Abstract
The effect of postharvest chitosan, gallic acid (GA) and chitosan gallate (CG) dipping treatments at different concentrations on quality parameters, antioxidant compounds, free radical scavenging capacity (FRSC) and enzymes activities of ‘Sukkari’ bananas were studied during storage (ripening) at 20 ± 2 °C and 60–70% RH for 13 days. Weight loss and peel color index (the change from green to yellow) increased while, membrane stability index of peel tissues, pulp firmness and acidity decreased during storage. CG and GA treatments slowed down the changes in these parameters compared to control. Total soluble solids (TSS) concentration increased during storage and was lower at CG than other treatments. TSS/acid ratio increased during storage and showed higher value after storage than initial. This ratio was lower at 1% chitosan, 0.075% GA and CG treatments than control. Both vitamin C and total flavonoids concentrations decreased during storage and were not affected by the applied treatments. Total phenols concentration decreased during storage and was higher at acetic acid and the high rate of chitosan, GA and CG treatments than control. FRSC (DPPH IC50 values) of fruit peel ranged from 2.54 to 4.19 µg phenolics concentration among the treatments. FRSC was not affected by the applied treatments but increased (lower IC50 value) during shelf life. The possible relations of these biochemical changes with the activities of the enzymes α-amylase, xylanase, polygalacturonase, peroxidase and polyphenoloxidase were discussed. It is concluded that postharvest CG and GA treatments delayed ripening and maintained better quality parameters of ‘Sukkari’ bananas during 13 days of shelf life than control.
Keywords: Banana, Edible coatings, Chitosan, Gallic acid, Quality, Enzymes
Introduction
Banana (Musa spp.) is one of the most popular tropical and subtropical fruit that marketed and consumed worldwide due to its high functional nutritional value (Mohapatra et al. 2010). Banana fruit represent a typical climacteric type of fruit in which ethylene plays a critical role in the ripening process (Duan et al. 2007). The post-climacteric life of bananas at ambient conditions is very short (a few days) due to rapid softening, oxidation damages, peel spotting and fungal decay (Duan et al. 2007; Hailu et al. 2013). In addition, the cold storage of banana fruit is limited by high sensitivity to low temperature and pathogens (Cano et al. 1997). Modified or controlled atmosphere storage techniques have been reported to relatively extend the shelf life of banana fruit (Yousaf et al. 2006; Bhande et al. 2008). However, the use of such technology is also limited by the sensitivity of bananas to CO2 injuries and development of off-flavor by anaerobic respiration and ethanol production (Yousaf et al. 2006; Bhande et al. 2008). Bioactive natural edible coatings such as chitosan is attracting a worldwide interest as an alternative to chemical preservatives to control decay and extend fruit shelf life (Romanazzi et al. 2013). Postharvest dipping of ‘Embul’ bananas in 1% chitosan (Jinasena et al. 2011) or ‘Cavendish’ bananas in 2% chitosan (Suseno et al. 2014) delayed ripening, decreased weight loss and maintained higher firmness and vitamin C concentration with lower anthracnose incidence than control. In addition to its function as protective barrier, chitosan coating can work as carrier for bioactive compounds, such as antioxidants and antimicrobials (Sun et al. 2014). In this respect, gallic acid (GA) (3,4,5-trihydroxybenzoic acid) is a phenolic acid, widely distributed among the plant kingdom, showed a promising potential for inclusion in chitosan matrix since it proved antioxidants and antimicrobial activities (Yen et al. 2002; Chanwitheesuk et al. 2007). It was also reported that the incorporation of GA into the chitosan matrix not only improved the elasticity and physical properties of chitosan film (Hager et al. 2012; Sun et al. 2014) but also increased the hydroxyl radical scavenging capacity of the produced films (Pasanphan et al. 2010). Accordingly, it is hypothesized that the use of chitosan in conjunction with GA would be beneficial for fruit preservation. GA was selected in the present study owing to the fact that it is a natural water soluble antioxidant compound. Chitosan as the film forming agent, is soluble in aqueous acetic acid. Since GA (pKa = 4.5) is more acidic than acetic acid (pKa = 4.76), therefore it is anticipated that mixing aqueous solutions of both GA in water and chitosan in acetic acid would result in a homogeneous solution in which GA would replace acetic acid in the composite to produce chitosan-gallic acid formulation [refereed to as chitosan gallate (CG)] as shown below.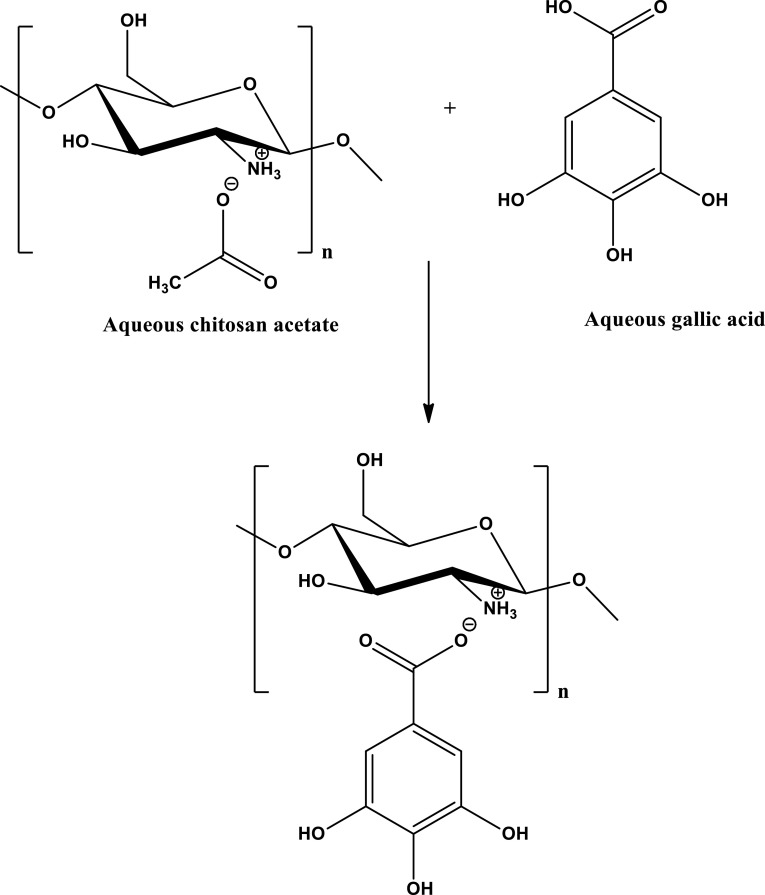
To the best of our knowledge, there is no published information on the response of banana fruit to postharvest GA and CG (as a coating formulation) treatments. Therefore, this study aim to evaluate the effectiveness of postharvest dipping in chitosan, GA and CG on shelf life and quality, FRSC, antioxidant compounds and enzymes activities of ‘Sukkari’ bananas.
