Abstract
The study optimized the hydrolysis conditions for the production of fish collagen peptides from skin of Malabar grouper (Epinephelus malabaricus) using response surface methodology. The hydrolysis was done with enzymes pepsin, papain and protease from bovine pancreas. Effects of process parameters viz: pH, temperature, enzyme substrate ratio and hydrolysis time of the three different enzymes on degree of hydrolysis were investigated. The optimum response of degree of hydrolysis was estimated to be 10, 20 and 28% respectively for pepsin, papain and protease. The functional properties of the product developed were analysed which showed changes in the properties from proteins to peptides. SDS-PAGE combined with MALDI TOF method was successfully applied to determine the molecular weight distribution of the hydrolysate. The electrophoretic pattern indicated that the molecular weights of peptides formed due to hydrolysis were nearly 2 kDa. MALDI TOF spectral analysis showed the developed hydrolysate contains peptides having molecular weight in the range below 2 kDa.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2490-2) contains supplementary material, which is available to authorized users.
Keywords: Fish collagen peptide, Optimization, Degree of hydrolysis, Response surface methodology
Introduction
Fish protein hydrolysates from various sources have been studied extensively and described by several researchers (Gómez et al. 2010; Kittiphattanabawon et al. 2012; Klompong et al. 2007; Kristinsson and Rasco 2000; Li et al. 2007; Bhaskar et al. 2008; Ovissipour et al. 2009). Collagen is often used as ingredients and additives of functional food and nutraceuticals. But collagen is hard to absorb through oral intake because of its high molecule weight. One way to solve this problem is to hydrolyze the collagen with appropriate proteases. Enzymatic hydrolysis process can produce small fragments of collagen peptides. Furthermore, some of its bioactivity increased (Huo and Zhao 2009) and its antigenicity decreased after hydrolysis (Suetsuna et al. 2000). Collagens containing yellow tail fish bone and swine skin wastes were used as raw materials for production of protein hydrolysates and peptides (Jayathilakan et al. 2012).
The bioactive properties of collagen-derived peptides, and also their resistance to proteolytic digestion in the stomach, make them potential ingredients of health promoting foods (Nagai and Suzuki 2000). Bioactive peptides usually contain 2–20 amino acid residues per molecule (Pihlanto-Leppala 2000); lower the molecular weight, the higher their chances of crossing the intestinal barrier and exerting a biological effect (Roberts et al. 1999). There are a number of methods by which peptides with biological activity can be produced from precursor proteins. The most common ones are (a) enzymatic hydrolysis with digestive enzymes, (b) by means of the microbial activity of fermented foods, (c) through the action of enzymes derived from proteolytic microorganisms (Bhat et al. 2015). Depending on the specificity of the enzymes, nature of substrate, environmental conditions, and the extent of hydrolysis, wide variety of peptides will be generated. The resultant protein hydrolysate will possess particular properties according to the new peptides generated (Zhang et al. 2010). The functional properties of fish proteins may be improved by the use of specific enzymes and by choosing a defined set of hydrolysis conditions. Several factors like pH, time, enzyme activity and temperature influence enzyme function, offering possibilities to control the process (Gómez et al. 2010).
Interest in nutraceuticals is growing rapidly worldwide as they are a safe alternative to pharmaceutical drugs, whose use sometimes limited by toxicity or intolerance reactions. Collagen and collagen hydrolysates could be attractive nutraceuticals for their interesting bioactive properties.
Present study aims to the optimization of the process parameters for the development of fish collagen peptides (FCP) from the skin of fish Malabar grouper using enzymatic hydrolysis method. Hydrolysis was done with three different enzymes viz pepsin, papain and protease from bovine pancreas (PP). Response surface methodology (RSM) was used in the optimisation process where combinations of four main factors [pH, temperature, the ratio of enzyme to substrate (E/S) and time] at three levels for each enzyme were optimized. The degree of hydrolysis (DH) was set as response to evaluate the efficiency of hydrolysis. Functional properties like solubility, viscosity and amino acid compositions of the developed product were analysed. SDS-PAGE combined with MALDI TOF method was successfully applied to determine the molecular weight distribution of the hydrolysate.
