Abstract
The effect of bovine serum albumin (BSA), whey protein isolate (WPI), whey protein hydrolysate (WPH), sodium caseinate (SC), carboxymethylcellulose sodium (CMC), fish gelatin (FG), high methoxyl apple pectin (HMAP), low methoxyl apple pectin (LMAP), gum Arabic (GA), ι-carrageenan (CGN), and hydroxypropyl chitosan (HPCTS) on physical stability of internal or external aqueous phase of water-in-oil-in-water (W/O/W) emulsions was evaluated. WPI and CGN in the internal aqueous phase, and GA, HPCTS, and CMC in the external phase reduced the size of emulsion droplets. BSA, WPI, SC, FG, CGN, and HPCTS improved the dilution stability of W/O/W emulsions, but HMAP had a negative effect. BSA, WPI, SC, FG, LMAP, GA, CGN, HPCTS, or CMC significantly improved the thermal stability of W/O/W emulsions. Results also indicated that the addition of CGN (1.0%), HMAP (1.0%), WPH (1.0%), or HPCTS (1.0%) in internal aqueous phase significantly increased the viscosity of emulsions, however, addition to the external aqueous phase had insignificant effects. A protein-knockout experiment confirmed that proteins as biomacromolecules, were the key factor in improving physical stability of emulsions.
Keywords: W/O/W emulsions, Biomacromolecules, Physical stability, Rheological properties
Introduction
Water-in-oil-in-water (W/O/W) emulsions are types of complex, structured liquid multiphase systems known as “emulsions of emulsions”, in which, the droplets in the dispersed phase contain even smaller dispersed droplets (Dickinson 2011; Garti and Bisperink 1998; Zhang et al. 2014). Generally, W/O/W emulsions are prepared by a two-step emulsification reduced using two surfactants. A hydrophobic emulsifier with a low hydrophilic-lipophilic balance to stabilize the internal interface of the primary emulsion (W/O), and an outer interface of oil-in-water emulsions (O/W) stabilized by a hydrophilic emulsifier with a high hydrophilic-lipophilic balance (Benichou et al. 2004; Garti 1997a; Garti and Aserin 1996; Garti and Bisperink 1998; Giroux et al. 2013; Li et al. 2014; Palencia and Rivas 2011; Zhang et al. 2014).
Over the past few decades, W/O/W emulsions have been proven to be useful vehicles for encapsulating and delivering active components in agricultural and food industries, pharmaceuticals, biomedical sciences, and cosmetics, or for fat reduction in food (Akhtar and Yazan 2008; Benichou et al. 2002; Dickinson 2011; Ferreira et al. 1995; Frasch-Melnik et al. 2010; Hernández-Marín et al. 2013; Khopade and Jain 1999; Li et al. 2013, 2014; Lindenstruth and Muller 2004; Matos et al. 2014, 2015; Miladi et al. 2015; Muschiolik 2007; Okochi and Nakano 2000; Regan and Mulvihill 2009; Sela et al. 1995; Wroński et al. 2012). However, compared with simple emulsions such as O/W and W/O, W/O/W emulsions are thermodynamically unstable. One of major challenges of generating W/O/W emulsions is the low stability arising from the excess free energy associated with the high droplet surface of the primary and secondary emulsions (Matos et al. 2014, 2015). In particular, the use of two different emulsifiers, a lipophilic emulsifier to stabilize the W/O emulsions and a hydrophilic emulsifier to stabilize the oil droplets, leads to several interactions and instabilities in the W/O/W emulsions (Kanouni et al. 2002). On the one hand, water molecules can diffuse from the inner water phase (W1) into the outer water phase (W2) and vice versa. These movements change the amount of water encapsulated in the oil droplets. On the other hand, coalescence, both between oil droplets in W1/O emulsions or between two W1 droplets, as well as between W1 droplets and the W2 phase, can change the structure stability of W/O/W emulsions, and also change the amount of water encapsulated in the oil droplets, resulting in low encapsulation efficiency and/or fast release of the encapsulated component, and limits the further application of W/O/W emulsions. All these instabilities can occur during processing, as well as storage of the emulsions (Florence and Whitehill 1981; Pays et al. 2001; Schuch et al. 2014). As mentioned previously, formulation and stabilization of the structures of W/O/W emulsion are still challenging and become much more difficult than that of simple emulsions (Dickinson 2011).
Previous studies showed that numerous parameters determine the stability of W/O/W emulsions, including the emulsification method (Hemar et al. 2010; Sugiura et al. 2004; Surh et al. 2007; Vandergraaf et al. 2005), the droplet size distribution characteristics of both the W1/O droplets and the external O/W2 droplets (Bonnet et al. 2009, 2010a, b; Garti 1997b; Weissa and Muschiolik 2007), the type and concentration of different emulsifiers (Chávez-Páez et al. 2012; Garti 1997b; Jager-Lezer et al. 1997; Kanouni et al. 2002; Pays et al. 2001, 2002; Schmidts et al. 2010a, b), the phase volume fraction (Bonnet et al. 2010a, b; Herzi et al. 2014; Weissa and Muschiolik 2007), the osmotic pressure (Delample et al. 2014; Garti and Aserin 1996; Guery et al. 2009; Mezzenga et al. 2004; Schmidts et al. 2010a, b; Wen and Papadopoulos 2001), and the properties of oil phase (Bonnet et al. 2009; Garti and Aserin 1996; Weiss et al. 2005).
Some studies showed that the W/O/W emulsions became more stable if the surfactants have a large molecular size. The large molecules are able to ‘‘anchor” themselves to the respective interface (so the energy required to remove large molecules from an interface is high), so that made themselves less likely to diffuse between interfaces (Benichou et al. 2007; Dickinson 2011; Garti 1997b). Moreover, the additive of some biomacromolecules (Benichou et al. 2004; Bonnet et al. 2010a, b; Dickinson 2011; Garti 1997a; Garti and Aserin 1996; Garti and Bisperink 1998; Michaut et al. 2003; Muschiolik 2007; Pays et al. 2002) can improve the stability of W/O/W emulsions. For example, globular proteins such as bovine serum albumin (BSA) or hemoglobin severed as active co-emulsifier were effective to improve the stability of W/O/W emulsions (Al Haushey et al. 2007; Benichou et al. 2004; Gaiti et al. 1994; Meng et al. 2004; Rojas and Papadopoulos 2007). Our previous study found similar conclusions that BSA or whey protein isolate (WPI) added into the internal phase improved the stability and controlled release properties of W/O/W emulsions (Li et al. 2014). However, still very less studies have provided a convincing theoretical justification or experimental evidence to explain the observed improvements from biomacromolecules. Even in some experiments, the conclusions have suggested that higher concentrations of BSA might play a negative role on long-term stability (Dickinson 2011). Furthermore, it’s more necessary to define a more general rule about the different effects of wide types of biomacromolecules on the stability if W/O/W emulsions, rather than a single type of biomacromolecules.
The present work was undertaken to investigate the effect of different hydrocolloids (bovine serum albumin, whey protein isolate, whey protein hydrolysate, sodium caseinate, carboxymethylcellulose, fish gelatin, high and low methoxyl pectin, gum arabic, carrageenan, and hydroxypropyl chitosan) on the physical stability of W/O/W emulsions.