Materials and methods
Plant materials and experimental procedure
This experiment was performed on bananas (cv. ‘Sukkari’) imported from Yemen, and purchased in a local commercial company in Jeddah, Kingdom of Saudi Arabia (KSA). Fruit were harvested, packed as hands in polyethylene film in perforated cardbox (about 30 kg) and transported from Yemen to Jeddah within 48 h at 15 °C. Bananas (at the ripening stage 1, according to color chart index (Soltani et al. 2010) were directly pre-treated with ethylene gas (about 0.01% by volume in air) at 18 °C and 85% RH for 24 h for ripening induction at a commercial airtight ground warehouses with a great deal of bananas. Then, uniform hands (at the ripening stage 2) were randomly selected at the warehouse and rapidly transported to the horticulture laboratory of King Abdulaziz University in Jeddah.
Preparation of chitosan gallate formulation (stock solution)
CG coating was prepared by mixing equal volumes of a clear solution of 1% (w/v) of GA with a clear solution of 2% (w/v) of chitosan (100,000–300,000 MW) (Acros Organic, New Jersey, USA) and stirring for 1 h at room temperature. GA solution was dissolved in distilled water and chitosan solution was dissolved in 2% acetic acid (v/v). This composite of chitosan: gallic acid: acetic acid (2:1:2) considered as a stock solution.
Fruit treatments
Bananas (at the color stage 2) carefully prepared in small uniform hands (about five fingers each, free of visual defects and with similar weight and size) were selected. A completely randomized experimental design with three replicates (six hands each) was established. Fruit of each treatment/replicate were soaked either into water (control), 1% acetic acid, 0.15 or 1% chitosan (dissolved in 2% acetic acid), 0.075 or 0.15% GA, and 25, 50 or 75 mL/L of CG solution (stock solution) for 5 min. A surfactant (Tween 20 at 0.5 mL/L) was added to all treatments. Following air draying of about 1 h, all treatments/replicates were weighted and stored at 20 ± 2 °C and 60–70% (RH) in perforated cardboard cartons for 13 days. Before applying the treatments, additional three samples (ten fingers of each) were randomly collected for initial quality and biochemical analyses as described below. After 3, 6, and 13 days of storage weight loss and peel color stage were recorded for each treatment/replicate as described below. After 6 and 13 days of storage samples (ten fingers of each) from each treatment/replicate were randomly collected for quality and biochemical analyses. Then, these fruit samples were peeled and the peel tissue was sliced and mixed. Random part of this peel was used for electrolyte leakage measurement and the remaining peel was kept at −80 °C for later enzyme, total flavonoids and phenols and antioxidant activity analysis. Pulp firmness was measured in each sample directly following peeling. The pulp tissue was later sliced and mixed and a random portion of it was used for TSS, titratable acidity, pH, and vitamin C determinations.
Weight loss determination
The total fruit weight loss was calculated on initial weight basis and expressed in percentage.
Peel color change estimation
Peel color stage was visually recorded for each samples (ten individual fingers of each) using a banana color chart index (scale 1–7, refers to dark green to full yellow color) (Soltani et al. 2010).
Firmness, TSS, acidity, pH and vitamin C measurements of fruit pulp
Fruit pulp firmness was measured independently in ten fingers (in the middle of each finger) per replicate by a digital basic force gauge, model BFG 50N (Mecmesin, Sterling, Virginia, USA) supplemented with a probe of 11 mm diameter and the results were expressed as Newton. A homogeneous sample was prepared from these ten fingers per replicate for measuring TSS, acidity, pH and vitamin C. TSS concentration was measured as a percentage in fruit pulp juice with a digital refractometer (Pocket Refractometer PAL 3, ATAGO, Japan). Titratable acidity was determined in fruit juice diluted in water at a ratio 1:2 by titrating with 0.1 N sodium hydroxide up to pH 8.2, using automatic titrator (HI 902, HANNA Instrument, USA) and the results expressed as a percentage of malic acid. Fruit juice pH was measured by a pH meter (WTW 82382, Weilheim, Germany). Vitamin C was measured by the oxidation of ascorbic acid with 2,6-dichlorophenol endophenol dye and the results expressed as g kg−1 on a fresh weight (FW) basis.
Leakage of ions from fruit peel
Leakage of ions, total phenols and flavonoids, free radical scavenging capacity and enzymes activities were measured in peel since the integrity of these tissues is critical for fruit storability and the biochemical changes in peel reflect the physiological and ripening status of whole fruit including flesh. Also, it is well known that the peel contain much higher level of antioxidant substances, antioxidant activity and enzymes activities than the flesh. Leakage of ions from peel disks was measured according to Sairam et al. (1997) with some modifications and was expressed as membrane stability index percentage (MSI%). Three grams of peel disks per replicate/treatment was randomly taken and placed in 30 ml of deionized water at ambient temperature for 4 h in a shaker. Conductivity before boiling (C1) was measured with an electrical conductivity digital meter (Orion 150A+, Thermo Electron Corporation, USA). The same disks were kept in a boiling water bath (100 °C) for 30 min to release all electrolytes, cooled to 22 ± 2 °C with running water, and conductivity after boiling was recorded (C2). MSI was expressed in percentage using the formula: [1 − (C1/C2)] × 100.
