||||
| ∑ UFA | 47.22 ± 1.2 | |||||||
| ∑ MUFA | 12.38 ± 0.31 | |||||||
| ∑ PUFA | 35.54 ± 0.89 | |||||||
Minerals are also essential micronutrients that are needed in small amounts to maintain proper health and optimum physical performance (Soetan et al. 2010). There are a number of methods to analyze the mineral content in plant sample and among these techniques inductively coupled plasma optical emission spectrometer (ICP-OES) has become one of the most convenient analytical tools for the determination of trace elements in a wide variety of sample types (Hou and Jones 2000; Rodrigues et al. 2015). The mineral composition of R. imbricata has been depicted in Table 4. It was found to contain thirteen important mineral elements required for human nutrition. Calcium (11034.17 mg/kg) was quantified in highest amount tracking down to potassium (2143.25 mg/kg), iron (1441.17 mg/kg), magnesium (581.99 mg/kg), phosphorous (376.72 mg/kg), sodium (109.75 mg/kg), manganese (75.78 mg/kg), zinc (16.27 mg/kg), chromium (7.27 mg/kg), nickel (4.89 mg/kg), copper (3.49 mg/kg), cobalt (2.98 mg/kg) and molybdenum (2.65 mg/kg). These micronutrients could be beneficial for normal growth and development for human and other animals.
Table 4.
Mineral content of R. imbricata root estimated by ICP-OES (n = 3)
| Sr. no. | Minerals | Symbols | Content (mg/kg) |
|---|---|---|---|
| 1. | Calcium | Ca | 11034.17 ± 332.04 |
| 2. | Potassium | K | 2143.25 ± 65.37 |
| 3. | Iron | Fe | 1441.17 ± 27.98 |
| 4. | Magnesium | Mg | 581.99 ± 17.40 |
| 5. | Phosphorous | P | 376.72 ± 11.87 |
| 6. | Sodium | Na | 109.75 ± 3.32 |
| 7. | Manganese | Mn | 75.78 ± 2.21 |
| 8. | Zinc | Zn | 16.27 ± 0.54 |
| 9. | Chromium | Cr | 7.27 ± 0.32 |
| 10. | Nickel | Ni | 4.89 ± 0.21 |
| 11. | Copper | Cu | 3.49 ± 0.12 |
| 12. | Cobalt | Co | 2.98 ± 0.06 |
| 13. | Molybdenum | Mo | 2.65 ± 0.05 |
The cold-arid region of Indian trans-Himalaya possesses unique ecology and environment that have endowed with a mosaic of sparse vegetation pattern. Plants of this high altitude cold desert areas therefore show stress tolerant properties with the capacity of producing certain bioactive metabolites such as mineral ions, amino acids, fatty acids and other bioactive secondary metabolites which are acting as osmolyte, ion transport regulator, modulating stomatal opening, detoxification of heavy metals, modification of membrane fluidity, synthesis and activity of some enzymes, gene expression, and redox-homeostasis (Rai 2002; Upchurch 2008). Therefore, various amino acids, fatty acids and mineral elements found in R. imbricata may reflect stress adaptation of this plant in the harsh terrain of trans-Himalayan cold desert. Although the root of R. imbricata had been extensively studied for its pharmacological and therapeutic potentials, data on its free amino acid, fatty acid and mineral content is very scarce and therefore limits the scope for comparison of present analysis with other reports on this plant. In this report, we are primarily focusing on quantification of amino acids, fatty acids and mineral elements in R. imbricata root because these components are the basic nutritional source for human. Other compounds like sugars, sugar alcohols, low-complexity carbohydrates, tertiary amines, sulfonium compounds, starch etc. may also be interesting to quantify and future studies would be performed to explore the role of these metabolites in stress adaptation of this plant. In future, research efforts should also be concentrated on the effect of seasonal, geographical and altitudinal variations on the bioactive composition of R. imbricata. In addition, changes in bioactive metabolites with respect to the course of development will also provide new insights for isolation of novel compounds from this plant.
