Abstract
Bioactive properties of fungi considerably differ between the fruiting body (FB) and the submerged culture as regards mycelia (M) and the fermentation broth (F). Antioxidant properties of hot-water extracts obtained from three different fungal origins: FB, M and F of two autochthonous fungal species (Northern Serbia), Coprinus comatus and Coprinellus truncorum were investigated. Free radical scavenging capacity (RSC) was evaluated in vitro by the DPPH assay and reducing power ability (FRAP assay). Considering possible bioactive properties of different compounds present in fungal extracts, the content of total proteins (TP), phenols (TC) and flavonoids (TF) were investigated colorimetrically. The chemical characterisation of the examined extracts was evaluated using the HPLC–MS/MS method. C. comatus showed the strongest RSC activity; more precisely, fermentation broth extract (FCc) on DPPH radicals (IC50 = 5.06 μg mL−1) and fruiting body extract (FBCc) for the FRAP assay (42.86 mg ascorbic acid equivalents (AAE)/g). Submerged M extract of both species showed the highest TC (MCc 81.95 mg gallic acid eq (GAE)/g d.w.; MCt 81.64 mg GAE/g d.w.), while FB extracts contained the highest content of TP. Comparing LC–MS phenolic profiles between species—interspecifically and among different fungal origins—intraspecifically (fruiting bodies and submerged cultures), high variations were noticed. In submerged M or F extracts of C. comatus, vanillic, gallic, gentisic and cinnamic acids were detected, as opposed to FB. Considering that diverse phenolic profiles of detected antioxidant compounds were obtained by submerged cultivation, this type of cultivation is promising for the production of antioxidant substances.
Keywords: Coprinus comatus, Coprinellus truncorum, Antioxidant activity, Submerged cultures, HPLC–MS/MS
Introduction
Fungi are widespread organisms on earth with high biodiversity and strong biochemical potentials. Because of high water content (70–95%), a small amount of fat (1–8% of unsaturated fatty acids) and many bioactive compounds with diverse biological activities, such as antioxidant (Karaman et al. 2014), antitumor and immune-modulating properties (Zhao et al. 2014), fungi represent excellent sources of nutraceuticals (dietary supplements with a potential therapeutic application or health benefits) that may be derived from the mycelium (M) or the fruiting body (FB) of fungi (Chang and Miles 2004). Recent studies have proved nutritional values of fungi and promoted their use in a well-balanced diet since bioactive compounds may enhance human’s immune system and possess valuable antioxidative properties (Cohen et al. 2014). Furthermore, awareness of a healthy lifestyle is rising, so preference is given to naturally originated antioxidants worldwide.
Members of the genus Coprinus are saprobes with a cosmopolitan distribution, typically growing on meadows in waste areas in clusters or near rotting tree stumps (Powell 2014). They contain up to 25% of protein when young, and they are rich in polysaccharides, triglycerides and essential amino acids (Powell 2014).
Coprinus comatus (O.F. Müll.) Pers. 1797 (Ph. Basidiomycota, Cl. Agaricomycetes, O. Agaricales, Fam. Agaricaceae) is an edible fungal species, but only young mushrooms, before the gills start to turn black. It is well known for its bioactive properties, such as antimicrobial (Zenkova et al. 2003), antioxidant (Asatiani et al. 2010), hypoglycemic (De Silva et al. 2012), immunomodulator, antitumor (Zhao et al. 2014) etc. In China, this fungus has been cultivated in a large amount, and its production was amounted to almost 382.000 tons in 2006 (Li et al. 2010).
C. comatus is taxonomically closely linked with the species Coprinellus truncorum (Scop.) Redhead et al. 2001, regarding position in systematic (Ph. Basidiomycota, Cl. Agaricomycetes, O. Agaricales, Fam. Psathyrellaceae). However, according to analysed ITS1-5.8S-ITS2 sequences (Ko et al. 2001), it has been recently suggested that C. truncorum is conspecific with the species Coprinelus micaceus (Bull.) Fr. 1838, thus they might be used as synonyms. Coprinelus micaceus is considered conditionally edible given the fact that consumption with alcoholic beverages may produce symptoms such as reddening of the face, increased heart rate, vomiting, malaise, agitation and diarrhoea (Chang and Miles 2004). Nonetheless, the Coprinelus micaceus species contains some of unique bioactive chemical compounds, such as sterol micaceol with a “modest” antibacterial activity (Zahid et al. 2006). Likewise, the species Coprinus comatus generates 4,5-dihydroxy-2-methyoxy-benzaldehyde, also known as comatin, a compound with a hypoglycaemic effect (Ding et al. 2010). Additionally, previous studies have documented that fungi are very important sources of antioxidant components, mainly due to the presence of their secondary metabolites, such as phenolic compounds (Vaz et al. 2011; Karaman et al. 2014), although some polysaccharides showed similar effects (Wasser 2011).
Antioxidant activity is considered very important for the protection of aerobic organisms from reactive oxygen species (ROS), the production of which is in a constant balance with the antioxidant defence system in healthy organisms (Halliwell and Gutteridge 1990). As a consequence of disturbance of this balance, a serious disruption of normal cell homeostasis leads to oxidative stress. Although synthetic antioxidants can improve the defence system capacity, they have toxic and mutagenic effects (Singh et al. 2007). Therefore, research into naturally originated metabolites, including fungal species and their activity, is on the increase.