Preparation of the methanol extract of fruit peel
Two grams of fruit peel (randomly collected from ten fingers/replicate) were extracted by shaking at 150 rpm for 12 h with 20 mL methanol (80%) and filtered through filter paper No. 1. The filtrate designated as methanol extract that will be used for total phenols, total flavonoids and antioxidant activity estimations.
Estimation of total phenols
Total phenols concentration was measured according to Hoff and Singleton (1977). Fifty µL of the methanol extract was mixed with 100 µL Folin-Ciocalteu reagent, 850 µL of methanol and allowed to stand for 5 min at ambient temperature. A 500 µL of 20% sodium carbonate was added and allowed to react for 30 min. Absorbance was measured at 750 nm. Total phenols was quantified from a calibration curve obtained by measuring the absorbance of known concentrations of gallic acid and the results expressed as g kg−1 FW gallic acid equivalent.
Estimation of total flavonoids
Total flavonoids concentration was determined using a modified colorimetric method described previously by Zhishen et al. (1999). Methanol extract or standard solution (250 µL) was mixed with distilled water (1.25 mL) and 5% NaNO2 solution (75 µL). After standing for 6 min, the mixture was combined with 10% AlCl3 solution (150 µL), 1 M NaOH (0.5 mL) and distilled water (275 µL) were added to the mixture 5 min later. The absorbance of the solutions at 510 nm was then measured. Total flavonoids was quantified from a calibration curve obtained by measuring the absorbance of known concentrations of catechin and the results expressed as g kg−1 FW catechin equivalent.
Evaluation of DPPH radical scavenging assay of fruit peel
Free radical scavenging activity of methanol extract of fruit peel was determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method (Ao et al. 2008). A methanol extract (0.1 ml) was added to 0.9 ml of freshly prepared DPPH methanol solution (0.1 mM). An equal amount of methanol was used as a control. After incubation for 30 min at room temperature in the dark, the absorbance (Abs) was measured at 517 nm using a spectrophotometer. Activity of scavenging (%) was calculated using the following formula:
The inhibition concentration (IC50) was defined as µg phenolics of the test sample that decreases 50% of initial radical. The IC50 values were calculated from the dose responses curves.
Enzymes measurements of fruit peel
Crude extract
One gram of fruit peel (randomly collected from ten fingers/replicate) was homogenized with 20 mM Tris–HCl buffer, pH 7.2 using homogenizer. The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was designed as crude extract and stored at −20 °C for peroxidase, polyphenoloxidase, polygalacturonase, xylanase and α-amylase assay.
Peroxidase assay
Peroxidase (EC 1.11.1.7) activity (POD) was assayed according to Miranda et al. (1995). The reaction mixture containing in one ml: 0.008 mL of 0.97 M H2O2, 0.08 mL of 0.5 M guaiacol, 0.25 mL of 0.2 M sodium acetate buffer, pH 5.5 and least amount of enzyme preparation. The change in absorbance at 470 nm due to guaiacol oxidation was followed for 1 min using a spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme which increases the O.D. 1.0 per min under standard assay conditions.
Polyphenoloxidase assay
Polyphenoloxidase (EC 1.14.18.1) (PPO) activity was assayed with catechol as a substrate according to the spectrophotometric procedure of Jiang et al. (2002). The extract (0.2 mL) was rapidly added to 2.8 mL of 20 mM catechol solution prepared in 0.01 M sodium phosphate buffer (pH 6.8). The increase in absorbance at 400 nm was recorded for 3 min using a spectrophotometer. One unit of enzyme activity was defined as the amount of the enzyme that causes a change of 0.1 in absorbance per min.
Polygalacturonase, α-amylase and xylanase assays
Polygalacturonase (EC 3.2.1.15) (PG), α-amylase (EC 3.2.1.1) and xylanase (EC 3.2.1.8) activities were assayed by determining the liberated reducing end products using galacturonic acid, maltose and xylose, respectively as standards (Miller 1959). The reaction mixture (0.5 mL) containing 5 mg substrate, 0.25 mL of 0.2 M sodium acetate buffer pH 5.5 and a suitable amount of crude extract. Assays were carried out at 37 °C for 1 h. Then 0.5 mL dinitrosalicylic acid reagent was added to each tube and heated in a boiling water bath for 10 min. After cooling to room temperature, the absorbance was measured at 560 nm. Substrates used were polygalacturonic acid, starch and xylane for polygalacturonase, α-amylase and xylanase, respectively. One unit of enzyme activity was defined as the amount of enzyme which liberated 1 μM of reducing sugar per min under standard assay conditions.
Statistical analysis
The data were statistically analyzed as a completely randomized design with three replicates by analysis of variance (ANOVA) using the statistical package software SAS (SAS Institute Inc., 2000, Cary, NC., USA). Comparisons between means were made by F-test and the least significant differences (LSD) at P ≤ 5%.