Materials and methods
Materials
Polyglycerol polyricinoleate (PGPR) and polyglycerol fatty acid ester (PGFE) were obtained from Taiyo Kagaku Co., Ltd. (Yokkaichi, Japan). Gum arabic (GA) and ι-carrageenan (CGN) were purchased from Beijing Banxia Science and Technology Development Co., Ltd. (Beijing, China). Fish gelatin (FG) with two bloom value (280 and 180) was purchased from Hubei Jusheng Technology Co., Ltd (Wuhan, China). Both high methoxyl apple pectin (HMAP) and low methoxyl apple pectin (LMAP) were obtained from Herbstreith and Fox KG (Mannheim, Germany). According to the supplier, the degrees of esterification of the pectin samples were 68 and 38%, respectively. BSA (98% pure) was purchased from Roche (Basel, Switzerland). WPI (Provon® 292) and whey protein hydrolysate (WPH, Thermax® 690) powders were obtained from Glanbia Nutritionals, Inc. (Twin Falls, ID, USA). Sodium caseinate (SC) and carboxymethylcellulose sodium (CMC) were purchased from Sigma–Aldrich (St. Louis, MO, USA). The water-soluble hydroxypropyl chitosan (HPCTS) with a degree of substitution of more than 80% was purchased from Lushen Bioengineering Co., Ltd. (Nantong, China). Soybean oil was purchased from a local supermarket and used without further treatment.
Preparation of W/O/W emulsions
W/O/W emulsions were prepared using an established two-step procedure (Li et al. 2014). First, the stable primary W/O emulsion was prepared using PGPR as the hydrophobic emulsifier. Sodium chloride solution (NaCl, 0.1 M) was used as the internal aqueous phase. To investigate the effects of different macromolecules in the internal aqueous phase, they were added separately at various mass fractions (of the total W/O emulsion). Soybean oil containing PGPR was used as the oil phase. The mass ratio of the internal aqueous phase to the oil phase was 4:6. The mixture was preheated to 60 °C for 15 min, then the primary emulsions were prepared by homogenizing at 12,000 rpm for 3 min using a high-speed shearing mixer homogenizer (T25 digital ULTRA-TURRAX, IKA, Germany). The primary emulsions were then high-pressure homogenized (NS1001, GEA Niro Soavi, Italy) at 60 MPa to generate the final W/O emulsions. The mass fraction of PGPR of the whole W/O emulsion was 1.2%. Secondly, the primary W/O emulsion was dispersed in an aqueous solution of PGFE (which served as a hydrophilic emulsifier) with a mass ratio of the W/O emulsion to the external aqueous solution of 6:4. After mixing, the final W/O/W emulsion was prepared using the high-speed shear machine at 10,000 rpm for 3 min. The mass fraction of PGFE with respect to the whole W/O/W emulsion was 0.5%.
Light microscopy
After dilution in distilled water, W/O/W emulsion samples were observed at 100 × magnification using a light microscope (BX53, Olympus, Japan) equipped with a camera to observe the particle size of emulsion droplets. The light microscopy experiments were conducted to show visual images of particle size distribution.
Physical stability evaluation of W/O/W emulsions
The creaming index was measured to evaluate the physical stability of W/O/W emulsions via the method described earlier (Li et al. 2012, 2014). The emulsion samples were transferred into a centrifuge tube, and then were separated into an optically opaque “cream” layer at the top, and transparent (or turbid) “serum” layers at the bottom after dilution, heating, and freeze–thaw cycles. The entire transparent layer was defined as the serum layer, and the total height of the emulsion (H T) and the height of the serum layer (H S) were measured to calculate the creaming index (%), using equation that creaming index (%) = 100 × (H S /H T). To test dilution stability, 1.0 mL of emulsion sample was added to a centrifuge tube and was diluted with distilled water to a 5.0 mL. To evaluate thermal stability, emulsion samples were heated at 60 °C for 15 min in a water bath. To evaluate freeze–thaw stability, all samples were frozen at −18 °C for 12 h, and then thawed at room temperature. The entire freeze–thaw cycle was repeated thrice. The creaming index provided indirect information about the extent of droplet aggregation in the emulsion after various processing steps. With emulsions, a small creaming index indicates that the emulsion is more stable than one with a high creaming index.
Cryogenic scanning electron microscope
The microstructures of the W/O/W emulsions were analyzed by a HELIOS NanoLab 600i cryogenic scanning electron microscope (Cryo-SEM, FEI, Hillsboro, Oregon, USA). Before scanning, the sample was quickly frozen by liquid nitrogen, and then the frozen sample was fractured to expose an irregular fracture surface. This Cryo-SEM analysis was expected to show the detailed changes in emulsion morphology before and after freeze–thaw treatment.
Rheological properties of W/O/W emulsions
A coaxial cylinder rheometer (R/S-CC plus, BROOKFIELD, Harlow Essex, UK) was used to measure emulsion viscosity at increasing rotational speeds from 1 to 200 min−1 at room temperature.
Protein-knockout experiment
A biomacromolecule-knockout experiment was conducted to investigate the behavior and impact of the biomacromolecules present at the internal interface. Since our approach was limited to remove polysaccharide biomacromolecules by pH adjustment, we only conducted protein-knockout experiments. The detailed conditions of protein-knockout were shown in Table 1. Control groups were established that had no added protein with original pH or the pH adjusted to the pI of the protein (WPI/BSA). Treatment groups were established with 1.0% WPI or 1.0% BSA added to the internal aqueous phase. The pH value of the internal aqueous phase was either not adjusted or was adjusted to the pI of the protein.
Table 1.