Materials and methods
Raw materials
The species used for the study was Malabar grouper (Epinephelus malabaricus). The skin in iced condition was procured from Fort Cochin (9.9680°N, 76.2449°E), Kerala, India. Enzymes used for the hydrolysis process were pepsin from porcine gastric mucosa, ≥250 units/mg solid (EC Number 3.4.23.1), Papain from papaya latex lyophilized powder, ≥10 units/mg protein (EC Number 3.4.22.2), and protease from bovine pancreas Type I, ≥5 units/mg solid (MDL number MFCD00132092) and are procured from Sigma Chemicals (St. Louis, MO, USA).
Optimization of hydrolysis by RSM
Experimental design
Single factor experiments were done for establishing the range of independent variables (especially E/S ratio and time of hydrolysis) in RSM. Box-Behnken response surface design was formulated to evaluate and optimize the effects of four controlled independent variables viz: pH (X1), temperature (X2), time (X3), E/S ratio (X4) and their interaction on the measured response, DH (Y) for three different enzymes viz:: pepsin, papain and protease. The input variables were coded at three levels (−1, 0, +1,) and the complete design consisted of 27 experimental points including 3 replications at the centre for each enzyme. The original and coded levels of the independent variables used in the experimental design for each enzyme is listed in Table 1.
Table 1.
Experimental design with original and coded levels of independent variables
| Independent variables | Coded variables | Pepsin | Papain | Protease | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | −1 | 0 | 1 | −1 | 0 | 1 | ||
| pH | X1 | 1.6 | 2 | 2.4 | 5 | 6 | 7 | 6 | 7 | 8 |
| Temperature (°C) | X2 | 30 | 40 | 50 | 20 | 30 | 40 | 30 | 40 | 50 |
| Time (h) | X3 | 2 | 4 | 6 | 2 | 4 | 6 | 2 | 4 | 6 |
| E/S (mg%) | X4 | 0.5 | 4.25 | 8 | 0.5 | 4.25 | 8 | 0.05 | 0.425 | 0.8 |
Statistical analysis
The second-order polynomial regression model fitted to the DH (Y) as a function of independent variables and the adequacy of the fitted model was assessed by using R2 for each enzyme viz: pepsin, papain and protease. The functional form of the fitted model for each enzyme is given in the equation
where, “Y” is response variable, “β o” is intercept, “β i” is linear regression coefficients, “β ii” is quadratic regression coefficients, “β ij” is interaction regression coefficients and “e” is error term. Ridge analysis was carried out to predict the response variable at different radius from the centre of the design region. The optimization of response variables was done based on the ridge score and response surface plot of the response variables. The fitting of second-order polynomial regression model and numerical optimization was done by writing a SAS code in SAS 9.3.
The validation of the optimized condition was carried out for each enzyme with three replications once the optimization of pH (X1), temperature (X2), time (X3) and E/S ratio (X4) was done based on the fitted model for maximum DH.
Development of collagen hydrolysate
The selected fish skin was thoroughly washed, minced and mixed with 0.5 M sodium hydroxide solution and kept stirred for 24 h over a magnetic stirrer. The treated mass was strained through a coarse sieve. The process was repeated twice and the residue was washed twice with 30 volumes of chilled distilled water. The residue was homogenized with 30 volumes of 0.5 N acetic acid and the same was stirred over a magnetic stirrer for 24 h. The acid solution containing collagen was centrifuged. To the supernatant crystalline sodium chloride was added to the level of 5% and stirred for appropriate time to precipitate collagen. The precipitated collagen was separated and suspended in 0.5 molar Tris–glycine buffer (pH 7) and dialysed against the same buffer for 24 h to get pure fish skin collagen.
For hydrolysate preparation the purified fish skin collagen was homogenised in double distilled water and heated to 60 °C for 5 min, and then hydrolyzed enzymatically. Pepsin was dissolved in 10 mM HCl solution (pH 2), papain was dissolved in deionised water and protease from bovine pancreas (PP) dissolved in 10 mM sodium acetate buffer, pH 7.5. Three different proteolytic enzymes were treated consecutively under the conditions developed from experimental design. After hydrolysis, inactivation of enzymes was accomplished by boiling the mixture for 3 min. From the DH obtained from the experiments (shown in Tables 2, 3, and 4 of supplementary material), the hydrolysis conditions of pH, temperature, time and E/S ratio were optimised for the three enzymes by RSM. Validation studies under optimised conditions were conducted to confirm the optimisation process.