Results
Fruit weight loss was lower at all CG treatments and at the high concentration of GA than control and other treatments (Table 1). Fruit weight loss increased during storage. There were no significant interaction effects between treatment and storage period on fruit weight loss (Table 1). Peel color index was lower at all CG treatments, especially at the highest concentration, than other treatments. In this respect, chitosan at 1% and GA at both concentrations showed lower color index values than control and acetic acid treatments. Peel color index increased during storage and showed higher values than initial (Table 1). Membrane stability index of peel decreased during storage and was higher at CG and at the high rate of GA than other treatments (Table 1). The significant interaction effects between treatment and storage period on both color index and membrane stability index of peel (Table 2) revealed that CG was the only effective treatment in maintaining fruit color and membrane stability after 13 days of storage. Fruit firmness was higher at all CG treatments, especially at 50 and 75 mL/L, than other treatments including control (Table 3). In this respect, 1% chitosan and 0.075% GA treatments showed higher firmness values than control. Fruit firmness decreased during storage and showed greatly lower values than initial. TSS concentration was lower in all treatments than control and acetic acid treatments. In this respect, CG treatments showed lower TSS concentration than all other treatments. TSS concentration increased during storage and showed much higher concentration than initial. Acidity concentration was higher at acetic acid, the high concentration of GA and chitosan, and CG treatments than control. Acidity concentration decreased during storage and showed higher value after 6 days of storage than initial. TSS/acid ratio was lower at 1% chitosan, 0.075% GA and at all CG treatments than control. This ratio increased during storage and showed much higher value after storage than initial. There were no significant interaction effects between treatment and storage period on fruit firmness, TSS and acidity concentration, and TSS/acid ratio (Table 3). However, the pH value was not significantly affected by the applied treatments or shelf life period (Table 3). Vitamin C concentration was not affected by the applied treatments but, significantly decreased during shelf life and showed much lower values than initial (Table 3). However, the significant interaction effects between treatment and storage period on vitamin C concentration (Table 3) revealed that CG and the high rate of both chitosan and GA maintained higher level of vitamin C than other treatments only after 6 days of storage. Total flavonoids concentration was not affected by the applied treatments but significantly decreased during shelf life and showed lower values than initial (Table 4). There were no significant interaction effects between treatment and storage period on total flavonoids concentration (Table 4). Total phenols concentration was higher at acetic acid, 1% chitosan, 0.15% GA and at medium and high levels of CG treatments than control (Table 4). Total phenols concentration decreased during storage and showed higher value after 6 days but lower after 13 days of storage than initial. The significant interaction between treatment and shelf life period on total phenols concentration revealed that, after 13 days of storage, CG treatments showed similar level of total phenols as control. Also, after 6 days of shelf life, the low rate of CG gave similar total phenols value to control. FRSC mean values of fruit peel extract measured by the DPPH method (IC50 values) ranged from 2.54 to 4.19 µg phenolics concentration among all treatments. FRSC was not affected by the applied treatments but significantly increased (lower IC50 values) during shelf life. There were no significant interaction effects between treatment and shelf life period on the FRSC (Table 4). α-amylase activity was not affected by the applied treatments or shelf life period but, showed slightly higher values than initial (Table 5). Xylanase activity was lower at acetic acid, 0.15% chitosan, 0.15% GA and 50 mL/L CG than control. Xylanase activity was higher after 6 than 13 days of shelf life and showed higher values than initial (Table 5). There were no significant interaction effects between treatment and shelf life period on both α-amylase and xylanase activities (Table 5). PG activity decreased by all treatments compared to control except for acetic acid treatment. PG activity was higher after 6 than 13 days of shelf life (Table 5). There were significant interaction effects between treatment and shelf life period on PG activity (Tables 5, 6). POD activity was lower at both GA concentrations and at the low and medium concentrations of CG than other treatments (Table 5). POD activity was higher after 13 than 6 days of shelf life and showed higher values than initial (Table 5). There were significant interaction effects between treatment and shelf life period on POD activity (Tables 5, 6). PPO activity was significantly lower at 1% chitosan and the medium concentration of CG than other treatments, except for the low concentration of CG treatment (Table 5). While, PPO activity was higher at 0.075% GA and the high rate of CG than control. PPO activity was higher after 6 than 13 days of shelf life and showed higher values than initial (Table 5). The significant interaction effects between treatment and shelf life period on PPO activity revealed that PPO activity decreased during shelf life in all treatments except for, the low and medium concentrations of CG (Tables 5, 6).
Table 1.
Fruit weight loss, peel color and peel membrane stability index of ‘Sukkari’ bananas during storage as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| Fruit weight loss (%) | Peel color (index) | Peel membrane stability (index) | |
|---|---|---|---|
| Initial | 0.0 | 2.0 | 69.8 |
| Treatments (T) | |||
| Control | 5.16a | 6.2a | 12.9b |
| Acetic acid 1% | 4.65abcd | 6.1a | 14.1b |
| Chitosan (%) | |||
| 0.15 | 5.13a | 6.2a | 13.5b |
| 1 | 4.89ab | 5.7b | 15.2b |
| Gallic acid (%) | |||
| 0.075 | 4.85abc | 5.5b | 19.8b |
| 0.15 | 4.19cd | 5.1c | 29.0a |
| Chitosan gallate (mL/L)a | |||
| 25 | 4.39bcd | 4.5d | 36.4a |
| 50 | 4.16d | 4.3d | 36.3a |
| 75 | 4.19cd | 3.9e | 29.1a |
| F-test | * | *** | *** |
| LSD (0.05) | 0.67 | 0.36 | 7.7 |
| Storage period (SP) (days) | |||
| 3 | 1.54c | 3.8c | – |
| 6 | 3.02b | 5.2b | 33.4a |
| 13 | 9.48a | 6.8a | 12.5b |
| F-test | *** | *** | *** |
| LSD (0.05) | 0.39 | 0.21 | – |
| T × SP | |||
| F-test | NS | *** | ** |
NS not significant, – not calculated
Means within each column followed by the same letter are not significantly different at level P ≤ 0.05. *, ** and *** significant at P ≤ 0.05, 0.01 and 0.001, respectively
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Table 2.