While the antioxidant potential of fungi is strain specific and depends on the habitat (Karaman et al. 2014), life-cycle phase, solvent extraction method (Mushtaq et al. 2013) and others, in this research we used hot-water extracts of autochtonous fungal species to make a comparative study of antioxidative properties of different fungal origins: mature fruiting bodies (FB) and submerged culture mycelium (M) and fermentation broth (F) of the two Coprinus species: C. comatus and Coprinellus truncorum.
The aim of this study was to determine a comparative overview of antioxidative properties in vitro of different fungal origins as well as phenolic compound profiles of different extracts derived from FB, M and F of two autochtonous species originated from the grassland in Novi Sad and meadows in Sremski Karlovci (Northern Serbia).
Materials and methods
Standards and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was from Fluka Chemie (Switzerland), ascorbic acid and 2,4,6-tripyridyl-s-triazine (TPTZ) were from Merck, and butylated hydroxyanisole (BHA) was from Sigma-Aldrich (Germany). Deionised water was produced using a Millipore water purification system. Reference standards of the phenolic compounds were obtained from Sigma-Aldrich Chem (Steinheim, Germany), Fluka Chemie gmbh (Buchs, Switzerland) or from Chromadex (Santa Ana, USA). HPLC gradient grade methanol was purchased from J. T. Baker (Deventer, The Netherlands), and p.a. Formic acid and DMSO from Merck (Darmstadt, Germany).
Fungal material
Wild-growing autochthonous fungal species were collected at two different localities and times: C. truncorum on the grassland in Novi Sad (town) on 15th September in 2015 year and C. comatus in meadows of Sremski Karlovci on 24th November in 2014 year. Fungal species were determined and both voucher species (12-00704 and 12-00705) were deposited at BUNS Herbarium at Department of Biology and Ecology, Faculty of Science, University of Novi Sad. From fruiting bodies mycelia were isolated and preserved in fungal culture collection (FCC) at the Microbiological Laboratory at the Faculty of Sciences, University of Novi Sad.
Submerged cultivation
Isolated mycelia were cultivated on Malt agar (Torlak, Serbia) at 26 °C during 10-day cultivation. For the production of mycelia and fermentation filtrate, the culture was inoculated into a 300 mL flask, containing 100 mL of fermentation medium and incubated at 26 °C and 100 rpm (New Brunswick Scientific, Edison, USA). The liquid medium (1 L) consisted of 5 g peptone, 35 g glucose, 5 g yeast extract, 1 g K2HPO4, 0.5 g MgSO4 × 7H2O, 0.05 g, at pH 6.51. After 21 days, the mycelia was filtrated using Fioroni Filter, France, and both the mycelia biomass and filtrate were collected and lyophilised until we got dried samples (CHRIST ALPHA 2-4 LDplus, Freeze Dryer).
Extracts preparation
For extract preparation, 5 g lyophilised mass of FB (cap and stipe), as well as 5 g of submerged M and F biomass were re-dissolved in 100 mL of hot water for 18 h on a rotary shaker (100 rpm). After extraction, all six extracts (four from submerged culture and two from FB) were filtrated and lyophilised to dryness. After lyophilisation, water was added to make stock solution at concentration of 100 mg mL−1. All isolates, from both species FB, M and F were prepared in the same conditions according to Karaman et al. 2014.
Antioxidant activity
Antioxidant activities were assessed by in vitro scavenging methods with microplate assays: 1,1-diphenyl-2-picrylhydrazyl radicals (DPPH) and ferric reducing ability of plasma (FRAP). RSC capacity of investigated extracts was determined by the DPPH test according to the method by Espin et al. (2000). Spectrophotometric determination scavenging activity of extracts was based on monitoring the transformation of DPPH radicals in the presence of antioxidants (H-donor atoms), followed by measuring the change in absorbance. The concentrations of the tested extracts of fungi were ranged from 5 to 400 μg mL−1; 10 μL of extracts was transferred into a 96-well plate with 60 μL of freshly prepared methanolic solution containing DPPH radical. After incubation for 30 min in the dark at 25 °C absorbance were measured at 515 nm (Multiskan GO Thermo Scientific). For negative control treatments, 10 μL of H2O was applied, instead of extract.
Synthetic antioxidant butylated hydroxyanisole (BHA), at concentration 50 µg mL−1 was used as a positive control. The antioxidant activity of the extracts was expressed as IC50 values (a concentration of the extract that inhibited the DPPH radical formation by 50%) Espin et al. (2000).
The ferric reducing/antioxidant power (FRAP) assay was done according to Benzie and Strain (1996). The method is based on in vitro reduction of iron (II) 2,4,5-tripyridyl-s-triazine (TPTZ) complex at low pH. Reduction potential of fungal extracts was calculated using the calibration curve of the standard solution of ascorbic acid. Final concentration of ascorbic acid ranging from 0 to 38.91 μg mL−1 was used to create a standard curve. The concentrations of the tested extracts of fungi ranged from 250 to 2000 μg mL−1. 10 μL of the extracts of fungi were transferred into a 96 well plate with 225 μL of freshly prepared FRAP reagent (acetate buffer, TPTZ solution and FeCl3·6H2O in a ratio 10:1:1). 10 μL of solvent (H2O) with 225 μL of FRAP reagent was used as blank. Measurement of the absorbance was performed after 6 min incubation in the dark at 25 °C and all the samples were performed in triplicate. Values of reducing power were expressed as mg of ascorbic acid equivalents (AAE) per gram of dry weight, calculated according to the standard calibration curve.
