Abstract
Currently, there is a growing interest in the use of non-Saccharomyces yeast to enhance the aromatic quality of wine, with pure or mixed cultures, as well as sequential inoculation. Volatile components of wines were closely related to their sensory quality. Hence, to study the evolution of volatile compounds during fermentation was of great interest. For this, sampling methods that did not alter the volume of fermentation media were the most suitable. This work reports the usefulness of headspace sorptive extraction as non-invasive method to monitor the changes in volatile compounds during fermentation. This method allowed monitoring of 141 compounds throughout the process of fermentation by Saccharomyces cerevisiae and Lachancea thermotolerans strains. Both strains showed a similar ability to ferment a must with high sugar content. The S. cerevisiae strain produced higher amount of volatile compounds especially esters that constitutes fruity aroma than L. thermotorelans.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2499-6) contains supplementary material, which is available to authorized users.
Keywords: Volatile compounds, HSSE/GC–MS, Non-invasive, Real-time, Alcoholic fermentation, Lachancea thermotolerans
Introduction
Wine is a complex solution containing abundant volatile compounds which contribute to wine aroma (Boss et al. 2015). These aromatic components of wine are closely related to its sensory quality, which is determined by the consumer’s acceptability (Vilanova 2006). Compounds that constitute the volatile profile of wine have different origins. Primary aromas are grape-derived volatiles that pass through fermentation often unchanged, and are largely responsible for “varietal” aromas. Secondary aromas, which are by far the greatest pool of volatile molecules, are produced through the winemaking process, the great majority produced by yeast as metabolism by-products (Robinson et al. 2014). Tertiary aromas develop in finished wine through storage and maturation, and result from intermolecular chemical interactions and equilibrium effects as the wine matrix changes (Boss et al. 2015). Therefore, volatile profile of wine depends on primary the quality and variety of grape employed, fermentation process (yeast, temperature…) and maturation (in bottle or wood barrel), if it takes place.
One of the most important factors in the alcoholic fermentation process is the yeast strain involved. The choice of yeast strain is also a determinant of the final concentration of these volatile compounds (Callejón et al. 2010). For this reason, one of the new yeast selection criteria that have emerged is the appropriate enhancement of aroma via the production of volatile compounds such as esters and higher alcohols, along with the scant production of off-flavours (Suárez-Lepe and Morata 2012).
Some authors call “yeast bouquet” to the set of volatile compounds produced by yeast as secondary metabolites. Among them there are ethyl esters, acetate esters, fusel alcohols, carbonyls, and volatile fatty acids synthesized by a wide range of microbial species (Cordente et al. 2012).
It is well known that in the fermentation of grape must there is a sequential development of Saccharomyces and non-Saccharomyces species (Renault et al. 2015). The conditions of alcoholic fermentation favour the development of Saccharomyces cerevisiae, being these yeasts predominant during the latter stages of fermentation. Moreover, because non-Sacharomyces has been related to negative aromatic notes and off-flavour in wines (Benito et al. 2015), to ensure proper development of the alcoholic fermentation, winemakers commonly inoculate the grape must with Saccharomyces commercial strains.
Currently, conversely, different research have revealed that certain non-Saccharomyces yeasts can enhance the aroma and improve the wine quality (Benito et al. 2015; Gobbi et al. 2013; Jolly et al. 2014; Renault et al. 2015). This has led to a new perspective on the use of non-Saccharomyces strains in winemaking.
To perform an exhaustive study of volatile compounds, these have to be analysed by gas chromatography–mass spectrometry which requires a previous sample extraction process. Presently, the most extensively used extraction technique for volatile compounds in wines is the solid phase microextraction (SPME) (Boss et al. 2015; Renault et al. 2015). The other extraction technique that has showed successful results in volatile profile analysis of wine is stir bar sorptive extraction (SBSE). Although, it has been used in lesser extent, it has major extraction capacity (Lancas et al. 2009). In wine analysis, the device with the polymeric extraction phase has been used in immersion, SBSE (Martinez-Gil et al. 2013), as well as in headspace, named headspace sorptive extraction (HSSE) (Callejón et al. 2010) with satisfactory results.
The study of the changes of volatile compounds produced in alcoholic fermentation are performed primarily in two ways, analysing samples at the end of process (Romano et al. 2015; Synos et al. 2015) or sampling at different stages of fermentation (Concejero et al. 2016). The former is the most widely used. However, and to our understanding, the possibility of studying the evolution of volatile compounds during fermentation, using sampling methods that not alter the volume of fermentation media, is of great interest. In spite of this, non-invasive methods to monitoring the evolution of volatile profile during must fermentation or wine maturation have been seldom used. Among them, we can mention the recent monitoring study of fermentative aromas produced by evolved Saccharomyces cerevisiae strain (pilot scale) using an on-line gas chromatography (GC) special device in the headspace (HS) (Mouret et al. 2015). Callejón et al. (2012) monitored the effects of skin contact time on the partitioning, release, and formation of volatile compounds during fermentation of Cabernet Sauvignon grapes (laboratory scale), using a polydimethylsiloxane (PDMS) SPME fiber in HS. Silva Ferreira et al. (2014) carried out a study of the changes of volatile profile at microscale fermentation (5 mL) in fermentation media with different sources of assimilable nitrogen using again HS-SPME.