The interaction effect between treatments and storage periods on color and membrane stability index of ‘Sukkari’ banana fruit peel during shelf life as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| Treatments | Storage periods (days) | ||||
|---|---|---|---|---|---|
| Peel color (index) | Membrane stability (index) | ||||
| 3 | 6 | 13 | 6 | 13 | |
| Control | 5.0f | 6.7ab | 7.0a | 20.7defg | 5.1i |
| Acetic acid 1% | 5.0f | 6.3bc | 7.0a | 22.4de | 5.8i |
| Chitosan (%) | |||||
| 0.15 | 5.0f | 6.7ab | 7.0a | 18.2efgh | 8.8hi |
| 1 | 4.0g | 6.0cd | 7.0a | 22.1def | 8.3hi |
| Gallic acid (%) | |||||
| 0.075 | 4.0g | 5.7de | 7.0a | 29.8cd | 9.8hig |
| 0.15 | 3.0i | 5.3ef | 7.0a | 46.5ab | 11.5hgif |
| Chitosan gallate (mL/L)a | |||||
| 25 | 3.3hi | 3.7gh | 6.6ab | 45.2ab | 27.7de |
| 50 | 3.0i | 3.6gh | 6.3bc | 54.9a | 17.8ehgf |
| 75 | 2.3j | 3.3hi | 6.0cd | 40.6bc | 17.7ehgf |
For each parameter, means within and between columns followed by the same letter are not significantly different at level P ≤ 0.05
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Table 3.
Firmness, TSS, acidity, TSS/acid ratio, pH and vitamin C of ‘Sukkari’ bananas during storage as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| Firmness (N) | TSS (%) | Acidity (%) | TSS/acid (ratio) | pH | Vitamin C (g kg−1) | |
|---|---|---|---|---|---|---|
| Initial | 41.5 | 3.3 | 0.52 | 6.4 | 5.0 | 0.095 |
| Treatments (T) | ||||||
| Control | 6.2d | 16.5a | 0.47de | 41.8ab | 5.1 | 0.059 |
| Acetic acid 1% | 7.1cd | 16.1a | 0.61abc | 33.3bcd | 5.1 | 0.064 |
| Chitosan (%) | ||||||
| 0.15 | 6.4d | 13.8b | 0.34e | 48.0a | 5.2 | 0.059 |
| 1 | 7.7c | 14.0b | 0.68ab | 30.8cde | 5.2 | 0.064 |
| Gallic acid (%) | ||||||
| 0.075 | 7.7c | 13.2b | 0.53cd | 29.4cde | 5.2 | 0.064 |
| 0.15 | 7.3cd | 13.1b | 0.69a | 35.6bc | 5.5 | 0.069 |
| Chitosan gallate (mL/L)a | ||||||
| 25 | 11.1b | 8.4d | 0.55bcd | 24.6ef | 5.1 | 0.063 |
| 50 | 13.7a | 7.7d | 0.53cd | 18.1f | 4.7 | 0.066 |
| 75 | 13.6a | 10.8c | 0.69a | 25.8def | 4.8 | 0.064 |
| F-test | *** | *** | *** | *** | NS | NS |
| LSD (0.05) | 1.1 | 1.9 | 0.13 | 8.5 | – | – |
| Shelf life period (SP) (days) | ||||||
| 6 | 10.3a | 11.1b | 0.83a | 15.2a | 5.1 | 0.070a |
| 13 | 7.7b | 14.3a | 0.30b | 48.8b | 5.2 | 0.058b |
| F-test | *** | *** | *** | *** | NS | *** |
| T × SP | ||||||
| F-test | NS | NS | NS | NS | NS | *** |
NS not significant, – not calculated
Means within each column followed by the same letter are not significantly different at level P ≤ 0.05. ** and *** significant at P ≤ 0.01 and 0.001, respectively
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Table 4.
Total flavonoids and phenols concentration and free radical scavenging capacity (FRSC) of ‘Sukkari’ banana fruit peel during storage as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| Flavonoids (g kg−1) | Phenols (g kg−1) | FRSC (DPPH IC50 value) | |
|---|---|---|---|
| Initial | 0.23 | 0.33 | 2.52 |
| Treatments (T) | |||
| Control | 0.14 | 0.30c | 3.12 |
| Acetic acid 1% | 0.16 | 0.38a | 2.67 |
| Chitosan (%) | |||
| 0.15 | 0.13 | 0.28c | 2.54 |
| 1 | 0.15 | 0.36ab | 3.30 |
| Gallic acid (%) | |||
| 0.075 | 0.15 | 0.31bc | 4.19 |
| 0.15 | 0.14 | 0.38a | 3.72 |
| Chitosan gallate (mL/L)a | |||
| 25 | 0.12 | 0.30c | 2.96 |
| 50 | 0.14 | 0.38a | 2.69 |
| 75 | 0.14 | 0.40a | 2.81 |
| F-test | NS | *** | NS |
| LSD (0.05) | – | 0.048 | – |
| Storage period (SP) (days) | |||
| 6 | 0.18a | 0.45a | 4.46a |
| 13 | 0.10b | 0.24b | 0.95b |
| F-test | *** | *** | *** |
| T × SP | |||
| F-test | NS | *** | NS |
NS not significant, – not calculated
Means within each column followed by the same letter are not significantly different at level P ≤ 0.05. *** significant at P ≤ 0.001, respectively
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Table 5.