Antioxidant components
Total phenolic (TC) content was determined in vitro according to Singleton et al. (1999). The concentrations of the tested extracts of fungi for test of TC ranged from 3.12 to 1000 μg mL−1. TC was calculated on the basis of a calibration curve of standard solution of gallic acid, and the result is expressed as the mean value of three measurements ± standard deviation (mg gallic acid eq (GAE)/g d. w). Total flavonoid content (TF) was determined by in vitro method by Chang et al. (2002). The concentrations of all tested extracts of fungi ranged from 1242 to 9934 μg mL−1. The standard curve was constructed on the basis of the results obtained by measuring the absorbance of a series of different concentrations of quercetin solution. From the calculated calibration curve was obtained TF content of extracts which is expressed as mg of quercetin equivalents (Q) eq/g d.w. in the study. Total proteins (TP) were determined by original spectrophotometric method, according to Bradford (1976). Bovine Serum Albumin (BSA) was used for standard curve formation while the concentrations of the tested extracts of fungi ranged from 250 to 2000 μg mL−1. Values were expressed as mg eq of protein/mL of sample.
Absorbance for each test was measured at an appropriate wavelength using a Multiskan GO (Thermo Scientific) plate reader. Corresponding inhibition-concentration curves were drawn using the Origin software (OriginLab Corporation, Northampton, MA, USA), and IC50 values were determined by linear regression analysis.
HPLC–MS/MS determination of compounds
For HPLC–MS/MS determination of the phenolic profile, the method by Orčić et al. (2014) was used. All extracts were diluted with mobile phase solvents A (water) and B (methanol), premixed in 1:1 ratio, to obtain a final concentration of 2 mg mL−1. Fifteen working standards, ranging from 1.53 to 25.0.103 ng mL−1, were prepared by serial 1:1 dilutions of standard mixture with solvents A and B (1:1). Samples and standards were analysed using Agilent Technologies 1200 Series high-performance liquid chromatograph coupled with Agilent Technologies 6410A Triple Quad tandem mass spectrometer with electrospray ion source, and controlled by Agilent Technologies MassHunter Workstation software—Data Acquisition (ver. B.03.01). Five microlitres were injected into the system, and compounds were separated on Zorbax Eclipse XDB-C18 (50 mm × 4.6 mm, 1.8 µm) rapid resolution column held at 50 °C. Mobile phase was delivered at flow rate of 1 mL/min in gradient mode (0 min 30% B, 6 min 70% B, 9 min 100% B, 12 min 100% B, re-equilibration time 3 min). Eluted compounds were detected by ESI–MS, using the ion source parameters as follows: nebulisation gas (N2) pressure 40 psi, drying gas (N2) flow 9 L/min and temperature 350 °C, capillary voltage 4 kV, negative polarity. Data were acquired in dynamic MRM mode, using the optimised compound-specific parameters (retention time, precursor ion, product ion, fragmentor voltage, collision voltage) given in the previously published research by Orčić et al. (2014). For all the compounds, peak areas were determined using Agilent MassHunter Workstation software—Qualitative Analysis (ver. B.04.00.). Calibration curves were plotted and samples’ concentrations calculated using the OriginLabs Origin Pro (ver. 8.0) software.
Statistical analysis
All results were performed in triplicate and expressed as means and standard deviations (mean ± SD). One-way ANOVA with post hoc Tukey HSD test was used to determine significant differences of extracts at the level p < 0.01 and 0.05. Statistical data correlation was obtained by correlation coefficient (Excel 2010) to estimate the relationship between the antioxidant activity of extracts and TC, TF and TP contents.
Results and discussion
Submerged cultivation is the best method for growing fungal biomass and extracellular media to detect fungal physiology and biochemical properties (Elisashvili 2012). Previous studies concerning submerged cultivation have been aimed at investigation of fungal polysaccharides, whereas there are few studies dealing with antioxidant activities and the phenolic compounds profile of the mycelia and the fermentation broth separately (Zhong and Tang 2004; Elisashvili 2012).
Antioxidant assays
Results of antioxidant activity clearly revealed the difference in activity in different types of extracts (FB, M, F), statistically determined by Tukey HSD test (Table 1). In the DPPH test, according to obtained mean values which significantly different from each other two statistically significant groups were defined, while five groups were determined in the FRAP assay (Table 1). Based on the tests performed, C. comatus extracts expressed a higher antioxidant activity than C. truncorum, except for M extract in the FRAP assay. The best antiradical activity was obtained by FCc (5.06 ± 2.06 μg mL−1), which is an approximate to the value of tested synthetic antioxidant—BHA (IC50 2.09 ± 0.56 μg mL−1), although MCt extract exhibited a similar effect. This result points to a high antioxidant activity of F extract of C. comatus and according to our knowledge this is the first recorded observation of this potential.
Table 1.