The final hydrolysate was filtered and centrifuged at 10,000×g for 30 min at 4 °C. The resulting solution was adjusted to pH 7 with saturated NaOH or HCl. Finally, the neutralised solution was filtered through Whatman No. 4 filter paper. The filtered solution was then spray dried at an inlet air temperature of 60 °C, outlet temperature of 180 °C and feed flow rate of 0.5 mL/min. The powdered product formed was stored at 4 °C.
Determination of degree of hydrolysis (DH)
Properly diluted samples (125 μL) were mixed with 2.0 mL of 0.2125 M phosphate buffer, pH 8.2, followed by the addition of 1.0 mL of 0.01% TNBS solution. The mixtures were then placed in a water bath at 50 °C for 30 min in the dark. During incubation the test tubes must be covered with aluminum foil because the blank reaction is accelerated by exposure to light. The reaction was terminated by adding 2.0 mL of 0.1 M sodium sulfite. The mixtures were cooled down at ambient temperature for 15 min and the absorbance was measured at 340 nm. A TNBS standard calibration curve was prepared using amino acid glycine (11.25 mg/mL) and was found to be linear (R2 = 0.996). The amount of free amino group liberated was expressed in terms of glycine.
The DH was evaluated as
where C0 is glycine equivalent of sample at time = 0, Ct is glycine equivalent of sample at time t.
Characterization
Solubility
To determine solubility, 250 mg of developed powder was dispersed in 5 mL of deionized water. The mixture was stirred at 25 °C for 2 h and centrifuged at 2000g for 10 min. Protein content of the supernatant was determined by Lowry’s method. Solubility was calculated as protein content in supernatant over total protein content in the initial dispersion. Protein solubility was calculated as follows
Change in viscosity
Viscosity of collagen solution (1%) and collagen hydrolysate solution (1%) was determined by rotary viscometer test method (Brookfield Digital Viscometer, Model DV-E). In this test method, the solution is placed in a glass tube, housed in an insulated block at a fixed temperature (37 °C). A metal spindle (spindle number, zero) is then rotated in the solution at 100 rpm, and the torque required to rotate the spindle is measured. Based on the internal resistance to rotation provided by the shear stress of the solution, the solution’s absolute viscosity was determined. Absolute viscosity is represented in centipoise (cP).
Amino acid composition of hydrolysate
The developed collagen peptide powder was treated with 6 N HCl at 120 °C for 24 h. The filtered sample was injected to the amino acid analyzer (HPLC-LC 10 AS). The amino acid composition was determined as per the method of Ishida et al. (1981) using Model Hitachi L-2130 Elite La Chrome (Tokyo, Japan) amino acid analyser connected with cation exchange column (Shodex, CX Pak, 4.6 × 15 mm).
SDS page
The developed collagen peptide powder was analyzed by Tricine-SDS-PAGE according to Schagger and von Jagow (1987) with slight modifications. The approximate molecular weights of the hydrolysate were determined using appropriate prestained protein molecular weight marker 2–71 kDa (SBS Genetech Co., Ltd, Beijing, China).
MALDI-TOF mass spectrometric analysis
Using MALDI-TOF mass spectrometry, the molecular weight distributions of peptides from collagen hydrolysate was estimated. The analysis was done on positive ion mode MALDI TOF mass spectrometer. The mass spectrometer used for detection of analytes, was AB SCIEX TOF/TOF 5800, AB SCIEX Co., USA with Nd: YAG 1000-Hz laser with 355 nm wavelengths, 2.0 m long TOF tube and a sample target plate with 96 wells. CentriVap −50 °C cold trap, LABCONCO Ltd., USA was used for concentrating sample under speed vacuum. The running conditions were: Operating mode—reflector positive ion, linear positive ion, Laser intensity—3600, 4400 (reflector positive ion mode), 6000 (linear positive ion mode) units, Laser type—Nd: YAG 1000-Hz laser with 355 nm wavelength, Mass range—300–10,000 m/z (reflector positive ion mode), 300–20,000 m/z (Linear positive ion mode), Delayed extraction time—400 ns, MALDI Plate velocity—900, 1100 µm/s, Laser shots: 4000 per spectrum.