Hydrolytic and antioxidant enzymes activities (U min g FW) of ‘Sukkari’ banana peel during storage as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| α-Amylase | Xylanase | PG | POD | PPO | |
|---|---|---|---|---|---|
| Initial | 14.0 | 8.0 | 81 | 11.2 | 394 |
| Treatments (T) | |||||
| Control | 18.5 | 14.2a | 107a | 26.4abc | 581cd |
| Acetic acid 1% | 16.5 | 12.0bc | 102a | 29.0a | 597bcd |
| Chitosan (%) | |||||
| 0.15 | 14.1 | 10.2cd | 78b | 24.1bcd | 624abc |
| 1 | 17.5 | 13.3ab | 68bc | 27.0a | 495e |
| Gallic acid (%) | |||||
| 0.075 | 16.7 | 12.3ab | 61cd | 18.6e | 680a |
| 0.15 | 16.4 | 10.1d | 55d | 14.9f | 586cd |
| Chitosan gallate (mL/L)a | |||||
| 25 | 15.0 | 12.3ab | 62cd | 19.5e | 536de |
| 50 | 14.5 | 12.0bc | 67bcd | 21.7ed | 510e |
| 75 | 15.5 | 13.3ab | 74bc | 23.0cd | 657ab |
| F-test | NS | *** | *** | *** | *** |
| LSD (0.05) | – | 1.92 | 13 | 3.43 | 61 |
| Storage period (SP) (days) | |||||
| 6 | 15.0 | 13.2a | 93a | 12.4b | 678a |
| 13 | 17.2 | 11.1b | 57b | 32.9a | 492b |
| F-test | NS | *** | *** | *** | *** |
| T × SP | |||||
| F-test | NS | NS | *** | *** | *** |
NS not significant; – not calculated
PG, POD and PPO refereeing to polygalacturornase, peroxidase and polyphenoloxidase, respectively. Means within each column followed by the same letter are not significantly different at level P ≤ 0.05. *** significant at P ≤ 0.001
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Table 6.
The interaction effect between treatments and storage periods on polygalacturornase (PG), peroxidase (POD) and polyphenoloxidase (PPO) activities (U min g FW) of ‘Sukkari’ banana peel during storage as affected by postharvest chitosan, gallic acid and chitosan gallate dipping
| Storage periods (days) | ||||||
|---|---|---|---|---|---|---|
| PG | POD | PPO | ||||
| 6 | 13 | 6 | 13 | 6 | 13 | |
| Treatments | ||||||
| Control | 117a | 97bcd | 18.7e | 34.1bc | 744b | 419h |
| Acetic acid 1% | 104ab | 100abc | 19.5e | 38.5ab | 755ab | 439hg |
| Chitosan (%) | ||||||
| 0.15 | 81de | 76e | 17.0ef | 31.2cd | 838a | 410h |
| 1 | 88bcde | 48f | 13.5fg | 40.5a | 550ef | 441hg |
| Gallic acid (%) | ||||||
| 0.075 | 85cde | 37fg | 8.6hij | 28.5d | 791ab | 570e |
| 0.15 | 84cde | 26g | 9.5hig | 20.3e | 657cd | 515efg |
| Chitosan gallate (mL/L)a | ||||||
| 25 | 91bcde | 32fg | 7.5hi | 31.5cd | 553ef | 520efg |
| 50 | 85cde | 49f | 5.5i | 38.0ab | 481fhg | 539fe |
| 75 | 104ab | 44fg | 12hg | 34bc | 736bc | 578de |
For each parameter, means within and between columns followed by the same letter are not significantly different at level P ≤ 0.05
aChitosan gallate was prepared by mixing equal volumes of 1% (w/v) gallic acid with 2% (w/v) chitosan
Discussion
Weight loss occurs during fruit ripening and shelf life is due to respiration process and loss of water through fruit peel that possibly reduced by coatings (Ayranci and Tunc 2003; Maqbool et al. 2011). The incorporation of GA into chitosan matrix might enhanced the effectiveness of chitosan in decreasing weight loss via the improvement of the tensile strength of the coat and decreased the permeability of both water vapor and oxygen (Sun et al. 2014). Peel color changes from green to yellow reflects the advances of banana ripening process (Meng et al. 1997). After the ripening stage six, brown speckles develop on fruit peel with a sharp decrease in flesh firmness, indicating the beginning of the over ripe or senescence stage (Soltani et al. 2010). CG, GA and the high rate of chtitosan delayed peel color changes possibly by delaying the degradation/oxidation of chlorophylls and/or increasing the accumulation of carotenoids. The higher membrane stability index of peel tissue at CG and the high rate of GA compared to other treatments might be attributed to maintenance of peel cell wall integrity by the general antioxidant action of CG and GA (Pasanphan et al. 2010; Hager et al. 2012; Sun et al. 2014). In addition, CG treatments delayed fruit ripening as reflected by a higher firmness, a lower TSS and TSS/acid ratio and a higher vitamin C especially after 6 days of storage than other treatments (according to the interaction effects between treatments and storage period). These results might be also attributed to the concept that the edible coatings are selective barriers to O2 resulting in a lower O2 level in treated fruit and thus reduce respiration rate and delay fruit ripening (Maqbool et al. 2011; Velickova et al. 2013). Different edible coat formulations have been reported to limit oxygen permeability and maintain vitamin C during storage of bananas (Maqbool et al. 2011; Suseno et al. 2014). The retention of phenols by chitosan, GA and CG, especially at higher rate, partly confirms those of Simoes et al (2009) who found a higher phenols concentration in carrot sticks treated with edible coating containing chitosan than control. The observed decrease in total phenols concentration during storage are in accordance with those of Kondo et al (2005) who found that total phenols concentration decreased in the peel of ‘Namwa’ bananas stored at 6 °C but slightly changed in fruit stored at 12 °C for 6 days. Also, Wang et al (2014) found that phenols in the peel of ‘Brazil’ banana slightly increased during the first 10 days of storage at 7 °C then sharply decreased for the rest of storage period. The decrease of phenols concentration in fruit during ripening might be due to breakdown of cell structure because of the senescence phenomena during storage (Macheix et al. 1990). Our results showed that FRSC of fruit peel measured by DPPH assay was not affected by the applied treatments but increased (lower IC50 values) during storage. However, Kondo et al (2005) found that IC50 values of superoxide (O2−) and DPPH-radical scavenging capacity of ‘Namwa’ bananas peel stored at 6 and 12 °C decreased (higher antioxidant