Antioxidant activity of hot-water extracts of Coprinus fungi
| Extracts | DPPH (IC50) (μg mL−1) | FRAP (mg AAE g−1) |
|---|---|---|
| C. comatus | ||
| FBCc | 24.02 ± 1.64a | 42.86 ± 2.04a |
| MCc | 13.27 ± 5.95a | 30.76 ± 0.85b |
| FCc | 5.06 ± 2.06a | 13.78 ± 2.80c |
| C. truncorum | ||
| FBCt | 65.90 ± 2.13b** | 26.72 ± 0.47d |
| MCt | 7.52 ± 2.46a | 30.63 ± 0.88b |
| FCt | 42.39 ± 1.75b** | 6.03 ± 0.18e |
a,b,c,d,e Significant differences between extracts were determined by Tukey HSD test at p < 0.01. In each column different letters mean significant differences
** Significant differences between extracts were determined by Tukey HSD test at p < 0.05
FBCc fruiting body extract of C. comatus, MCc submerged mycelium extract of C. comatus, FCc fermentation broth extract of C. comatus, FBCt fruiting body extract of C. truncorum, MCt submerged mycelium extract of C. truncorum, FCt fermentation broth extract of C. truncorum
In general, a stronger antiradical activity was exhibited in the investigated submerged culture extracts (both M and F) compared to FB, which may be explained by a stronger physiological activity of vegetative fungal mycelia in the fermentation broth than in a generative sessile basidioms in a form of FB. In comparison to research data on antioxidant activity of submerged culture of mycelia (1.1 ± 0.2 mg mL−1) of C. comatus in Israel (Asatiani et al. 2007), MCc showed lower IC50 values (13.27 ± 5.95 μg mL−1), which means it achieved a higher antiradical activity. The difference in this activity might have been caused by the different procedures performed in preparation of the extracts, since we used lyophilised (freeze-dried) mycelial biomass for extract preparation, in comparison to oven-dried mycelial biomass (at 50 °C) used by Asatiani et al. (2007). This procedure of oven-drying could cause the disintegration of some active termolabile compounds with a potential antioxidant activity.
The antiradical activity of FB of C. comatus methanol extracts (3.76 mg mL−1) (Stojković et al. 2013), showed a weaker activity compared to the analysed FBCc extract. Furthermore, ethanolic polysaccharides fraction of FB C. comatus revealed a higher antioxidant activity than water polysaccharides fraction (Vaz et al. 2011), which may be explained by the presence of phenolic compounds: p-hydroxibenzoic, p-coumaric, cinammic phenolic acids (Vaz et al. 2011), similar to compounds detected in some other lignicolous fungal species from Serbia (Karaman et al. 2014).
Moreover, FB water extracts of C. micaceus from Korea (11% neutralisation of DPPH at 2 mg mL−1) (Nguyen et al. 2014) was less potent than the FBCt extract in the present study (Table 1). Between methanol and hot-water extracts of C. truncorum, better activity was exhibited in hot-water extracts (Nguyen et al. 2014). Generally, FB water extracts showed much higher antiradical DPPH activities than methanol ones (Nguyen et al. 2014), which indicates the importance of more polar compounds in demonstrated activities.
For the FRAP test, the most effective activity was recorded for C. comatus FBCc 42.86 ± 2.04 mg AAE g−1 (Table 1), followed by FBCt and M extracts of both species, while the lowest activity was obtained for F extracts. Although a high variability was noticed among extracts (Table 1), M extracts showed similar mild effects. A special importance of our results for the FRAP assay is that they have not been reported before for C. truncorum submerged culture extracts. In comparison with other studies dealing with antioxidant potential of naturally originated fungi from different areal, the differences in activity, regarding strain-specific properties or preparing the extract, have been noticed (Asatiani et al. 2007; Vaz et al. 2011, Karaman et al. 2014, Nguyen et al. 2014). Moreover, the results obtained within present study have pointed out the differences in activity among three different fungal origins (namely, fruiting body, mycelia and fermentation broth) of the same species, while the majority of the performed studies so far have been focussed strictly on one fungal origin, such as fruiting body. For instance, the investigation of bioactivity of the commercial product based on the fungus C. comatus (Popovic et al. 2010) gave different results in comparison with naturally originated extract of the same fungus reported herein.
Total content of phenols, flavonoids and proteins
Phenols are characterised as effective antioxidants due to the existence of hydroxyl groups and there are reports about their direct correlation with antioxidant activity of fungal extracts (Karaman et al. 2014). In the present study, TC was found in all investigated extracts which ranged from 42.27 to 81.95 mg GAE/g d.w., while the highest TC content was determined in both analysed M extracts (Table 2). Concentration of TC observed in cap and stipe of FBCc from China (Li et al. 2010), ranged from 3.60 to 20.00 mg g−1, which is slightly lower than TC in all FBCc analysed in our study (24.02 mg/g GAE/g d.w.). Overall, TC content was richer for cap extracts than for stipe ones (Li et al. 2010).
Table 2.
Contents of total phenols (TC), total flavonoids (TF) and total proteins (TP) in analysed Coprinus extracts
| Extracts | Total phenols (mg gallic acid eq/g d.w.) | Total flavonoids (mg quercetin eq/g d.w.) | Total proteins (mg eq protein/ml of sample) |
|---|---|---|---|
| C. comatus | |||
| FBCc | 59.88 ± 1.75ab | 0.81 ± 0.30c | 10.95 ± 0.68a |
| FCc | 69.48 ± 3.51a | 1.63 ± 0.56a | n.d |
| MCc | 81.95 ± 2.62a | 1.40 ± 0.22b** | 8.04 ± 1.68b |
| C. truncorum | |||
| FBCt | 42.27 ± 1.00b | 1.58 ± 0.12a | 11.24 ± 0.96a |
| FCt | 45.32 ± 4.98b | 0.26 ± 0.11c | n.d |
| MCt | 81.64 ± 2.26a | 0.48 ± 0.20c | 6.87 ± 1.16b |
a,b,c,d,eSignificant differences between two extracts were determined by Tukey HSD test at p < 0.01
**Significant differences between extracts were determined by Tukey HSD test at p < 0.05
FBCc fruiting body extract of C. comatus, MCc submerged mycelium extract of C. comatus, FCc fermentation broth extract of C. comatus, FBCt fruiting body extract of C. truncorum, MCt submerged mycelium extract of C. truncorum, FCt fermentation broth extract of C. truncorum, n.d. not detected
In the DPPH antiradical test, the high correlations between obtained antioxidant activity (DPPH) and TC, TF and TP contents indicates that phenolic compounds could make a significant contribution to the activity of C. truncorum extracts (Table 3). These correlations are in accordance with Karaman et al. (2014) for FB extracts of some lignicolous fungal species. Furthermore, a strong correlation was noticed between FRAP activity and TC, TF and TP content for submerged M and F extracts for both investigated species (Table 3).