Sample preparation A solution containing acetonitrile: 0.1% trifluoro acetic acid in deionised water in 1:1 v/v (diluent) was prepared for reconstituting peptide powder. Five aliquotes of samples were evaporated to dryness by using CentriVap −50 °C cold trap and reconstituted in diluent. These samples were further diluted to 10× and 100× concentration followed by aliquoting in 10 µL volume each. The reconstituted samples were spotted on MALDI target plate, sandwiched with matrices (CHCA, sinapinic acid) followed by drying. Each reconstituted sample was mixed with each of matrices in 1:1 v/v, separately and vortexed. These resulted samples were spotted on MALDI target plate and dried. All the spotted samples were analyzed by AB SCIEX TOF/TOF 5800 system. Standard calmix peptide mixture was used for validating method for data acquisition.
Results and discussion
In the study, a three step hydrolysis reaction was adopted, and experimental points were designed to optimize processing conditions to obtain smaller peptides, which might possess potent bio activity. The degree of hydrolysis, DH (i.e. percentage of peptide bond cleaved) is a true reflection of the progress of hydrolysis and thus it is selected as the primary indicator for the control of hydrolysis. Second-order polynomial regression was used to approximate and predict DH as a function of independent variable. The maximum DH was optimized using ridge analysis and evaluation of response surface plot.
Single factor experiments
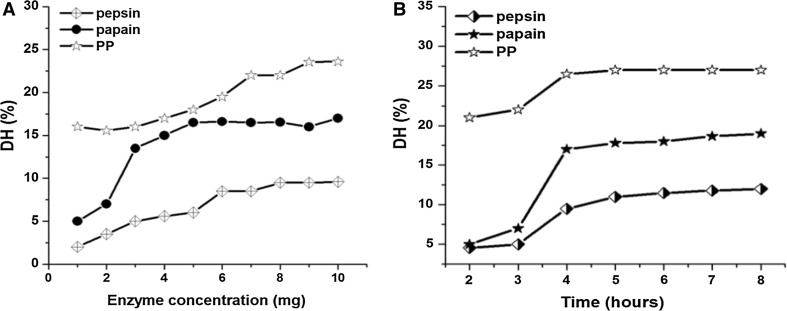
In single factor experiments, level of one factor varied and levels of three of four factors (pH, temperature, and time and enzyme substrate concentration) were fixed to evaluate its effect on hydrolysis. This was done to fix the range of time and enzyme substrate ratio in the hydrolysis process. At zero enzyme concentration, the degree of hydrolysis was nearly 1%.
The changes in DH are shown in Fig. 1a and b. An increase in the DH can be seen from the graphs, with the increase of enzyme concentration and time. By studying the trend of graphs, the range of enzyme concentration for setting experimental design was limited near to 6 mg for pepsin and papain and near to 2 mg for protease. The time of hydrolysis is ranged near to 6 h.
Fig. 1.
Effect of enzyme concentration (a) and reaction time (b) on the degree of hydrolysis
Optimization of enzymatic hydrolysis using RSM
Response surface analysis
The experiment was carried out based on the box-behnken design of experiment with 27 experimental runs.
The total variability in the DH for each enzyme was broken into variance due to linear, quadratic and interaction effect of independent parameters using analysis of variance (ANOVA). Second order polynomial regression model was fitted to the response variable as a function of input variables and the significance of linear (X1, X2, X3, X4), quadratic and interaction terms (X21, X22, X23, X24, X1X2, X1X3, X1X4, X2X3, X2X4, X3X4,) were assessed at 5% level of significance. The linear, quadratic and interaction effect of input factors was found to be significant at 5% level of significance for all three enzymes except for some two way interaction effect for papain and protease. The reliability of fitted model was assessed using coefficient of determination (R2) and root mean square error (RMSE). The R2 and RMSE value of fitted model was 0.86 and 1.55 for pepsin, 0.95 and 2.39 for papain and 0.95 and 3.63 for PP respectively. Based on the evaluation of R2 value which is close to 1 and minimum RMSE value, it is inferred second order response model fitted well to the experimental data (Chauhan and Gupta 2004).