Conditions of the protein-knockout experiment
| Groups | pH of the internal aqueous phase | Addition of protein |
|---|---|---|
| CG 1 | Normal | None |
| CG 2 | 5.0 | None |
| CG 3 | 4.7 | None |
| TG 1-a | Normal | WPI, 1.0% (w/w) |
| TG 1-b | 5.0 | WPI, 1.0% (w/w) |
| TG 2-a | Normal | BSA, 1.0% (w/w) |
| TG 2-b | 4.7 | BSA, 1.0% (w/w) |
CG control groups, TG treatment groups
Results and discussion
Particle size distribution of W/O/W emulsions
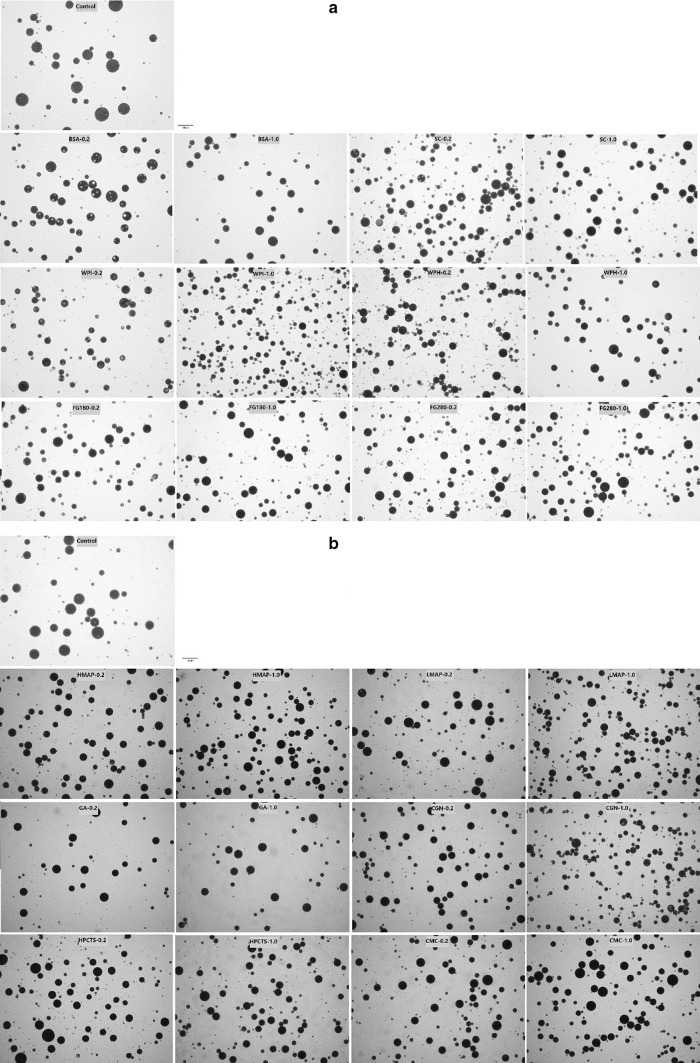
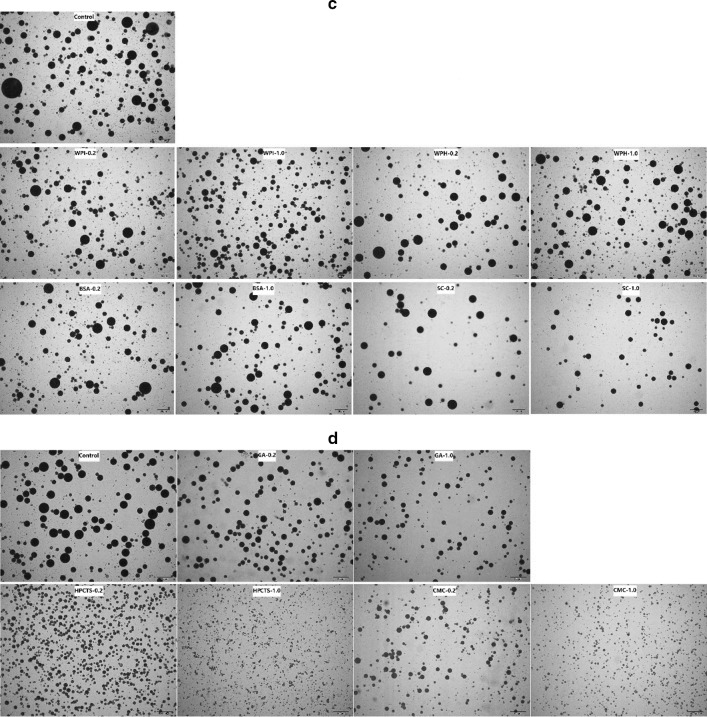
The droplet size in emulsions decreases slightly by most of biomacromolecules (Fig. 1a, b). Amongst all of the biomacromolecules tested, WPI or CGN at concentration of 1.0% decreased the droplet size to the highest extent. The results also showed that the droplet size of emulsions was not dependent on the concentration of biomacromolecules as the emulsions with 0.2 and 1.0% biomacromolecules showed similar droplet size, which may be because that W/O/W system was such a complex system, that each macromolecule had its own mass fraction range to play its positive role on droplet size.
Fig. 1.
Microscope images of W/O/W emulsions with proteins or polysaccharide in the internal or external aqueous phase. a Protein in the internal aqueous phase; b polysaccharide in the internal aqueous phase; c protein in the external aqueous phase; d polysaccharide in the external aqueous phase
The effect of different biomacromolecules in external aqueous phase on emulsion droplet size was also investigated. FG (both bloom values 280 and 180), HMAP, LMAP, and CGN increased the viscosity of the emulsions to a level that it became difficult to attain complete emulsification. Hence, the effect of other biomacromolecules in the external aqueous phase was evaluated. Figure 1 showed that different biomacromolecules showed variable effects on the droplet size distribution. BSA, WPI, WPH, and SC slightly decreased the droplet size, whereas GA, HPCTS and CMC significantly decreased the same. It was found that as the concentration of biomacromolecules increased, droplet size decreased.
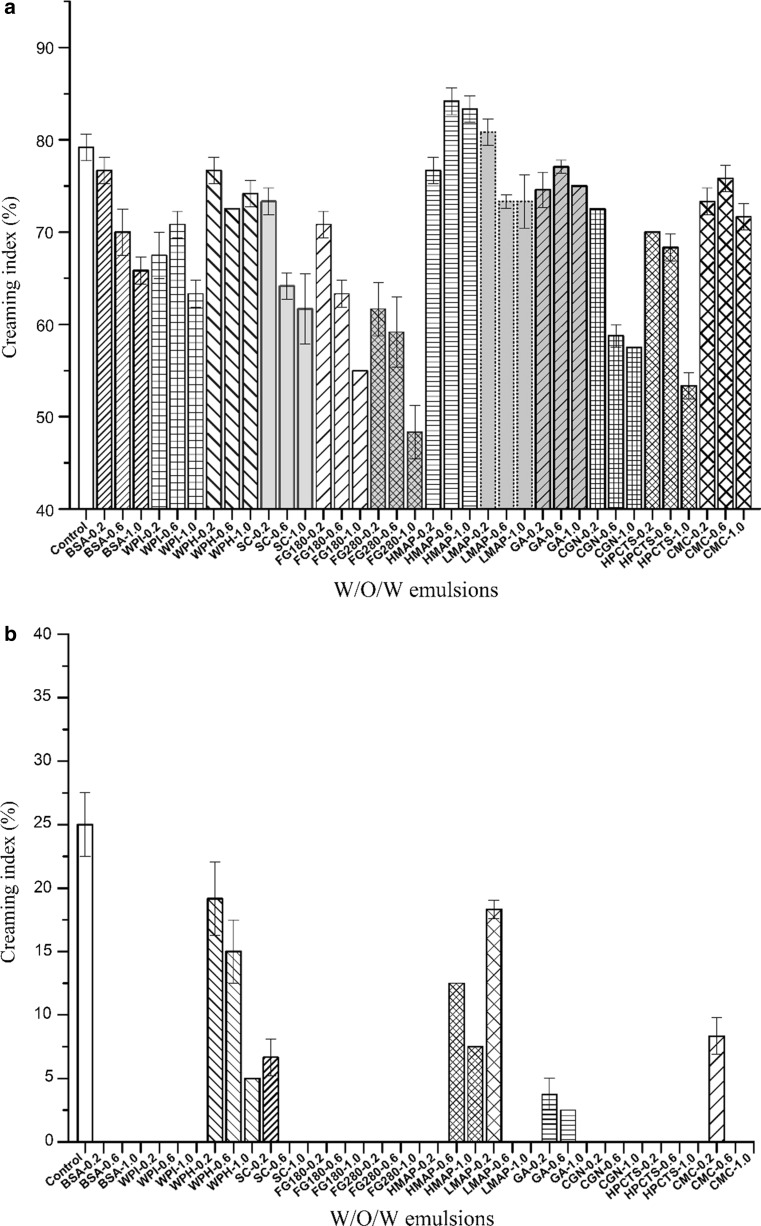
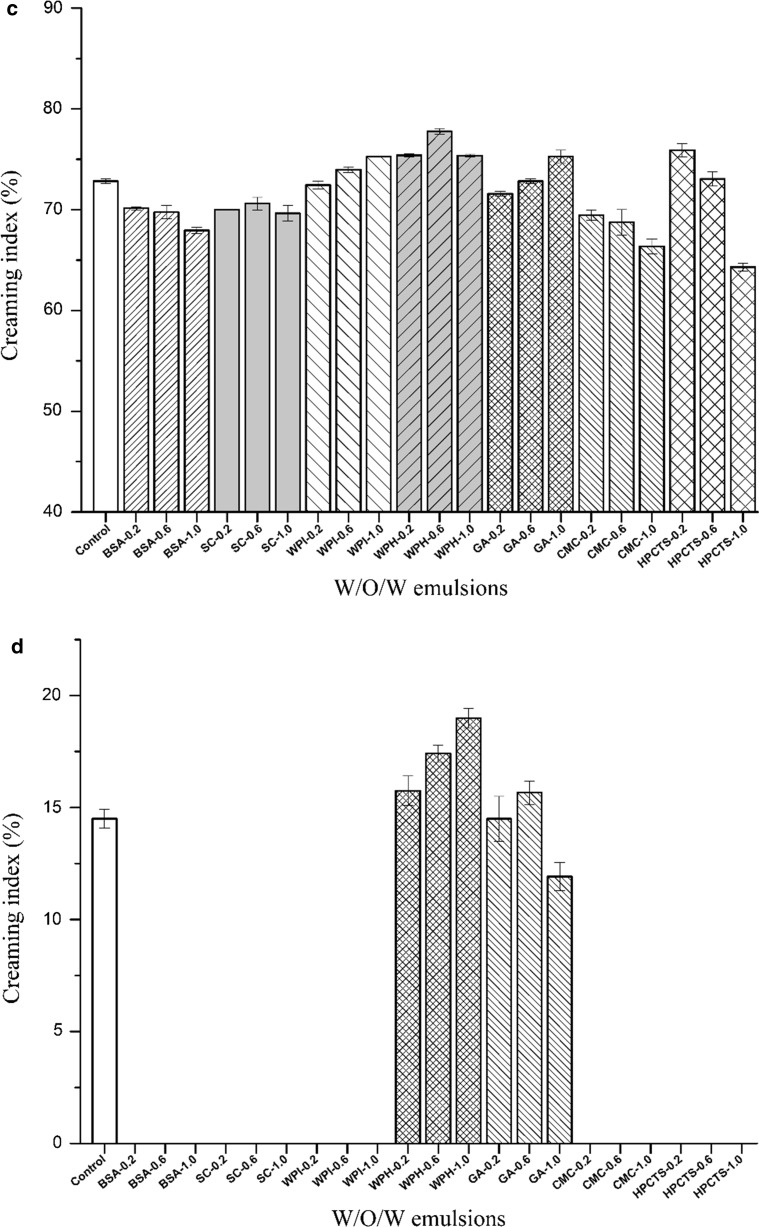
Physical stability of W/O/W emulsions
Physical instability such as creaming, sedimentation, flocculation, coalescence, or phase separation directly determines the shelf life of W/O/W emulsion, and thus is considered as key factor for further application. In the present study, the effects of biomacromolecules in the internal and external aqueous phases on dilution stability and thermal stability of W/O/W emulsions were also investigated. Figure 2a showed that the data could be categorized into two groups. One was a protein group that contained BSA, WPI, WPH, SC, FG280, and FG180, the other was a polysaccharose group that contained HMAP, LMAP, GA, CGN, HPCTS, and CMC. Adding proteins could slightly improve the dilution stability, while the dosage of proteins was a key factor. Our results also indicated that when the concentration of BSA, SC, and FG (both 280 and 180) increased from 0.2 to 1.0% an improvement in stability of W/O/W emulsions was observed. WPH, a protein hydrolysate of WPI, and is composed of amino acids and peptides (with relatively low molecular weights). Thus, adding WPH showed slight improvement to the dilution stability of W/O/W