Table 3.
Correlation coefficient (r2) between the contents of TC, TF and TP and antioxidant activities of extracts
| Total phenols | Total flavonoids | Total proteins | |
|---|---|---|---|
| DPPH | r2 | ||
| C. comatus | |||
| FBCc | 0.40 | 0.89 | 0.97* |
| FCc | 0.13 | 0.37 | n.d. |
| MCc | 0.74 | 0.80 | 0.82 |
| C. truncorum | |||
| FBCt | 0.99* | 0.75 | 0.74 |
| FCt | 0.97* | 0.98* | n.d. |
| MCt | 0.91* | 0.95* | 0.94* |
| FRAP | r2 | ||
| C. comatus | |||
| FBCc | 0.28 | 0.79 | 0.99* |
| FCc | 0.94* | 0.99* | n.d. |
| MCc | 0.99* | 0.99* | 0.99* |
| C. truncorum | |||
| FBCt | 0.70 | 0.99* | 0.99* |
| FCt | 0.99* | 0.99* | n.d. |
| MCt | 0.99* | 0.99* | 0.99* |
*Significant correlation coefficient (r2), n.d. not detected
FBCc fruiting body extract of C. comatus; MCc submerged mycelium extract of C. comatus; FCc fermentation broth extract of C. comatus, FBCt fruiting body extract of C. truncorum, MCt submerged mycelium extract of C. truncorum, FCt fermentation broth extract of C. truncorum
TF contents were detected in all analysed extracts of C. comatus and C. truncorum which ranged from 0.26 to 1.63 mg Q eq/g d.w. The species C. comatus showed a higher TF content in all three types of extracts in comparison to C. truncorum. The comparison between TF of FBCc investigated extract and the same FB water extract from China, points to a lower activity of analysed FBCc in our study (Li et al. 2010).
Total protein content (TP) in M and FB extracts which ranged from 6.87 to 11 mg eq protein/mL, while F extracts of both species contained no protein (Table 2). FB extracts of both species were the richest in the content of TP. TP for wild-growing and cultivated C. comatus were spotted at 11.84 g/100 g (Stojković et al. 2013), which correlates with our results. A high correlation between TP and antioxidant activity of extracts (DPPH, FRAP tests) were recorded (Table 3), which could be possibly explained by a synergistic antioxidant activity of the protein-polysaccharide complex (Zhang et al. 2011).
Determination of phenolic profile
High-performance liquid chromatography coupled with triple-quadrupole mass spectrometer (HPLC–MS/MS) was performed in this study in order to compare phenolic profiles of different fungal origins, namely FB, M and F of autochtonous fungal strains. It was used to analyse the contents of flavonoids and phenolic acids in all selected fungal samples. A total of 37 compounds were identified and quantified in the 6 examined extracts using the method optimised for quantification of 45 phenols (Orčić et al. 2014). Eighteen (18) flavonoid aglycones and glycosides, isoflavonoids, coumarins, 6-hydroxybenzoic and 5-hydroxybenzoic acids, quinic acid and 5-O-caffeoylquinic acid were found in the examined extracts (Table 4).
Table 4.
Concentrations of phenolic compounds (P) (µg P/g d.w.)
| Class | Compound | Extracts | |||||
|---|---|---|---|---|---|---|---|
| FBCc H2O | FCc H2O | MCc H2O | FBCt H2O | FCt H2O | MCt H2O | ||
| Flavones | Apigenin | 1.41 | 1.61 | 1.03 | 1.25 | 0.29 | 0.57 |
| Baicalein | 5.44 | 3.72 | 2.69 | 2.06 | n.d.a | n.d. | |
| Crysoeriol | 1.43 | 1.53 | 1.37 | 1.25 | 0.57 | 1.08 | |
| Vitexin | 1.93 | 2.77 | 1.61 | 1.09 | 0.76 | 1.61 | |
| Apigenin-7-O-glucoside | 2.01 | 1.81 | 2.38 | 1.26 | 0.61 | 1.23 | |
| Luteolin-7-O-glucoside | 0.70 | 0.38 | 0.34 | 0.15 | 0.37 | 0.37 | |
| Apiin | 1.70 | 2.11 | 1.19 | 1.78 | 0.34 | 0.97 | |
| Baicalin | 8.98 | 8.29 | 6.68 | 6.88 | 4.70 | 6.88 | |
| Flavonols | Quercetin | 30.10 | 27.80 | 28.60 | 28.30 | 27.90 | 27.90 |
| Isorhamnetin | 5.82 | 6.38 | 5.94 | 4.58 | 4.64 | 5.04 | |
| Quercitrin | 1.08 | 0.96 | 0.75 | 0.25 | n.d. | n.d. | |
| Kaempferol-3-O-glucoside | 1.82 | 1.58 | 1.78 | 1.51 | 0.61 | 1.49 | |
| Hyperoside | 0.26 | 0.35 | n.d. | n.d. | 0.26 | 0.18 | |
| Quercetin-3-O-glucoside | 1.05 | 0.49 | 0.22 | 0.40 | 0.49 | n.d. | |