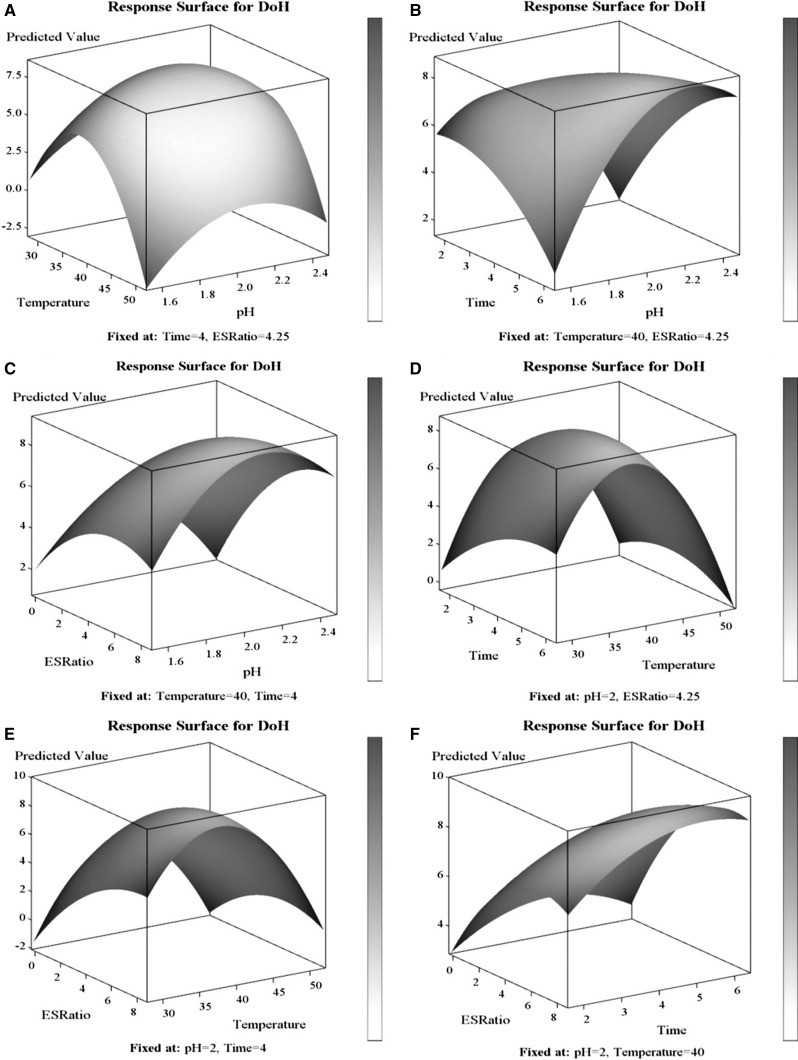
Three-dimensional response surface plots presenting the different combinations of input factors on the DH were evaluated (shown in Fig. 2 for pepsin). Each of the plots was drawn to predict the value of DH by varying the levels of two input factors at a time and by fixing the levels of other two factors constant. As shown in Figure, if the selected variable’s value was in the optimum range, the DH increased until combination of the time, temperature, and E/S ratio reached a maximum yield of the product. But if the conditions that selected for the hydrolysis were out of this range, even though it has higher value for each of the variables, the DH cannot reach the high value, and it will stay at the lower point of DH. This confirmed that extremes of pH and temperature are affecting the enzyme activity. The shape of the hydrolysis curve has been associated with enzyme inactivation, product inhibition by hydrolysis products formed at high degrees of hydrolysis, a low Km value for the soluble peptides that act as effective substrate competitors to the unhydrolyzed protein, and possibly auto digestion of the enzyme (Rebeca et al. 1991).
Fig. 2.
Pepsin: Effects of different variables on the degree of hydrolysis presented in response surface (3D) plots
These graphs met well with the results of ANOVA and described the statistical data visually. In each of the plots, there was a peak of the curved surface that revealed an optimal condition of the factors on the response, which mean that any level of the condition lower or higher than that would produce a negative influence on hydrolyzing process with the result of diminishing the DH.
Optimization and validation study
To maximize the DH, the conditions of four factors in this study were optimized based on the evaluation of response surface, ridge score desirability function score. According to the ridge analysis and desirability score, the optimized conditions for getting maximum DH are: 2.1 of pH; 36.62 °C of temperature; 3.6% of E/S ratio; and 5.47 h of time for pepsin; 6.38 of pH; 26.22 °C of temperature; 4.5% of E/S ratio; and 4.25 h of time for papain and 6.3 of pH; 39.86 °C of temperature; 1.8% of E/S ratio; and 4.25 h of time for protease. Under the optimized condition, the predicted response of DH was estimated to be 10, 20 and 28% respectively for pepsin, papain and protease.