emulsions. Similarly, when the effect of two types of FG on the stability were compared, the FG with a higher bloom value produced a greater improvement in stability than FG with the lower bloom value, because higher bloom value corresponded to a higher molecular weight of fish gelatin. In the polysaccharose group, results showed that HMAP had a negative effect on the stability. LMAP, GA, and CMC showed very little positive effects. CGN and HPCTS showed the larger improvements in stability, especially at higher concentrations.
Fig. 2.
The influence of the biomacromolecules type and concentration in the internal or external aqueous phase on the physical stability of W/O/W emulsions. a Dilution stability for internal aqueous phase; b thermal stability for internal aqueous phase; c dilution stability for external aqueous phase; d thermal stability for external aqueous phase
Figure 2b showed the effects of biomacromolecules in the internal aqueous phase on the thermal stability of W/O/W emulsions. In the protein group, adding BSA, WPI, SC (at high concentration), FG280, and FG180 improved the thermal stability significantly, while no creaming effect was found. WPH played slightly role in improving thermal stability, which was also affected by the concentration. In the polysaccharose group, all of the additives could improve thermal stability. In particular, LMAP (0.6 or 1.0%), GA (1.0%), CGN, HPCTS, and CMC (0.6 or 1.0%) did not cause any creaming.
As shown in Fig. 2c and Fig. 2d, the biomacromolecules added to the external aqueous phase affected the physical stability of W/O/W emulsions. Slightly different from the trend shown in Fig. 2a or b, it was difficult to divide the biomacromolecules into protein and polysaccharose group when present in the external aqueous phase. The addition of the various biomacromolecules in the external aqueous phase played less positive role on the dilution stability (Fig. 2c), sometimes even resulted in an increase of creaming index, indicating the decrease of dilution stability. While investigated the effect of biomacromolecules in the external aqueous phase on the thermal stability, results showed that BSA, SC, WPI, CMC, and HPCTS resulted in better thermal stability of W/O/W emulsions, without creaming was observed. GA addition to the external aqueous phase had virtually no effect on the thermal stability of W/O/W emulsions. However, WPH had a negative effect on thermal stability, which increased when the usage was increased.
The above results indicated that some types of biomacromolecules did possess the ability to improve the physical stability of W/O/W emulsions. Our findings were consistent with the previous studies (Garti 1997a; Garti and Aserin 1996; Garti and Bisperink 1998). They have suggested that a polymeric surfactant (such as a biomacromolecule) in the internal aqueous phase probably forms a thick, strongly, and gelled film, which was a “complex” with the monomeric lipophilic surfactant, to impart internal interface elasticity and resistance to rupturing of the inner droplets (Benichou et al. 2004; Garti 1997a; Garti and Aserin 1996; Garti and Bisperink 1998). Thereby, the complex can improve the mechanical and steric stability of double emulsions. However, in the external aqueous phase, biomacromolecules served only as a protective colloid at the outer interface.