| Rutin | 1.46 | 0.77 | 0.39 | n.d. | n.d. | n.d. | |
| Flavanones | Naringenin | 2.59 | 2.83 | 1.98 | 1.54 | 0.90 | 1.26 |
| Flavanols | Catechin | 4.54 | 10.10 | 6.09 | n.d. | 6.79 | 4.37 |
| Epicatechin | 3.36 | 3.92 | n.d. | 3.92 | 9.33 | 6.24 | |
| Biflavonoids | Amentoflavone | 4.84 | 3.47 | 3.61 | 3.32 | 2.14 | 1.92 |
| Isoflavonoids | Daidzein | 0.61 | 31.50 | 24.60 | n.d. | 12.10 | 5.28 |
| Genistein | 0.23 | 16.70 | 21.00 | n.d. | 5.40 | 1.50 | |
| Hydroxybenzoic acids | p-Hydroxybenzoic acid | 9.28 | 121 | 467 | 45.20 | 42.30 | 25.40 |
| Protocatechuic acid | 4.80 | 8.32 | 48.60 | 8.46 | 0.40 | 20.90 | |
| Vanillic acid | n.d. | n.d. | 22.60 | 24.20 | n.d. | n.d. | |
| Gallic acid | n.d. | 5.25 | 5.46 | 2.59 | 2.36 | 6.92 | |
| Gentisic acid | n.d. | 1.15 | n.d. | 0.21 | n.d. | 0.73 | |
| Syringic acid | 3.56 | 10.10 | n.d. | n.d. | n.d. | n.d. | |
| Hydroxycinnamic acids | Cinnamic acid | n.d. | n.d. | 51.40 | n.d. | n.d. | n.d. |
| p-Coumaric acid | 1.85 | 1.35 | 36.10 | 4.08 | 10.80 | 1.49 | |
| o-Coumaric acid | 1.16 | 1.05 | 1.30 | 1.08 | 0.89 | 0.89 | |
| Caffeic acid | 1.58 | 1.65 | 1.39 | 1.18 | 1.39 | 1.13 | |
| Ferulic acid | 1.49 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Coumarins | Esculetin | 1.48 | 0.75 | 0.57 | 0.60 | 0.33 | 0.54 |
| Scopoletin | 1.97 | 1.08 | n.d. | 1.11 | n.d. | 1.33 | |
| Umbeliferon | 1.60 | 1.30 | n.d. | 0.80 | n.d. | n.d. | |
| Cyclohexanecarboxylic acids | Quinic acid | 146 | 34.8 | 61.00 | 215 | 49.70 | 20.80 |
| Chlorogenic acids | 5-O-Caffeoylquinic acid | 5.54 | 2.25 | 1.93 | 1.12 | 2.49 | 1.16 |
aNot detected, peak not observed, the concentration is lower than the LOD, bold numbers indicates the highest values of compound
FBCc fruiting body extract of C. comatus, MCc submerged mycelium extract of C. comatus, FCc fermentation broth extract of C. comatus; FBCt fruiting body extract of C. truncorum, MCt submerged mycelium extract of C. truncorum; FCt fermentation broth extract of C. truncorum
Quinic acid was detected in all tested fungal extracts, while the richest content was found in FB extracts of both analysed species (FBCc and FBCt). This acid has been proven as a potent antioxidative agent since it nutritionally supports the synthesis of tryptophan and nicotinamide in the gastrointestinal tract, which afterwards have influence on DNA repair (Pero et al. 2009). As opposed to FB for all analysed M and F submerged extracts, the most common compounds in our study were hydroxybenzoic acids, with the compound p-hydroxybenzoic acid, which was defined as antioxidant (McDonald et al. 2001).
For the analysed FBCc extract 33 phenolic compounds were spotted and among them 6 phenolic acids were detected, while for the FB extract for the same species from Portugal only two phenolic acids were identified (Vaz et al. 2011). Furthermore, phenolic profile of water extract was richest compared to methanol (Stojković et al. 2013).
For C. truncorum FB extract we detected 29 phenolic compounds, while for the species C. micaceus from South Korea only 4 phenolic compounds were determined (protocatechuic acid, chlorogenic acid, (-)-epicatechin and naringin) (Nguyen et al. 2014).
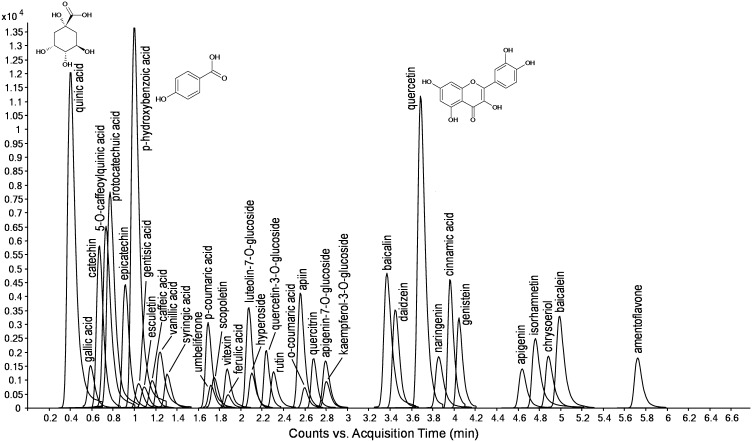
Among all analysed extracts, the highest TC content was detected for MCc extract (Table 4) obtained by both LC–MS chromatography and spectrophotometry (81.95 ± 2.62 mg GAE/g d.w.) (Table 2). Furthermore, FCc extract was the most diverse in detected phenolic compounds (34 detected of 37 analysed), which can be related to the best achieved anti-DPPH radical activity. LC–MS detection of phenolic profiles in submerged culture extracts and it’s comparation with FB extracts phenolic profiles have not been done so far in case of species C. truncorum. Moreover, until now there has been no evidence of detection of phenolic profiles of submerged M and F extracts of the species C. truncorum (Fig. 1).