Once an empirical model has been developed and optimized the process parameters using the developed model, it is very much important to validate the model by extrapolating the optimized conditions by conducting the actual experiment. Accordingly to confirm the validity of the developed model, three experiments were performed under the recommended conditions given above. The experimental values of DH obtained for pepsin, papain and protease were 11.1 ± 1.4 and 21 ± 1.03 and 28.6 ± 0.68% respectively. The experimental values agreed with the value predicted by the model within a 95% confidence interval. The above results confirmed that the model was powerful and suitable for the estimation for experimental values.
Development and characterisation of collagen hydrolysate
The process resulted in a fine powder of collagen hydrolysate having degree of hydrolysis and nitrogen recovery respectively of 56 ± 0.73 and 76.11 ± 1.03%.
Lyophilized hydrolysate was almost 100% soluble over a wide range of pH values (3–9). The viscosity of collagen solution was 96 cP and the viscosity of the developed peptide solution was 1.42 cP.
Hydrolysate is known to have excellent solubility at a high degree of hydrolysis (Gbogouri et al. 2004). Hydrolysis potentially influenced the molecular structure, hydrophobic nature and polar groups of the hydrolysate (Kristinsson and Rasco 2000). Higher DH means smaller peptides, which were expected to have proportionally more polar residues and the ability to form hydrogen bonds with water, thereby improving the solubility (Gbogouri et al. 2004).
Amino acid composition analysis
When the amino acid composition of the developed peptide was analyzed, it was rich in proline, glycine, alanine and hydroxyproline residues and small amounts of tyrosine, histidine, and methionine, residues. The results are in agreement with those reported by Zague et al. (2011) and Wang et al. (2008). Zague et al. (2011) reported that collagen hydrolysate had high contents of glycine (24.5%), glutamic acid (10.1%), arginine (8.1%), proline (13.8%), and hydroxyproline (7.4%) residues and small amounts of tyrosine, cysteine, histidine, and methionine residues.
SDS page
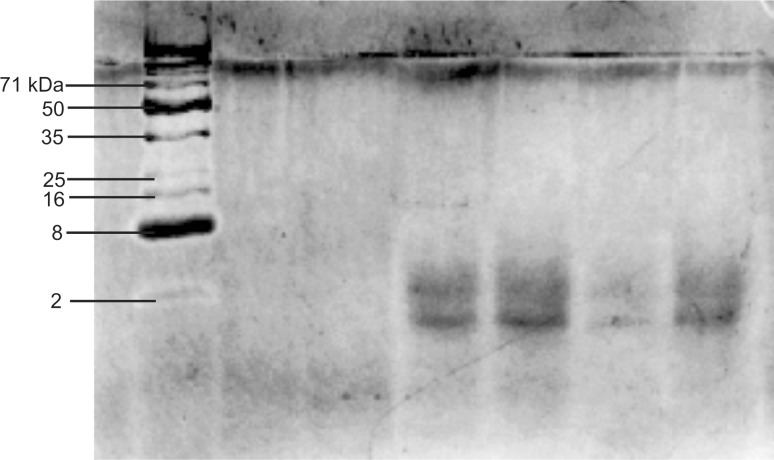
The electrophoretic pattern of the developed hydrolysate presented in Fig. 3 indicated that the molecular weights of peptides formed due to hydrolysis were nearly 2 kDa. This result indicated that the hydrolysis process had successfully cleaved the peptide bonds, resulting in lower molecular weight.
Fig. 3.