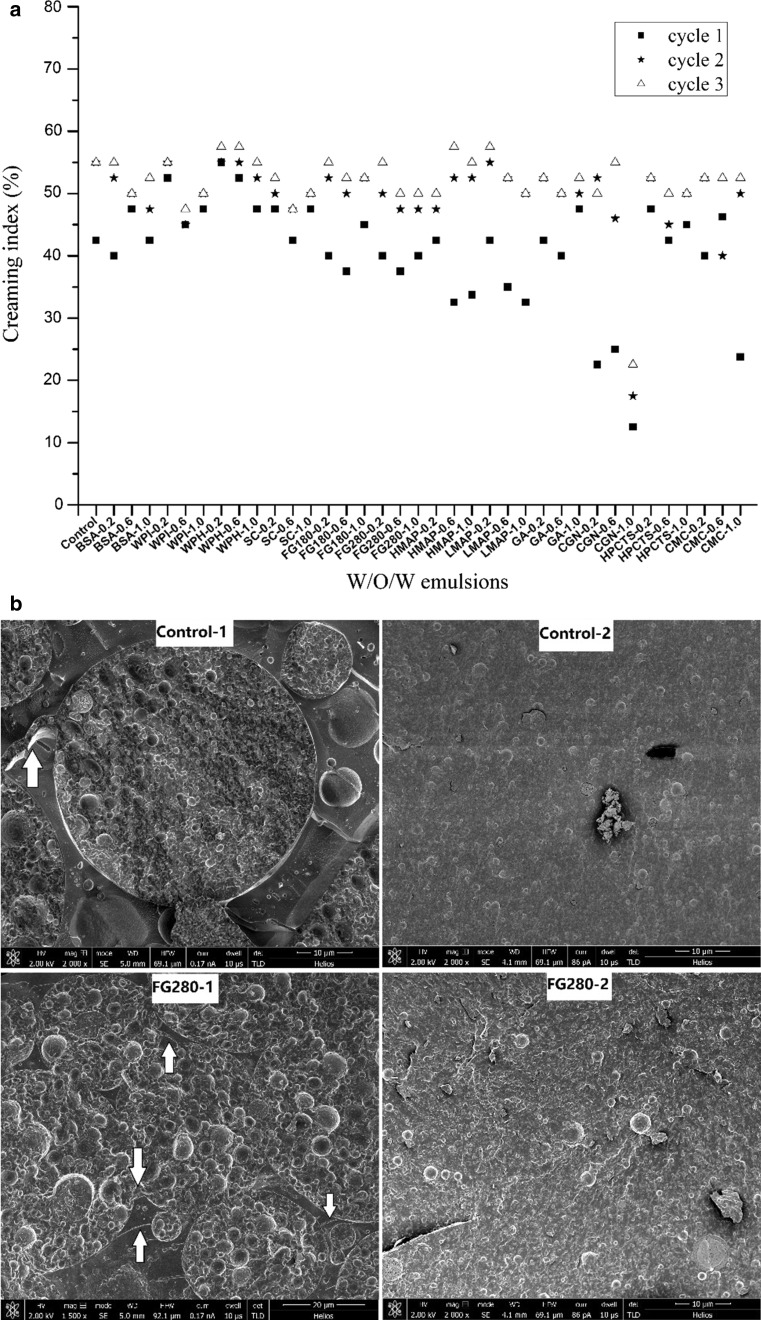
Freeze–thaw stability is an indicator of storage stability of W/O/W emulsions. Figure 3a showed that when biomacromolecules were added to the internal aqueous phase, two trends could be observed: i) the stability decreased as freeze–thaw cycles proceeded, and ii) no matter how stable the original emulsions were or how stable the single freeze–thaw cycle processed emulsions were, the final emulsions with three freeze–thaw cycles displayed similar level of creaming index. The explanation for the above results in Fig. 3a is reflected Fig. 3b. When compared the images before and after freeze–thaw treatments, we found that the external interface disappeared, and the amount of internal W1/O droplets decreased. These were mainly the consequence of interface disruption and emulsion coalescence. The arrows in the images clearly indicated channels between the two external interfaces of two neighboring emulsion droplets.
Fig. 3.
The influence of freeze–thaw treatment on the stability of W/O/W emulsions when biomacromolecules present in the internal aqueous phase. a Creaming index, b Cryo-SEM images before (−1) and after (−2) freeze–thaw treatment
Rheological properties of W/O/W emulsions
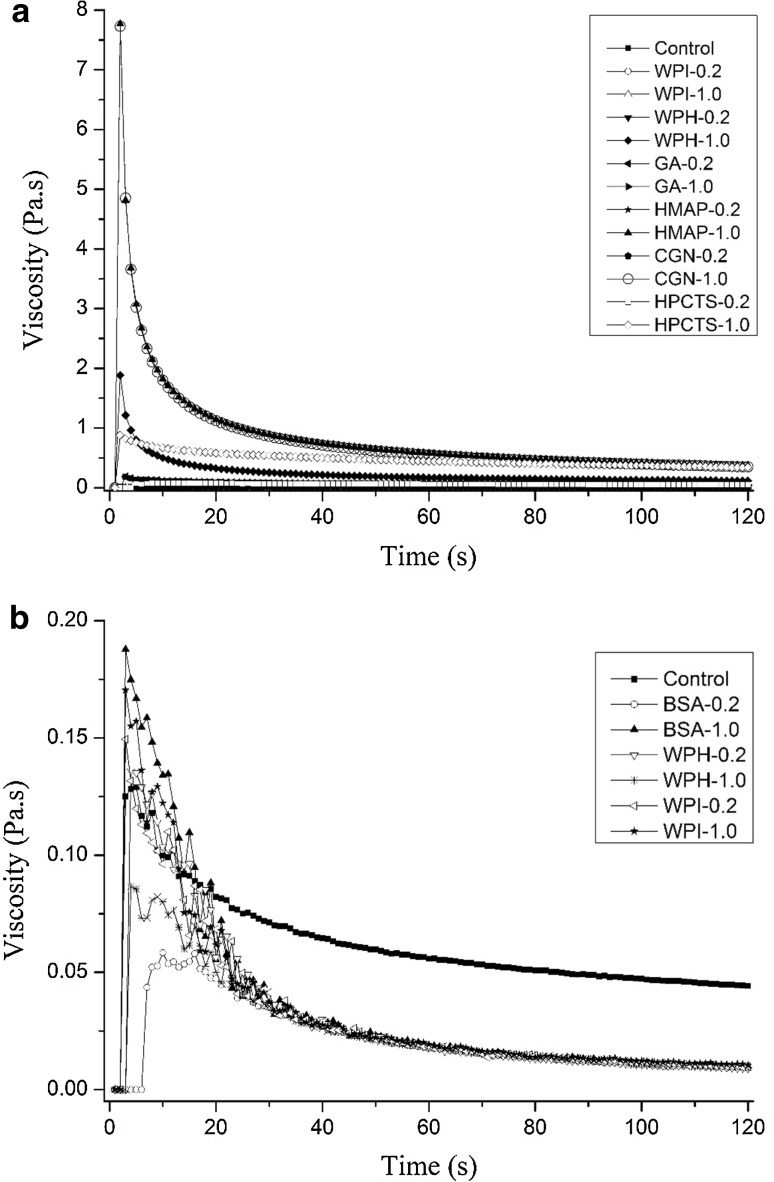
To investigate the effect of biomacromolecules on rheological properties of W/O/W emulsions, seven types of biomacromolecule were added to the internal aqueous phase, and three types of biomacromolecule were added to the external aqueous phase. Figure 4a indicated that different biomacromolecule showed different effects on the rheological properties. Generally, the order of the effect on the apparent viscosity of W/O/W emulsions was: CGN (1.0%) = HMAP (1.0%) ≫ WPH (1.0% > HPCTS (1.0%) ≫ Control. The effects of remaining biomacromolecules were almost the same as the control. However, Fig. 4b indicated that adding biomacromolecule to the external aqueous phase had only a little effects on the apparent viscosity of W/O/W emulsions.