Conclusion
To the best of our knowledge, this is the first document reporting the use of RP-HPLC with pre-column derivatization for amino acid analysis in roots of R. imbricata from trans-Himalayan cold desert. The present study revealed the presence of high concentration of essential amino acids in the plant root. Fatty acid analysis carried out using GC-FID with FAMEs derivatization also showed the occurrence of vital fatty acids in the plant root for the first time. A good number of dietary minerals were also determined by ICP-OES technique. Therefore, it can be concluded that R. imbricata root contains different health promoting bioactive nutritional attributes like essential amino acids, fatty acids and dietary mineral elements. The plant root could therefore be utilized for development of herbal supplements, nutraceutical products and functional foods to combat health issues associated with nutrition deficiency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1. GC-FID chromatogram of a. 37 FAMEs standards, b. FAMEs of R. imbricata root. 1: Butyric acid methyl ester (C4:0); 2: Caproic acid methyl ester (C6:0); 3: Caprylic acid methyl ester (C8:0); 4: Capric acid methyl ester (C10:0); 5: Undecanoic acid methyl ester (C11:0); 6: Lauric acid methyl ester (C12:0); 7: Tridecanoic acid methyl ester (C13:0); 8: Myristic acid methyl ester (C14:0); 9: Myristoleic acid methyl ester (C14:1); 10: Pentadecanoic acid methyl ester (C15:0); 11: cis-10-Pentadecenoic acid methyl ester (C15:1); 12: Palmitic acid methyl ester (C16:0); 13: Palmitoleic acid methyl ester (C16:1); 14: Heptadecanoic acid methyl ester (C17:0); 15: cis-10-Heptadecenoic acid methyl ester (C17:1); 16: Stearic acid methyl ester (C18:0); 17: Elaidic acid methyl ester (C18:1n9t); 18: Oleic acid methyl ester (C18:1n9c); 19: Linolelaidic acid methyl ester (C18:2n6t); 20: Linoleic acid methyl ester (C18:2n6c); 21: Arachidic acid methyl ester (C20:0); 22: cis-11-Eicosenoic acid methyl ester (C20:1); 23: α-Linolenic acid methyl ester (C18:3n3); 24: Heneicosanoic acid methyl ester (C21:0); 25: cis-11,14-Eicosadienoic acid methyl ester (C20:2); 26: Behenic acid methyl ester (C22:0); 27: cis-8,11,14-Eicosatrienoic acid methyl ester (C20:3n6); 28: Erucic acid methyl ester (C22:1n9); 29: cis-11,14,17-Eicosatrienoic acid methyl ester (C20:3n3); 30: Arachidonic acid methyl ester (C20:4n6); 31: Tricosanoic acid methyl ester (C23:0); 32: cis-13,16-Docosadienoic acid methyl ester (C22:2); 33: Lignoceric acid methyl ester (C24:0); 34: Nervonic acid methyl ester (C24:1); 35: cis-4,7,10,13,16,19-Docosahexaenoic acid methyl ester (C22:6n3); 36: γ-Linolenic acid methyl ester (C18:3n6); 37: cis-5,8,11,14,17-Eicosapentaenoic acid methyl ester (C20:5n3) (TIFF 9802 kb)
Acknowledgements
We express our sincere thankfulness to Defence Research and Development Organisation (DRDO) for supporting the study. The authors are also grateful to Debasmita Ghosh Dhar, Maharaja Manindra Chandra College, Kolkata for her important contribution in copyediting and proofreading of the manuscript.
Conflict of interest
The authors declare no conflict of interest for this work.
Footnotes
Amol B. Tayade and Priyanka Dhar have contributed equally to this work.