Tricine SDS PAGE pattern of collagen hydrolysate. First lane shows sigma’s low range molecular markers. Lane 2, 3, 4, 5, 6 and 7 are 0.5, 1, 1.5, 2, 2.5, and 3 mg/mL concentrations of collagen peptide respectively
MALDI TOF mass spectrum analysis
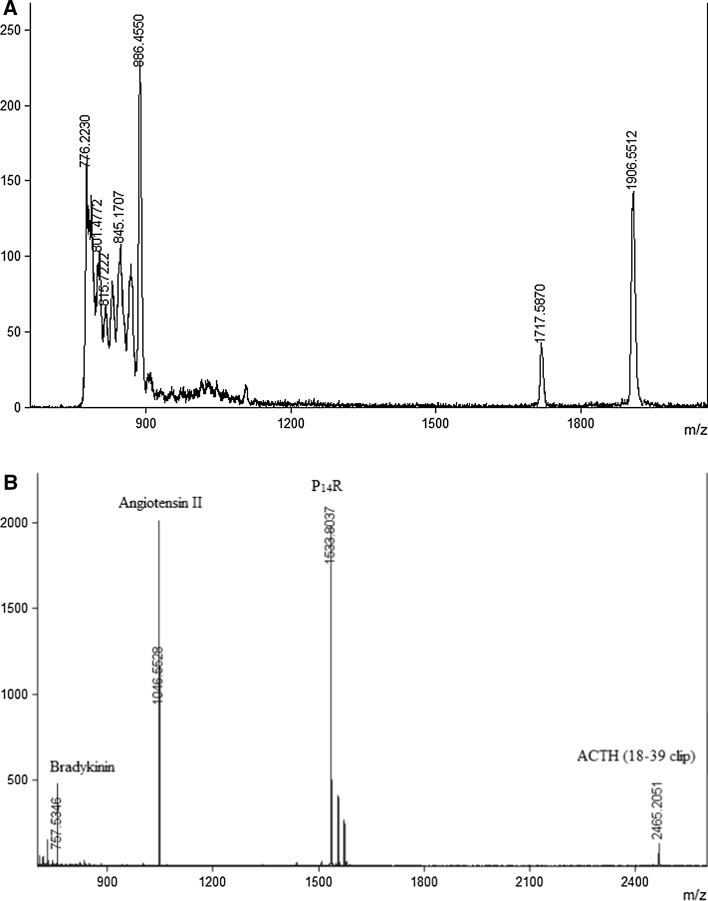
Molecular weight distribution of the hydrolysate was obtained from MALDI TOF mass spectrometer with resolving capabilities in the order of 400–1000 and accuracy ranging from ±0.2–0.005%. The mass spectra (shown in Fig. 4a) indicated that the molecular weight of the most active peptides in the range below 2 kDa. No signals were obtained above 2000 m/z. Standard Calmix peptide mixture was used for calibrating the instrument (spectra shown in Fig. 4b)
Fig. 4.
a Mass spectra obtained for collagen peptide. Spectra acquired till 20,000 m/z. However, no signals were obtained above 2000 m/z. b Mass spectra obtained for standard Calmix peptide mixture (Bradykinin, Angiotensin II, P14R, ACTH 18-39 clip). This mixture was used for calibration of instrument
Discussion and conclusion
Interest in nutraceuticals is growing rapidly worldwide, as they are a safe alternative to pharmaceutical drugs, whose use is sometimes limited by toxicity or intolerance reactions. Collagen and collagen hydrolysates could be attractive nutraceuticals for their interesting bioactive properties. The beneficial effect of collagen or gelatine hydrolysates on different diseases has been reported in animal or clinical studies. Moreover, hydrolysed collagen products have received GRAS status (generally recognized as safe) from the US Food and Drug Administration (FDA) Although mammalian gelatines are widely used in the field of nutraceuticals, the use of gelatines from marine-discarded sources for preparing protein hydrolysates is nowadays increasing, as they are not associated with the risk of outbreaks of bovine spongiform encephalopathy. Histological and immunological tests confirmed that marine collagen has no cytotoxic reaction in vitro and in vivo experiments (Subhan et al. 2015).
The resistance of some collagen-derived peptides to proteolytic digestion in the stomach is one of the most interesting properties of collagen hydrolysates. Several studies focused on the effect of oral intake in both animal and human models have revealed the excellent absorption of Hyp-containing peptides. Some of these collagen-derived peptides have revealed biological activity in vivo after absorption from the digestive tract.
The study optimized the hydrolysis conditions for the production of fish collagen peptides from skin of Malabar grouper (Epinephelus malabaricus) using response surface methodology. Using the optimised process conditions, we have developed fish skin collagen peptide extract having degree of hydrolysis and nitrogen recovery respectively of 56 ± 0.73 and 76.11 ± 1.03%. The hydrolysed peptide solution is purified and spray dried to form fish collagen powder.
Characterisation studies were conducted to analyse the functional properties and the molecular weight distribution of the developed collagen peptide powder. It is 100% soluble over a wide range of pH values and viscosity of the solution is greatly reduced as compared to the collagen solution. Several analysis were done for checking the quality control of the product; Filtration, the molecular weight distribution, the total nitrogen content, amino acid composition and the presence of toxic compounds (heavy metal contamination and pathogens). SDS-PAGE combined with MALDI TOF method was successfully applied to determine the molecular weight distribution of the hydrolysate and the results shows that the developed peptide extract is containing peptides in the range below 2 kDa.