Fig. 4.
The influence of the biomacromolecules type and concentration in the internal aqueous phase (a) or external aqueous phase (b) on the rheological properties of W/O/W emulsions
Biomacromolecule behaviors
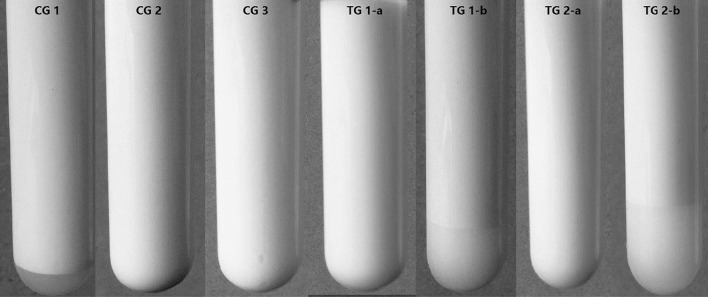
A protein-knockout experiment to identify the impact of biomacromolecules in the internal aqueous phase. As shown in Fig. 5, if the pH value of the internal aqueous phase was normal (no adjustment), no creaming layer was found when WPI or BSA were added, indicating a large stability improvement compared with the control (CG1), consisted with the results shown in Fig. 2. However, when the pH value was adjusted to the protein’s pI values (5.0 for WPI or 4.7 for BSA), the result was reversed, with no improvement in stability and the appearance of an obvious creaming layer. By comparing the control group of normal internal aqueous phase pH with the control group that the internal aqueous phase pH at the pI values, both in the presence and absence of proteins, it was clear that the reversal in stability increase was not only caused by pH adjustment, but also by the removal of proteins through protein aggregation at their pI. These results in the protein-knockout experiment indicated that the physical stability improvements obtained were caused by biomacromolecule addition, mainly by protein.
Fig. 5.
The observation of emulsion stability during protein-knockout experiment. CG 1 control group with normal pH value and without protein added in the internal aqueous phase; CG 2 control group with pH value at 5.0 and without protein added in the internal aqueous phase; CG 3 control group with pH value at 4.7 and without protein added in the internal aqueous phase; TG 1-a treatment group with normal pH value and WPI added in the internal aqueous phase; TG 1-b treatment group with pH value at 5.0 and WPI added in the internal aqueous phase; TG 2-a treatment group with normal pH value and BSA added in the internal aqueous phase; TG 2-b treatment group with pH value at 4.7 and BSA added in the internal aqueous phase
Conclusion
Our studies indicate that, 1.0% WPI or CGN decreases the emulsions droplet size to the greatest extent in comparison to all the macromolecules added in the internal aqueous phase. The addition of BSA, WPI, SC, FG280, or FG180 slightly improved the dilution stability and thermal stability of W/O/W emulsions. The dosage and molecular weight of the biomacromolecules were the key factors for emulsion stabilization. For the polysaccharide group, the results showed that HMAP had a negative effect on dilution stability, while LMAP, GA, and CMC had a positive effect, and CGN and HPCTS had a relatively larger effect, especially at higher concentrations. We also found that adding polysacchar could improve thermal stability, especially when LMAP (0.6 or 1.0%), GA (1.0%), CGN, HPCTS, or CMC (0.6 or 1.0%) were added. For the external aqueous phase, GA, HPCTS, and CMC significantly reduced the droplet size of W/O/W emulsions. The effects of GA, HPCTS, and CMC on droplet size distribution were especially pronounced when they were added at high mass fractions. Furthermore, adding various biomacromolecules to the external aqueous phase had no effect on dilution stability. However, BSA, SC, WPI, CMC, and HPCTS had a positive effect on thermal stability. The results also indicated that the effect of biomacromolecules added to the internal aqueous phase, on rheological properties were in the following order: CGN (1.0%) = HMAP (1.0%) ≫ WPH (1.0%) > HPCTS (1.0%) ≫ Control. The effect of the remaining biomacromolecules was similar to the control. In addition, biomacromolecules added to the external aqueous phase did not affect the rheological properties of W/O/W emulsions too much.
A biomacromolecule-knockout experiment confirmed that the improvement in the physical stability of emulsions seen were related to biomacromolecule, especially protein. In conclusion, all biomacromolecules did not have a positive impact on the physical stability of W/O/W emulsions suggesting their careful selection.
Acknowledgements
This research was conducted with the financial support of the National Natural Science Foundation (Project Nos. 31501487, 31371723). The authors also acknowledge the support from Talents Project of Organization Department of Beijing Municipal CPC Committee (2014000020124G030).