References
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Food Composition Additives, Natural Contaminants, Association of Official Analytical Chemists; 1990. pp. 1096–1097. [Google Scholar]
- AOAC (2002) AOAC Official methods of analysis, 17th edn, method 996.01; Fat (Total, Saturated, Unsaturated, and Monounsaturated) in cereal products: acid hydrolysis capillary gas chromatographic method, AOAC, Arlington, VA
- Avasthi AS, Bhatnagar M, Sarkar N, Kitchlu S, Ghosal S. Bioassay guided screening, optimization and characterization of antioxidant compounds from high altitude wild edible plants of Ladakh. J Food Sci Technol. 2016 doi: 10.1007/s13197-016-2300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z, Nan P, Zhong Y. Chemical composition of the essential oil of Rhodiola quadrifida from Xinjiang, China. Chem Nat Comp. 2005;41:418–419. doi: 10.1007/s10600-005-0166-z. [DOI] [Google Scholar]
- Ballabh B, Chaurasia OP, Ahmed Z. Herbal products from high altitude plants of Ladakh Himalaya. Curr Sci. 2007;92:1664–1665. [Google Scholar]
- Bharate SS, Bharate SB. Non-enzymatic browning in citrus juice: chemical markers, their detection and ways to improve product quality. J Food Sci Technol. 2014;51:2271–2288. doi: 10.1007/s13197-012-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrul KK, Srivastava B. Enantiomeric separation in pharmaceutical analysis: a chromatographic approach. J Chem Pharm Res. 2010;2:923–934. [Google Scholar]
- Chang NW, Huang PC. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids. 1998;33:481–487. doi: 10.1007/s11745-998-0231-9. [DOI] [PubMed] [Google Scholar]
- Chaurasia OP, Singh SB, Ballabh B, Stobdan T, Tayade AB, Saurav SK, Sharma D. A formulation of a novel herbal antioxidant supplement for nutraceutical value, 635/DEL/2009. Pat Off J. 2011;2009:738. [Google Scholar]
- Choudhary A, Kumar R, Srivastava RB, Surapaneni SK, Tikoo K, Singh IP. Isolation and characterization of phenolic compounds from Rhodiola imbricata, a trans-Himalayan food crop having antioxidant and anticancer potential. J Func Food. 2015;16:183–193. doi: 10.1016/j.jff.2015.04.013. [DOI] [Google Scholar]
- Darmstadt GL, Mao-Qiang M, Chi E, Saha SK, Ziboh VA, Black RE, Santosham M, Elias PM. Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr. 2002;91:546–554. doi: 10.1111/j.1651-2227.2002.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Dhar P (2013) Autonomic responses in high altitude and adaptogenic efficacy of phytococktail from trans-Himalayan plants. PhD Thesis awarded from Bharathiar University, Coimbatore
- Dhar P, Tayade AB, Bajpai PK, Sharma VK, Das SK, Chaurasia OP, Srivastava RB, Singh SB. Antioxidant capacities and total polyphenol contents of hydro-ethanolic extract of phytococktail from trans-Himalaya. J Food Sci. 2012;77:156–161. doi: 10.1111/j.1750-3841.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- Dhar P, Bajpai PK, Tayade AB, Chaurasia OP, Srivastava RB, Singh SB. Chemical composition and antioxidant capacities of phytococktail extracts from trans-Himalayan cold desert. BMC Complement Altern Med. 2013;13:259. doi: 10.1186/1472-6882-13-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar P, Tayade AB, Kumar J, Chaurasia OP, Srivastava RB, Singh SB. Nutritional profiling of phytococktail from trans-Himalayan plants. PLoS ONE. 2013;8:e83008. doi: 10.1371/journal.pone.0083008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donno D, Boggia R, Zunin P, Cerutti AK, Guido M, Mellano MG, Prgomet Z, Beccaro GL. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: an innovative technique in food supplement quality control. J Food Sci Technol. 2016;53:1071–1083. doi: 10.1007/s13197-015-2115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW, Shahidi F. The chemistry, processing and health benefits of highly unsaturated fatty acids. In: John WJ, Shahidi F, editors. Omega-3 fatty acids, chemistry, nutrition and health effects. Washington: American Chemical Society; 2001. pp. 258–279. [Google Scholar]
- Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- Hou X, Jones BT. Inductively coupled plasma/optical emission spectrometry. In: Meyers RA, editor. Encyclopedia of analytical chemistry. Chichester: Wiley; 2000. pp. 9468–9985. [Google Scholar]
- Huang YS (2007) Biocatalysis and biotechnology for functional foods and industrial products. In: Hou CT, Shaw JF (eds) CRC Press, Boca Ratón, pp 11–21
- Ilisz I, Aranyi A, Pataj Z, Péter A. Recent advances in the direct and indirect liquid chromatographic enantioseparation of aminoacids and related compounds: a review. J Pharm Biomed Anal. 2012;69:28–41. doi: 10.1016/j.jpba.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Chiavaroli L, Wong JM, Kendall C, Lewis GF, Vidgen E, Connelly PW, Leiter LA, Josse RG, Lamarche B. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. CMAJ. 2010;182:1961–1967. doi: 10.1503/cmaj.092128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Tayade A, Kumar A, Chaurasia OP, Srivastava RB. Phytochemical and pharmacological analysis of Rhodiola sp.: a review. In: Srivastava RB, Selvamurthy W, editors. Innovations in agro animals technologies. Delhi: Satish Serial Publishing House; 2012. pp. 171–183. [Google Scholar]
- Letawe C, Boone M, Pierard GE. Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin Exp Dermatol. 1998;23:56–58. doi: 10.1046/j.1365-2230.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- Maro AD, Dosi R, Ferrara L, Rocco M, Sepe J, Ferrari G, Parente A. Free amino acid profile of Malus domestica Borkh cv. Annurca from the Campania Region and other Italian vegetables. Aus J Crop Sci. 2011;5:154–161. [Google Scholar]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Nevin KG, Rajamohan T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin Biochem. 2004;37:830–835. doi: 10.1016/j.clinbiochem.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Noctor G, Novitskaya L, Lea PJ, Foyer CH. Co-ordination of leaf minor amino acid contents in crop species: significance and interpretation. J Exp Bot. 2002;53:939–945. doi: 10.1093/jexbot/53.370.939. [DOI] [PubMed] [Google Scholar]
- Peyrat-Maillard MN, Cuvelier ME, Berset C. Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)s-induced oxidation: synergistic and antagonistic effects. J Am Oil Chem Soc. 2003;80:1007–1012. doi: 10.1007/s11746-003-0812-z. [DOI] [Google Scholar]
- Pooja KR, Anil K, Khanum F, Bawa AS. Phytoconstituents and antioxidant potency of Rhodiola rosea—a versatile adaptogen. J Food Biochem. 2006;30:203–214. doi: 10.1111/j.1745-4514.2006.00055.x. [DOI] [Google Scholar]
- Rai VK. Role of amino acids in plant responses to stresses. Biol Plant. 2002;45:481–487. doi: 10.1023/A:1022308229759. [DOI] [Google Scholar]
- Rodrigues DMF, Freitas AC, Rocha-Santos TAP, Vasconcelos MW, Roriz M, Rodríguez-Alcalá LM, Gomes AMP, Duarte AC. Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J Food Sci Technol. 2015;52:6927–6939. doi: 10.1007/s13197-015-1826-z. [DOI] [Google Scholar]
- Ruan X, Hou P, Zhou J, Wang Q, Li G. Analysis on the trace element and mino acid content in xinjiang 6 series Rhodiola L. plant. Guang Pu Xue Yu Guang Pu en Xi. 2001;21:542–544. [PubMed] [Google Scholar]
- Seppänen-Laakso T, Laakso I, Hiltunen R. Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal Chim Acta. 2002;65:39–62. doi: 10.1016/S0003-2670(02)00397-5. [DOI] [Google Scholar]
- Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants: a review. Afr J Food Sci. 2010;4:200–222. [Google Scholar]
- Soh K, Choo YM, Cheng SF, Ma AN. Recovery and conversion of palm olein- derived used frying oil to methyl esters for biodiesel. LOH J Oil Palm Res. 2006;18:247–252. [Google Scholar]
- Tayade AB (2015) Phytochemical and pharmacological evaluation of Rhodiola imbricata Edgew. from trans-Himalayan cold desert region of Ladakh, India. PhD Thesis awarded from Jaypee University of Information Technology, Waknaghat, Solan
- Tayade AB, Dhar P, Kumar J, Sharma M, Chauhan RS, Chaurasia OP, Srivastava RB. Chemometric profile of root extracts of Rhodiola imbricata Edgew. with hyphenated gas chromatography mass spectrometric technique. PLoS ONE. 2013;8:e52797. doi: 10.1371/journal.pone.0052797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayade AB, Dhar P, Kumar J, Sharma M, Chaurasia OP, Srivastava RB. Sequential determination of fat- and water-soluble vitamins in Rhodiola imbricata root from trans-Himalaya with rapid resolution liquid chromatography/tandem mass spectrometry. Anal Chim Acta. 2013;789:65–73. doi: 10.1016/j.aca.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Tayade AB, Dhar P, Sharma M, Chauhan RS, Chaurasia OP, Srivastava RB. Antioxidant capacities, phenolic contents, and GC/MS analysis of Rhodiola imbricata Edgew. root extracts from trans-Himalaya. J Food Sci. 2013;78:402–410. doi: 10.1111/1750-3841.12054. [DOI] [PubMed] [Google Scholar]
- Terés S, Barceló-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, Escriba PV. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc Nat Acad Sci. 2008;105:13811–13816. doi: 10.1073/pnas.0807500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- Zhao J, Williams CC, Last RL. Induction of Arabidopsis trypthophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell. 1998;10:359–370. doi: 10.1105/tpc.10.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1. GC-FID chromatogram of a. 37 FAMEs standards, b. FAMEs of R. imbricata root. 1: Butyric acid methyl ester (C4:0); 2: Caproic acid methyl ester (C6:0); 3: Caprylic acid methyl ester (C8:0); 4: Capric acid methyl ester (C10:0); 5: Undecanoic acid methyl ester (C11:0); 6: Lauric acid methyl ester (C12:0); 7: Tridecanoic acid methyl ester (C13:0); 8: Myristic acid methyl ester (C14:0); 9: Myristoleic acid methyl ester (C14:1); 10: Pentadecanoic acid methyl ester (C15:0); 11: cis-10-Pentadecenoic acid methyl ester (C15:1); 12: Palmitic acid methyl ester (C16:0); 13: Palmitoleic acid methyl ester (C16:1); 14: Heptadecanoic acid methyl ester (C17:0); 15: cis-10-Heptadecenoic acid methyl ester (C17:1); 16: Stearic acid methyl ester (C18:0); 17: Elaidic acid methyl ester (C18:1n9t); 18: Oleic acid methyl ester (C18:1n9c); 19: Linolelaidic acid methyl ester (C18:2n6t); 20: Linoleic acid methyl ester (C18:2n6c); 21: Arachidic acid methyl ester (C20:0); 22: cis-11-Eicosenoic acid methyl ester (C20:1); 23: α-Linolenic acid methyl ester (C18:3n3); 24: Heneicosanoic acid methyl ester (C21:0); 25: cis-11,14-Eicosadienoic acid methyl ester (C20:2); 26: Behenic acid methyl ester (C22:0); 27: cis-8,11,14-Eicosatrienoic acid methyl ester (C20:3n6); 28: Erucic acid methyl ester (C22:1n9); 29: cis-11,14,17-Eicosatrienoic acid methyl ester (C20:3n3); 30: Arachidonic acid methyl ester (C20:4n6); 31: Tricosanoic acid methyl ester (C23:0); 32: cis-13,16-Docosadienoic acid methyl ester (C22:2); 33: Lignoceric acid methyl ester (C24:0); 34: Nervonic acid methyl ester (C24:1); 35: cis-4,7,10,13,16,19-Docosahexaenoic acid methyl ester (C22:6n3); 36: γ-Linolenic acid methyl ester (C18:3n6); 37: cis-5,8,11,14,17-Eicosapentaenoic acid methyl ester (C20:5n3) (TIFF 9802 kb)