From the results it is concluded that the enzymatic hydrolysis resulted in the development of fish collagen peptides. The developed product from the study is safe and can be recommended for further bio activity studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The financial assistance for the study from Department of Biotechnology is thankfully acknowledged. Acknowledgements are also due to Director, CIFT for providing facilities to carry out this study.
References
- Bhaskar N, Benila T, Radha C, Lalitha RG. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Biores Tech. 2008;99(10):4105. doi: 10.1016/j.biortech.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF. Bioactive peptides of animal origin: a review. J Food Sci Technol. 2015;52(9):5377–5392. doi: 10.1007/s13197-015-1731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan B, Gupta R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004;39:115–212. doi: 10.1016/j.procbio.2003.11.002. [DOI] [Google Scholar]
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J Food Sci. 2004;69(8):615–622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Gómez GMC, López Caballero ME, Alemán A, López de Lacey A, Giménez B, Garía Pilar M. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. Sea by-products as real material: new ways of application. Signpost: Transworld Research Network; 2010. pp. 89–115. [Google Scholar]
- Huo JX, Zhao Z. Study on enzymatic hydrolysis of Gadusmorrhua skin collagen and molecular weight distribution of hydrolysates. Agric Sci China. 2009;8(6):723–729. doi: 10.1016/S1671-2927(08)60271-0. [DOI] [Google Scholar]
- Ishida Y, Fugita T, Asai K. New detection and separation methods for amino acids by high performance liquid chromatography. J Chromatogr. 1981;204:143–148. doi: 10.1016/S0021-9673(00)81650-7. [DOI] [PubMed] [Google Scholar]
- Jayathilakan K, Sultana Khudsia, Radhakrishna K, Bawa AS. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol. 2012;49(3):278–293. doi: 10.1007/s13197-011-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F. Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: antioxidant activity and its potential in model systems. Food Chem. 2012;135(3):1118–1126. doi: 10.1016/j.foodchem.2012.05.080. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102(4):1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000;40(1):43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Li B, Chen F, Wang X, Ji B, Wu Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007;102(4):1135–1143. doi: 10.1016/j.foodchem.2006.07.002. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Isolation of collagen from fish waste material—skin, bone and fins. Food Chem. 2000;68:277–281. doi: 10.1016/S0308-8146(99)00188-0. [DOI] [Google Scholar]
- Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem. 2009;115:238–242. doi: 10.1016/j.foodchem.2008.12.013. [DOI] [Google Scholar]
- Pihlanto-Leppala A. Bioactive peptides derived from bovine wheyproteins: opioid and ace-inhibitory peptides. Trends Food Sci Tech. 2000;11:347–356. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Rebeca BD, Pena-Vera MT, Diaz-Castaneda M. Production of fish protein hydrolysates with bacterial proteases; yield and nutritional value. J Food Sci. 1991;56:309–314. doi: 10.1111/j.1365-2621.1991.tb05268.x. [DOI] [Google Scholar]
- Roberts PR, Burney JD, Black KW, Zaloga GP. Effect of chain length on absorption of biologically active peptides from the gastro intestinal tract. Digestion. 1999;60(4):332–337. doi: 10.1159/000007679. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Subhan F, Ikram M, Shehzad A, Ghafoor A. Marine collagen: an emerging player in biomedical applications. J Food Sci Technol. 2015;52(8):4703–4707. doi: 10.1007/s13197-014-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem. 2000;11(3):128–131. doi: 10.1016/S0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Wang L, An X, Yang F, Xin Z, Zhao L, Hu Q. Isolation and characterization of collagens from the skin, scale and bone of deep-sea red fish (Sebastes mentella) Food Chem. 2008;108(2):616–623. doi: 10.1016/j.foodchem.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Zague V, de Freitas V, da Costa Rosa M, Geórgia A, de Castro I, Jaeger RG, Machado-Santelli GM. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J Med Food. 2011;14(6):618–624. doi: 10.1089/jmf.2010.0085. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kouguchi T, Shimizu K, Sato M, Takahata Y, Morimatsu F. Chicken collagen hydrolysate reduces proinflammatory cytokine production in C57BL/6.KOR-ApoEshl Mice. J Nutr Sci Vitaminol. 2010;56:208–210. doi: 10.3177/jnsv.56.208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.