capacity) during the first 2 days and then gradually increased (lower antioxidant capacity) during the following 8 days of storage. Also, Fernando et al (2014) reported that total antioxidant activities (mmol TE/100 g fresh weight) measured by DPPH and FRAP of ‘Hom Thong’ and ‘Khai’ bananas flesh during ripening at 25 °C for 10 days increased with ripening but rapidly decreased with senescence. The decrease in total phenols concentration with the increase in FRSC during ripening might suggest qualitative changes in phenolic classes toward higher antioxidant potential. It was reported that the antioxidant capacity of phenolics possibly has a concentration saturation limit above which the activity could not increase further with the concentration (Dani et al 2012). Also, phenolic compounds are possibly not the only factor that contributes to FRSC of fruit but it might work synergistically with several vitamins and minerals (Dani et al 2012). Parallel several assays should be applied to investigate the principles of antioxidant/oxidation activity of a certain horticultural commodity. In the present study, α-amylase activity of fruit peel showed no significant response to the applied treatments or to storage period. Surprisingly, α-amylase activity measured in pulp of some fruit samples, where starch is mostly located, showed even much lower values (ranged from 0.29 to 0.53 U/min/g FW) than in peel and was slightly increased during ripening. Starch degradation process in banana fruit involve two main enzymatic pathways, the amylolytic enzymes (α-amylase, β-amylase, α-glucosidases) and phosphorolytic enzymes (sucrose synthase and sucrose-phosphate synthase) (Purgatto et al. 2001). It has been reported that the small amount of α-amylase detected in banana tissues would be responsible for the initial attack on entire starch granules to produce the sub-substrate needed for other enzymes such as β-amylase that makes the decisive role in the following starch hydrolysis process (Purgatto et al. 2001). However, the possible impact of the relatively high α-amylase activity in peel on starch degradation in pulp needs to be elucidated. The decrease in xylanase activity during storage suggest a possible involvement in the polysaccharides hydrolysis during banana ripening. Chitosan, GA and especially CG treatments decreased PG activity and delayed the ripening process, and maintained higher membrane stability index and green color compared to control. In confirmation, Mirshekari et al. (2015) reported that hot water treatment at 50 °C inhibited PG, pectin methylesterase and pectate lyase activities in the flesh, delayed the disassembling of pectin fraction, delayed the ripening process and prolonged shelf life of ‘Berangan’ bananas compared to control. POD is an antioxidant enzyme that play a role in controlling the level of reactive oxygen species (ROS) in plant cells. In the current study, GA and CG delayed ripening and showed lower POD activity compared to other treatments. POD activity increased during storage and showed higher values than initial. In confirmation, Wang et al (2014) reported that POD activity gradually increased in ‘Brazil’ bananas peel during 20 days of storage at 7 °C. However, other antioxidant enzymes such as superoxide dismutase (SOD) activity decreased in ‘Namwa’ bananas peel during 10 days of storage at 6 and 12 °C (Kondo et al. 2005). Fruit ripening and senescence is an oxidative process in which the transition from mature stage into ripening/senescence stage is accompanied by a progressive shift toward an oxidative state as suggested for ‘Williams’ bananas (Yang et al. 2008). Accordingly, excessive ROS production could participate in the oxidation of lipids and proteins of cell membrane that are involved in banana ripening induction. Indeed a steady decrease in membrane stability index, as measured by the leakage of ions, was observed upon the progression of fruit ripening, which indicates a gradual loss of membrane’s stability due to changes occurring in the biochemical and biophysical properties of cell membranes. A possible involvement of both POD and PPO in fruit browning process during ripening/senescence was suggested as PPO could act as a promoter of peroxidase activity in other fruit as peaches (Ferrer et al. 2005). In conclusion, postharvest CG and GA treatments delayed ripening and maintained better quality of ‘Sukkari’ bananas during 13 days of shelf life than control.
Acknowledgements
The authors would like to thank Dr. Mohamed Ibraheem; Nageeb Al-Masoudi, MSc. and Nour Gamal, BSc. at Arid land Agriculture Department, Faculty of Meteorology, Environment and Arid land Agriculture, King Abdulaziz University, for their indispensable technical support.
Compliance with ethical standards
Conflict of interest
None.
References
- Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control. 2008;19:940–948. doi: 10.1016/j.foodcont.2007.09.007. [DOI] [Google Scholar]
- Ayranci E, Tunc S. A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 2003;80:423–431. doi: 10.1016/S0308-8146(02)00485-5. [DOI] [Google Scholar]
- Bhande SD, Ravindra MR, Goswami TK. Respiration rate of banana fruit under aerobic conditions at different storage temperatures. J Food Eng. 2008;87:116–123. doi: 10.1016/j.jfoodeng.2007.11.019. [DOI] [Google Scholar]
- Cano MP, De Ancos B, Matallana C, Camara M, Reglero G, Tabera J. Difference among Spanish and latin-american banana cultivars: morphological, chemical and sensory characteristics. Food Chem. 1997;59:411–419. doi: 10.1016/S0308-8146(96)00285-3. [DOI] [Google Scholar]
- Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007;100:1044–1048. doi: 10.1016/j.foodchem.2005.11.008. [DOI] [Google Scholar]