References
- Akhtar N, Yazan Y. Formulation and in vivo evaluation of a cosmetic multiple emulsion containing vitamin C and wheat protein. Pak J Pharm Sci. 2008;21(1):45–50. [PubMed] [Google Scholar]
- Al Haushey L, Bolzinger MA, Bordes C, Gauvrit JY, Briançon S. Improvement of a bovine serum albumin microencapsulation process by screening design. Int J Pharm. 2007;344(1–2):16–25. doi: 10.1016/j.ijpharm.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Benichou A, Aserin A, Garti N. Double emulsions stabilized by new molecular recognition hybrids of natural polymers. Polym Adv Technol. 2002;13(10–12):1019–1031. doi: 10.1002/pat.270. [DOI] [Google Scholar]
- Benichou A, Aserin A, Garti N. Double emulsions stabilized with hybrids of natural polymers for entrapment and slow release of active matters. Adv Colloid Interface Sci. 2004;108–109:29–41. doi: 10.1016/j.cis.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Benichou A, Aserin A, Garti N. W/O/W double emulsions stabilized with WPI–polysaccharide complexes. Colloids Surf A. 2007;294(1–3):20–32. doi: 10.1016/j.colsurfa.2006.07.056. [DOI] [Google Scholar]
- Bonnet M, Cansell M, Berkaoui A, Ropers MH, Anton M, Leal-Calderon F. Release rate profiles of magnesium from multiple W/O/W emulsions. Food Hydrocolloids. 2009;23(1):92–101. doi: 10.1016/j.foodhyd.2007.11.016. [DOI] [Google Scholar]
- Bonnet M, Cansell M, Placin F, David-Briand E, Anton M, Leal-Calderon F. Influence of ionic complexation on release rate profiles from multiple water-in-oil-in-water (W/O/W) emulsions. J Agric Food Chem. 2010;58(13):7762–7769. doi: 10.1021/jf100917w. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Cansell M, Placin F, Monteil J, Anton M, Leal-Calderon F. Influence of the oil globule fraction on the release rate profiles from multiple W/O/W emulsions. Colloids Surf B. 2010;78(1):44–52. doi: 10.1016/j.colsurfb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Chávez-Páez M, Quezada CM, Ibarra-Bracamontes L, González-Ochoa HO, Arauz-Lara JL. Coalescence in double emulsions. Langmuir. 2012;28(14):5934–5939. doi: 10.1021/la205144g. [DOI] [PubMed] [Google Scholar]
- Delample M, Da Silva F, Leal-Calderon F. Osmotically driven gelation in double emulsions. Food Hydrocolloids. 2014;38:11–19. doi: 10.1016/j.foodhyd.2013.11.009. [DOI] [Google Scholar]
- Dickinson E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011;6(1):1–11. doi: 10.1007/s11483-010-9188-6. [DOI] [Google Scholar]
- Ferreira LAM, Seiller M, Grossiord JL, Marty JP, Wepierre J. Vehicle influence on in vitro release of glucose: w/o, w/o/w and o/w systems compared. J Controlled Release. 1995;33(3):349–356. doi: 10.1016/0168-3659(94)00099-G. [DOI] [Google Scholar]
- Florence AT, Whitehill D. Some features of breakdown in water-in-oil-in-water multiple emulsions. J Colloid Interface Sci. 1981;79(1):243–256. doi: 10.1016/0021-9797(81)90066-7. [DOI] [Google Scholar]
- Frasch-Melnik S, Spyropoulos F, Norton IT. W1/O/W2 double emulsions stabilised by fat crystals–Formulation, stability and salt release. J Colloid Interface Sci. 2010;350(1):178–185. doi: 10.1016/j.jcis.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Gaiti N, Aserin A, Cohen Y. Mechanistic considerations on the release of electrolytes from multiple emulsions stabilized by BSA and nonionic surfactants. J Controlled Release. 1994;29(1–2):41–51. doi: 10.1016/0168-3659(94)90120-1. [DOI] [Google Scholar]
- Garti N. Double emulsions—scope, limitations and new achievements. Colloids Surf A. 1997;123–124:233–246. doi: 10.1016/S0927-7757(96)03809-5. [DOI] [Google Scholar]
- Garti N. Progress in stabilization and transport phenomena of double emulsions in food applications. LWT Food Sci Technol. 1997;30(3):222–235. doi: 10.1006/fstl.1996.0176. [DOI] [Google Scholar]
- Garti N, Aserin A. Double emulsions stabilized by macromolecular surfactants. Adv Colloid Interface Sci. 1996;65:37–69. doi: 10.1016/0001-8686(95)00289-8. [DOI] [Google Scholar]
- Garti N, Bisperink C. Double emulsions: progress and applications. Curr Opin Colloid Interface Sci. 1998;3(6):657–667. doi: 10.1016/S1359-0294(98)80096-4. [DOI] [Google Scholar]
- Giroux HJ, Constantineau S, Fustier P, Champagne CP, St-Gelais D, Lacroix M, Britten M. Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. Int Dairy J. 2013;29(2):107–114. doi: 10.1016/j.idairyj.2012.10.009. [DOI] [Google Scholar]
- Guery J, Baudry J, Weitz DA, Chaikin PM, Bibette J. Diffusion through colloidal shells under stress. Phys Rev E Stat Nonlinear Soft Matter Phys. 2009;79(6 Pt 1):60402. doi: 10.1103/PhysRevE.79.060402. [DOI] [PubMed] [Google Scholar]
- Hemar Y, Li Jiang C, Oliver CM, Luz S, Augustin M. Encapsulation of resveratrol using water-in-oil-in-water double emulsions. Food Biophys. 2010;5:120–127. doi: 10.1007/s11483-010-9152-5. [DOI] [Google Scholar]
- Hernández-Marín NY, Lobato-Calleros C, Vernon-Carter EJ. Stability and rheology of water-in-oil-in-water multiple emulsions made with protein-polysaccharide soluble complexes. J Food Eng. 2013;119(2):181–187. doi: 10.1016/j.jfoodeng.2013.05.039. [DOI] [Google Scholar]
- Herzi S, Essafi W, Bellagha S, Leal-Calderon F. Influence of the inner droplet fraction on the release rate profiles from multiple W/O/W emulsions. Colloids Surf A. 2014;441:489–495. doi: 10.1016/j.colsurfa.2013.09.036. [DOI] [Google Scholar]
- Jager-Lezer N, Terrisse I, Bruneau F, Tokgoz S, Ferreira L, Clausse D, Seiller M, Grossiord J. Influence of lipophilic surfactant on the release kinetics of water-soluble molecules entrapped in a W/O/W multiple emulsion. J Controlled Release. 1997;45(1):1–13. doi: 10.1016/S0168-3659(96)01507-6. [DOI] [Google Scholar]
- Kanouni M, Rosano HL, Naouli N. Preparation of a stable double emulsion (W1/O/W2): role of the interfacial films on the stability of the system. Adv Colloid Interface Sci. 2002;99(3):229–254. doi: 10.1016/S0001-8686(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Khopade AJ, Jain NK. Multiple emulsions containing rifampicin. Pharmazie. 1999;54(12):915–919. [PubMed] [Google Scholar]
- Li JL, Cheng YQ, Wang P, Zhao WT, Yin LJ, Saito M. A novel improvement in whey protein isolate emulsion stability: generation of an enzymatically cross-linked beet pectin layer using horseradish peroxidase. Food Hydrocolloids. 2012;26(2):448–455. doi: 10.1016/j.foodhyd.2010.11.015. [DOI] [Google Scholar]