Fig. 1.
LC-MS MRM chromatogram of compounds quantified in the examined extracts
Based on our comparative analysis of phenolic profiles (Table 4), it can be seen that certain phenolic compounds occur only in submerged culture extracts, among which many are closely associated with nutritional quality of food (Ho et al. 1992). One of the most biologically active dietary flavonol—quercetin (Ho et al. 1992) was detected in a great amount in all investigated fungal extracts. Moreover, we identified vanillic acid only in M type of extract for the species C. comatus and only in FB extract for C. truncorum (Table 4). This acid is one of the most popular flavouring agents and it is also an intermediate in the production of vanillin from ferulic acid, which possesses important nutritional values (Ho et al. 1992). It implicates the fact that submerged cultivation of C. comatus would be a recommended procedure for vanillic acid production, which has not been detected before in FB extracts of this species (Vaz et al. 2011). Moreover, flavonol hyperoside together with flavanol catechin and isoflavonoids daidzein and genistein, were detected in the present study only in M and F extracts for species C. truncorum. In addition daidzein and genistein were produced in a much greater amount in submerged extracts than in FB for C. comatus. These two compounds are already well known as antioxidants (Han et al. 2009) and anti-tumour agents (Birt et al. 2001). Moreover, genistein was characterised as a beneficial agent for prevention of diabetes (Fraga 2009). This and similar compounds can contribute to the significance of fungi as nutraceuticals, hence submerged cultivation of C. comatus and C. truncorum confirmed its importance as a natural source of valuable antioxidants.
Conclusion
The most potent in antioxidant activity were extracts of the species C. comatus (FCc extract for DPPH and FBCc extract for FRAP test). For both Coprinus species, M and FB extracts were the richest in TC and TP content, respectively. Antiradical DPPH activity was in a high correlation with all analysed total content compounds (TC, TF and TP) for the species C. truncorum. Futhermore, both species submerged extracts (M and F) showed a FRAP activity in a high correlation with all inverstigated total contents (TC, TF and TP), indicating them as important constituents in antioxidant activity. HPLC–MS/MS revealed that submerged extracts were more potent in production of certain phenolic compounds, which can recommend investigated submerged cultures of Coprinus and Coprinellus for further biotechnological processes in production of these highly valuable natural antioxidants.
Acknowledgements
This study was carried out within the Project supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia Nos. 172058 and 172053 and is a partial fulfilment of Ph. D. thesis of M.Sc Kristina Tešanović.
Abbreviations
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- F
Fermentation broth
- FB
Fruiting body
- FBCc
Fruiting body extract of C. comatus
- FBCt
Fruiting body extract of C. truncorum
- FCc
Fermentation broth extract of C. comatus
- FCt
Fermentation broth extract of C. truncorum
- FRAP
Ferric reducing ability of plasma
- HPLC–MS/MS
Liquid chromatography–mass spectrometry
- M
Mycelia
- MCc
Submerged mycelium extract of C. comatus
- MCt
Submerged mycelium extract of C. truncorum
- ROS
Reactive oxygen species
- RSC
Free radical scavenging capacity
- TC
Total phenol content
- TF
Total flavonoid content
- TP
Total protein content
Contributor Information
Boris Pejin, Phone: + 381 11 2078 490, Email: brspjn@gmail.com, Email: borispejin@imsi.rs.
Maja Karaman, Phone: +381 21 485 2682, Email: maja.karaman@dbe.uns.ac.rs.
References
- Asatiani M, Elisashvili V, Wasser S, Reznick A, Eviatar N. Free-radical scavenging activity of submerged mycelium extracts from higher basidiomycetes mushrooms. Biosci Biotechnol Biochem. 2007;71:3090–3092. doi: 10.1271/bbb.70280. [DOI] [PubMed] [Google Scholar]
- Asatiani MD, Elisashvili V, Songulashvili G, Reznick AZ, Wasser SP. Higher basidiomycetes mushrooms as a source of antioxidants. In: Rai M, Kövics G, editors. Progres in Mycology. 1. Jodhpur: Rajasthan Law Book Binding Works; 2010. pp. 311–326. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/S0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of quantities microgram of protein utilizing the principle of dye-binding protein. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang ST, Miles PG. Mushrooms: cultivation, nutritional value, medicinal effect, and environmental impact. Boca Raton: CRC Press; 2004. [Google Scholar]
- Chang CC, Yang HM, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Cohen N, Cohen J, Asatiani MD, Varshney VK, Yu HT, Yang YC, Li YH, Mau JL, Wasser SP. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int J Med Mushrooms. 2014;16:273–291. doi: 10.1615/IntJMedMushr.v16.i3.80. [DOI] [PubMed] [Google Scholar]
- De Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012;56:1–29. doi: 10.1007/s13225-012-0187-4. [DOI] [Google Scholar]
- Ding Z, Lu T, Lu Z, Lv F, Wang Y, Bie X, Wang F, Zhang K. Hypoglycaemic effect of comatin, an antidiabetic substance separated from Coprinus comatus broth, on alloxan-induced-diabetic rats. Food Chem. 2010;121:39–43. doi: 10.1016/j.foodchem.2009.12.001. [DOI] [Google Scholar]