- Dani C, Oliboni LS, Pra D, Bonatto D, Santos CEI, Yoneama ML, Dias JF, Salvador M, Henriques JAP. Mineral content is related to antioxidant and antimutagenic properties of grape juice. Genet Mol Res. 2012;11:3154–3163. doi: 10.4238/2012.September.3.4. [DOI] [PubMed] [Google Scholar]
- Duan XW, Joyce DC, Jiang YM. Postharvest biology and handling of banana fruit: review. Fresh Prod. 2007;1:140–152. [Google Scholar]
- Fernando HRP, Srilaong V, Pongprasert N, Boonyaritthongchai P, Jitareerat P. Changes in antioxidant properties and chemical composition during ripening in banana variety ‘Hom Thong’ (AAA group) and ‘Khai’ (AA group) Int Food Res J. 2014;21:749–754. [Google Scholar]
- Ferrer A, Remon S, Negueruela AI, Oria R. Changes during the ripening of the very late season Spanish peach cultivar Calanda feasibility of using CIELAB coordinates as maturity indices. Sci Hortic. 2005;105:435–446. doi: 10.1016/j.scienta.2005.02.002. [DOI] [Google Scholar]
- Hager AS, Vallons KJR, Arendt EK. Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J Agric Food Chem. 2012;60:6157–6163. doi: 10.1021/jf300983m. [DOI] [PubMed] [Google Scholar]
- Hailu M, Workneh TS, Belew D. Review on postharvest technology of banana fruit. Afr J Biotechnol. 2013;12:635–647. [Google Scholar]
- Hoff JF, Singleton KI. A method for determination of tannin in foods by means of immobilized enzymes. J Food Sci. 1977;42:1566–1569. doi: 10.1111/j.1365-2621.1977.tb08427.x. [DOI] [Google Scholar]
- Jiang YM, Zhang ZQ, Joyce DC, Ketsa S. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.) Postharvest Biol Technol. 2002;26:241–252. doi: 10.1016/S0925-5214(02)00047-9. [DOI] [Google Scholar]
- Jinasena D, Pathirathna P, Wickramarachchi S, Marasinghe E (2011) Use of chitosan to control anthracnose on “Embul” banana. International Conference on Asia Agriculture and Animal, IPCBEE, 13, 56-60, IACSIT Press, Singapore
- Kondo S, Kittikorn M, Kanlayanarat S. Preharvest antioxidant activities of tropical fruit and the effect of low temperature storage on antioxidants and jasmonates. Postharvest Biol Technol. 2005;36:309–318. doi: 10.1016/j.postharvbio.2005.02.003. [DOI] [Google Scholar]
- Macheix JJ, Fleuriet A, Billot J. Fruit phenolics. Boca Raton: CRC Press Inc; 1990. [Google Scholar]
- Maqbool M, Ali A, Alderson PG, Zahid N, Siddiqui Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J Agric Food Chem. 2011;59:5474–5482. doi: 10.1021/jf200623m. [DOI] [PubMed] [Google Scholar]
- Meng L, David CS, James FT. Optical chlorophyll sensing system for banana ripening. Postharvest Biol Technol. 1997;12:273–283. doi: 10.1016/S0925-5214(97)00059-8. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal Chem. 1959;31:426–429. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Miranda MV, Fernandez Lahor HM, Cascone O. Horseradish peroxidase extraction and purification by aqueous two-phase partition. Appl Biochem Biotechnol. 1995;53:147–154. doi: 10.1007/BF02788604. [DOI] [Google Scholar]
- Mirshekari A, Ding Ph, Ghazali HM. Enzymatic activity and microstructural changes of hot water treated banana during ripening. J Agric Sci Technol. 2015;17:949–962. [Google Scholar]
- Mohapatra D, Mishra S, Singh CB, Jayas DS. Post-harvest processing of Banana: opportunities and challenges. Food Bioprocess Technol. 2010;4:327–339. doi: 10.1007/s11947-010-0377-6. [DOI] [Google Scholar]
- Pasanphan W, Buettner GR, Chirachanchai S. Chitosan gallate as a novel potential polysaccharide antioxidant: an EPR study. Carbohydr Res. 2010;345:132–140. doi: 10.1016/j.carres.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgatto E, Lajolo FM, Nascimento JRO, Cordenunsi BR. Inhibition of β-amylase activity, starch degradation and sucrose formation by IAA during banana ripening. Planta. 2001;212:823–828. doi: 10.1007/s004250000441. [DOI] [PubMed] [Google Scholar]
- Romanazzi G, Feliziani E, Santini M, Landi L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol Technol. 2013;75:24–27. doi: 10.1016/j.postharvbio.2012.07.007. [DOI] [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–177. doi: 10.1111/j.1439-037X.1997.tb00486.x. [DOI] [Google Scholar]
- Simoes ADN, Tudela JA, Allende A, Puschmann R, Gil MI. Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Biol Technol. 2009;51:364–370. doi: 10.1016/j.postharvbio.2008.08.012. [DOI] [Google Scholar]
- Soltani M, Alimardani R, Omid M. Prediction of banana quality during ripening stage using capacitance sensing system. Aust J Crop Sci. 2010;4:443–447. [Google Scholar]
- Sun X, Wang Z, Kadouh H, Zhou K. The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT Food Sci Technol. 2014;57:83–89. doi: 10.1016/j.lwt.2013.11.037. [DOI] [Google Scholar]
- Suseno N, Savitri E, Sapei L, Padmawijaya KS. Improving shelf-life of Cavendish banana using chitosan edible coating. Procedia Chem. 2014;9:113–120. doi: 10.1016/j.proche.2014.05.014. [DOI] [Google Scholar]
- Velickova E, Winkelhausen E, Kuzmanova S, Alves VD, Moldao-Martins M. Impact of chitosan beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv. Camarosa) under commercial storage conditions. LWT Food Sci Technol. 2013;52:80–92. doi: 10.1016/j.lwt.2013.02.004. [DOI] [Google Scholar]
- Wang Y, Luo Z, Huang X, Yang K, Gao S, Du R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic. 2014;168:132–137. doi: 10.1016/j.scienta.2014.01.022. [DOI] [Google Scholar]
- Yang S, Su X, Prasad KN, Yang B, Cheng G, Chen Y, Yang E, Jiang Y. Oxidation and peroxidation of postharvest banana fruit during softening. Pak J Bot. 2008;40:2023–2029. [Google Scholar]
- Yen G, Duhb P, Tsaia H. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–313. doi: 10.1016/S0308-8146(02)00145-0. [DOI] [Google Scholar]
- Yousaf MS, Yusof S, Yazid M, Abd-Aziz S. Physico-chemical, biochemical and sensory characteristics of Berangan and Mas banana (Musa sapientum) cultivars and their suitability for value added processing. J Food Technol. 2006;4:229–234. [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]