- Li JL, Qiao ZH, Tatsumi E, Saito M, Cheng YQ, Yin LJ. A novel approach to improving the quality of bittern-solidified tofu by W/O controlled-release coagulant. 2: using the improved coagulant in tofu processing and product evaluation. Food Bioprocess Technol. 2013;6(7):1801–1808. doi: 10.1007/s11947-012-0849-y. [DOI] [Google Scholar]
- Li JL, Cheng YQ, Tatsumi E, Saito M, Yin LJ. The use of W/O/W controlled-release coagulants to improve the quality of bittern-solidified tofu. Food Hydrocolloids. 2014;35:627–635. doi: 10.1016/j.foodhyd.2013.08.002. [DOI] [Google Scholar]
- Lindenstruth K, Muller BW. W/O/W multiple emulsions with diclofenac sodium. Eur J Pharm Biopharm. 2004;58(3):621–627. doi: 10.1016/j.ejpb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Matos M, Gutiérrez G, Coca J, Pazos C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf A. 2014;442:69–79. doi: 10.1016/j.colsurfa.2013.05.065. [DOI] [Google Scholar]
- Matos M, Gutiérrez G, Iglesias O, Coca J, Pazos C. Enhancing encapsulation efficiency of food-grade double emulsions containing resveratrol or vitamin B12 by membrane emulsification. J Food Eng. 2015;166:212–220. doi: 10.1016/j.jfoodeng.2015.06.002. [DOI] [Google Scholar]
- Meng FT, Ma GH, Liu YD, Qiu W, Su ZG. Microencapsulation of bovine hemoglobin with high bio-activity and high entrapment efficiency using a W/O/W double emulsion technique. Colloids Surf B. 2004;33(3–4):177–183. doi: 10.1016/j.colsurfb.2003.10.003. [DOI] [Google Scholar]
- Mezzenga R, Folmer BM, Hughes E. Design of double emulsions by osmotic pressure tailoring. Langmuir. 2004;20(9):3574–3582. doi: 10.1021/la036396k. [DOI] [PubMed] [Google Scholar]
- Michaut F, Hébraud P, Perrin P. Amphiphilic polyelectrolyte for stabilization of multiple emulsions. Polym Int. 2003;52(4):594–601. doi: 10.1002/pi.995. [DOI] [Google Scholar]
- Miladi K, Sfar S, Fessi H, Elaissari A. Encapsulation of alendronate sodium by nanoprecipitation and double emulsion: from preparation to in vitro studies. Ind Crops Prod. 2015;72:24–33. doi: 10.1016/j.indcrop.2015.01.079. [DOI] [Google Scholar]
- Muschiolik G. Multiple emulsions for food use. Curr Opin Colloid Interface Sci. 2007;12(4–5):213–220. doi: 10.1016/j.cocis.2007.07.006. [DOI] [Google Scholar]
- Okochi H, Nakano M. Preparation and evaluation of w/o/w type emulsions containing vancomycin. Adv Drug Deliv Rev. 2000;45(1):5–26. doi: 10.1016/S0169-409X(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Palencia M, Rivas BL. Metal-ion retention properties of water-soluble amphiphilic block copolymer in double emulsion systems (w/o/w) stabilized by non-ionic surfactants. J Colloid Interface Sci. 2011;363(2):682–689. doi: 10.1016/j.jcis.2011.05.089. [DOI] [PubMed] [Google Scholar]
- Pays K, Giermanska-Kahn J, Pouligny B, Bibette J, Leal-Calderon F. Coalescence in surfactant-stabilized double emulsions. Langmuir. 2001;17(25):7758–7769. doi: 10.1021/la010735x. [DOI] [Google Scholar]
- Pays K, Giermanska-Kahn J, Pouligny B, Bibette J, Leal-Calderon F. Double emulsions: how does release occur? J Controlled Release. 2002;79(1–3):193–205. doi: 10.1016/S0168-3659(01)00535-1. [DOI] [PubMed] [Google Scholar]
- Regan JO, Mulvihill DM. Water soluble inner aqueous phase markers as indicators of the encapsulation properties of water-in-oil-in-water emulsions stabilized with sodium caseinate. Food Hydrocolloids. 2009;23(8):2339–2345. doi: 10.1016/j.foodhyd.2009.06.009. [DOI] [Google Scholar]
- Rojas EC, Papadopoulos KD. Induction of instability in water-in-oil-in-water double emulsions by freeze-thaw cycling. Langmuir. 2007;23(13):6911–6917. doi: 10.1021/la063533f. [DOI] [PubMed] [Google Scholar]
- Schmidts T, Dobler D, Guldan AC, Paulus N, Runkel F. Multiple W/O/W emulsions—using the required HLB for emulsifier evaluation. Colloids Surf A. 2010;372(1–3):48–54. doi: 10.1016/j.colsurfa.2010.09.025. [DOI] [Google Scholar]
- Schmidts T, Dobler D, Schlupp P, Nissing C, Garn H, Runkel F. Development of multiple W/O/W emulsions as dermal carrier system for oligonucleotides: effect of additives on emulsion stability. Int J Pharm. 2010;398(1–2):107–113. doi: 10.1016/j.ijpharm.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Schuch A, Wrenger J, Schuchmann HP. Production of W/O/W double emulsions. Part II: influence of emulsification device on release of water by coalescence. Colloids Surf A. 2014;461:344–351. doi: 10.1016/j.colsurfa.2013.11.044. [DOI] [Google Scholar]
- Sela Y, Magdassi S, Garti N. Release of markers from the inner water phase of W/O/W emulsions stabilized by silicone based polymeric surfactants. J Controlled Release. 1995;33(1):1–12. doi: 10.1016/0168-3659(94)00029-T. [DOI] [Google Scholar]
- Sugiura S, Nakajima M, Yamamoto K, Iwamoto S, Oda T, Satake M, Seki M. Preparation characteristics of water-in-oil-in-water multiple emulsions using microchannel emulsification. J Colloid Interface Sci. 2004;270(1):221–228. doi: 10.1016/j.jcis.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Surh J, Vladisavljevi CG, Mun S, McClements DJ. Preparation and characterization of water/oil and water/oil/water emulsions containing biopolymer-gelled water droplets. J Agric Food Chem. 2007;55(1):175–184. doi: 10.1021/jf061637q. [DOI] [PubMed] [Google Scholar]
- Vandergraaf S, Schroen C, Boom R. Preparation of double emulsions by membrane emulsification-a review. J Membr Sci. 2005;251(1–2):7–15. doi: 10.1016/j.memsci.2004.12.013. [DOI] [Google Scholar]
- Weiss J, Scherze I, Muschiolik G. Polysaccharide gel with multiple emulsion. Food Hydrocolloids. 2005;19(3):605–615. doi: 10.1016/j.foohyd.2004.10.023. [DOI] [Google Scholar]
- Weissa J, Muschiolik G. Factors affecting the droplet size of water-in-oil emulsions (W/O) and the oil globule size in water-in-oil-in-water emulsions (W/O/W) J Dispersion Sci Technol. 2007;28(5):703–716. doi: 10.1080/01932690701341819. [DOI] [Google Scholar]
- Wen L, Papadopoulos KD. Effects of osmotic pressure on water transport in W1/O/W2 emulsions. J Colloid Interface Sci. 2001;235(2):398–404. doi: 10.1006/jcis.2000.7384. [DOI] [PubMed] [Google Scholar]
- Wroński S, Vladimirov V, Adach A. Modelling of mass transfer from multiple emulsions. Int J Heat Mass Transf. 2012;55(15–16):4241–4245. doi: 10.1016/j.ijheatmasstransfer.2012.03.065. [DOI] [Google Scholar]
- Zhang Y, Gou J, Sun F, Geng S, Hu X, Zhang K, Lin X, Xiao W, Tang X. Impact of electrolytes on double emulsion systems (W/O/W) stabilized by an amphiphilic block copolymer. Colloids Surf B. 2014;122:368–374. doi: 10.1016/j.colsurfb.2014.07.008. [DOI] [PubMed] [Google Scholar]