- Elisashvili V. Submerged cultivation of medicinal mushrooms: bioprocesses and products (review) Int J Med Mushrooms. 2012;14:211–239. doi: 10.1615/IntJMedMushr.v14.i3.10. [DOI] [PubMed] [Google Scholar]
- Espin CJ, Soler-Rivas G, Wichers JH. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem. 2000;48:648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Fraga C. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. New Jersey: Wiley; 2009. [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]
- Han RM, Tian YX, Liu Y, Chen CH, Ai XC, Zhang JP, Skibsted LH. Comparison of flavonoids and isoflavonoids as antioxidants. J Agric Food Chem. 2009;57:3780–3785. doi: 10.1021/jf803850p. [DOI] [PubMed] [Google Scholar]
- Ho CT, Lee CY, Huang MT. Phenolic compounds in food and their effects on health I. Analysis occurrence & chemistry. Washington: ACS Publications; 1992. [Google Scholar]
- Karaman M, Stahl M, Vulić J, Vesić M, Čanadanović-Brunet J. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int J Food Sci Nutr. 2014;65:311–319. doi: 10.3109/09637486.2013.860584. [DOI] [PubMed] [Google Scholar]
- Ko KS, Lim YW, Kim YH, Jung HS. Phylogeographic divergences of nuclear ITS sequences in Coprinus species sensu lato. Mycol Res. 2001;105:1519–1526. doi: 10.1017/S0953756201005184. [DOI] [Google Scholar]
- Li B, Lu F, Suo X, Nan H, Li B. Antioxidant properties of cap and stipe from Coprinus comatus. Molecules. 2010;15:1473–1486. doi: 10.3390/molecules15031473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- Mushtaq MY, Choi YH, Verpoorte R, Wilson EG. Extraction for metabolomics: access to the metabolome. Phytochem Anal. 2013;25:291–306. doi: 10.1002/pca.2505. [DOI] [PubMed] [Google Scholar]
- Nguyen TK, Lee MW, Yoon KN, Kim HY, Jin GH, Choi JH, Im KH, Lee TS. In vitro antioxidant, anti-diabetic, anti-cholinesterase, tyrosinase and nitric oxide inhibitory potential of fruiting bodies of Coprinellus micaceus. J Mushrooms. 2014;12:330–340. doi: 10.14480/JM.2014.12.4.330. [DOI] [Google Scholar]
- Orčić D, Francišković M, Bekvalac K, Svirčev E, Beara I, Lesjak M, Mimica-Dukic Neda. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014;143:48–53. doi: 10.1016/j.foodchem.2013.07.097. [DOI] [PubMed] [Google Scholar]
- Pero RW, Lund H, Leanderson T. Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res. 2009;23:335–346. doi: 10.1002/ptr.2628. [DOI] [PubMed] [Google Scholar]
- Popović M, Vukmirović S, Stilinović N, Čapo I, Jakovljević V. Anti-oxidative activity of an aqueous suspension of commercial preparation of the mushroom Coprinus comatus. Molecules. 2010;15:4564–4571. doi: 10.3390/molecules15074564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. Medicinal mushrooms—a clinical quide. UK: Mycology Press; 2014. [Google Scholar]
- Redhead SA, Vilgalys R, Moncalvo JM, Johnson J, Hopple JS., Jr. Coprinus Persoon and the disposition of Coprinus species sensu lato. Taxon. 2001;50(1):203–241. doi: 10.2307/1224525. [DOI] [Google Scholar]
- Singh G, Maurya S, deLampasona MP, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650–1661. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Stojković D, Reis F, Barros L, Glamočija J, Ćirić A, Griensven L, Soković M, Ferreira I. Nutrients and non-nutrients composition and bioactivity of wild and cultivated Coprinus comatus (O. F. M Müll.) Pers. Food Chem Toxicol. 2013;59:289–296. doi: 10.1016/j.fct.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Vaz JA, Barros L, Martins A, Santos-Buelga C, Vasconcelos M, Ferreira I. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011;126:610–616. doi: 10.1016/j.foodchem.2010.11.063. [DOI] [Google Scholar]
- Wasser SP. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol. 2011;89:1323–1332. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- Zahid S, Udenigwe CC, Ata A, Eze MO, Segstro EP, Holloway P. New bioactive natural products from Coprinus micaceus. Nat Prod Res. 2006;20:1283–1289. doi: 10.1080/14786410601101829. [DOI] [PubMed] [Google Scholar]
- Zenkova VA, Efremenkova OV, Ershova EY, Tolstych IV, Dudnik YV. Antimicrobial activity of medicinal mushrooms from the genus Coprinus (Fr.) S. F. Gray (Agaricomycetideae) Int J Med Mushrooms. 2003;5:1–6. doi: 10.1615/IntJMedMushr.v5.i1.50. [DOI] [Google Scholar]
- Zhang M, Zhu L, Cui SW, Wang Q, Zhou T, Shen H. Fractionation, partial characterization and bioactivity of water-soluble polysaccharides and polysaccharide–protein complexes from Pleurotus geesteranus. Int J Biol Macromol. 2011;48:5–12. doi: 10.1016/j.ijbiomac.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Zhao S, Rong CB, Kong C, Liu Y, Xu F, Miao QJ, Wang SX, Wang HX, Zhang GQ. A novel laccase with potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from mycelia of mushroom Coprinus comatus. BioMed Res Int. 2014;2014:1–8. doi: 10.1155/2014/417461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JJ, Tang YJ. Submerged cultivation of medicinal mushrooms for production of valuable bioactive metabolites. Adv Biochem Eng Biotechnol. 2004;87:25–59. doi: 10.1007/b94367. [DOI] [PubMed] [Google Scholar]