This work had two aims; one of them was to test the use of HSSE as non-invasive method to monitor online the volatile compounds changes during fermentation. The other one was to study the influence of two types of yeast, Saccharomyces cerevisiae and Lachancea thermotolerans, on volatile profile throughout fermentation of a must with high sugar content.
Materials and methods
Yeast strains and media
Two different autochthonous yeast strains belonging to the collection housed in the Area de Edafología y Química Agricola (Univesity of Seville) were used for the fermentation assays. One corresponding to a Saccharomyces cerevisiae strain (coded as G263), and the other one to a Lachancea thermotolerans strain (coded as G234). Both of them were isolated from previous laboratory-scale fermentations with sun-dried Pedro Ximénez grape must and were identified at species level by PCR–RFLP of the 5.8S ribosomal region as described by Guillamón et al. (1998). Identification was corroborated by sequencing the D1/D2 variable domains of 26S rRNA gene according to Clavijo et al. (2011). In addition, isolates of S. cerevisiae were characterized at strain level by mitochondrial DNA restriction analysis following Querol et al. (1992). Yeast strains G234 and G263 were selected in order to their ability to ferment high sugar content grape must which was previously tested in laboratory assays.
Grape must for fermentation assays was kindly provided by local winery (Montalbán, Córdoba, Spain). It was obtained from sun-dried grapes of the “Pedro Ximénez” variety during 2014 vintage. Physical and chemical must parameters were the following: pH 4.51 ± 0.02, total acidity (g/L tartaric acid) 4.3 ± 0.1, and reducing sugar content 487 ± 20 g/L.
Fermentation assays
Duplicate fermentations were carried out under static conditions at 22 °C in 500 mL Erlenmeyer flasks containing 350 mL of sun-dried Pedro Ximénez must, previously pasteurized by 20 min heating at 100 °C. Erlenmeyer flasks were inoculated at a density of approximately 5.5 × 106 cell/mL from 48 h pure yeast cultures that were grown in the same grape must. Fermentation progress was monitored through measuring of turbidity at optical density of 660 nm (OD660), using a spectrophotometer Beckman DU 640, and sugar consumption control. Data of cell per millilitre was determined using polynomial function previously calculated, which relates OD660 values to cell/mL. Residual fermentable sugars were determined according to Rebelein procedure involving reaction of reducing sugars with copper(II) in alkaline solution (MAPA 1993). For this purpose, aliquot samples were taken from each flask, after extraction of volatile compounds, throughout the fermentation process. End of the fermentation was established when no sugar consumption was detected.
Online extraction of volatile compounds
The online sampling procedure was performed in headspace by PDMS Twisters (HSSE). A special device made of stainless wire was designed to maintain the Twister in the headspace, in the centre of the Erlenmeyer flask at 2.5 cm above the liquid surface.
Twister was exposed to headspace of must during 2 h at 22 °C of temperature (fermentation temperature). The extraction time was established in previous assays. After extraction, the stir bar was removed with tweezers and introduced in a 2 mL vial to be transported to the analysis laboratory where they were thermally desorbed in a gas chromatograph/mass spectrometer (GC/MS). The stainless wire devices, the tweezers and the vials to transport Twister were autoclaved to avoid contamination of flasks. Moreover, the insertion of Twisters into the flasks and their removal were performed in a laminal flow chamber.
A total of six extractions were accomplished for each replicate of the fermentation assay as follows: before inoculation (MT0), every 24 h after inoculation (T24, T48 and T72) and at 144 and 192 h after inoculation (T144 and T192, respectively).
Thermal desorption and GC conditions
Gas chromatography analysis was carried out using a 6890 Agilent GC system coupled to a quadrupole mass spectrometer Agilent 5975 inert and was equipped with a thermo desorption system (TDS2) and a cryo-focusing CIS-4 PTV injector (Gerstel). The thermal desorption was performed in splitless mode with a flow rate of 70 mL/min. The desorption temperature program was the following: the temperature was held at 35 °C for 0.1 min, was ramped at 60 °C/min to 210 °C and held for 5 min. The temperature of the CIS-4 PTV injector, with a Tenax TA inlet liner, was held at –35 °C using liquid nitrogen for the total desorption time and was then raised at 10 °C/s to 260 °C and held for 4 min. The solvent vent mode was used to transfer the sample to the analytical column. A CPWax-57CB column with dimensions 50 m × 0.25 mm and a 0.20 μm film thickness (Varian, Middelburg, Netherlands) was used, and the carrier gas was He at a flow rate of 1 mL/min. The oven temperature program was the following: the temperature was 35 °C for 4 min and was then raised to 220 °C at 2.5 °C/min (held 15 min). The quadrupole, source and transfer line temperatures were maintained at 150, 230 and 280 °C, respectively. The electron ionization mass spectra in the full-scan mode were recorded at 70 eV with the electron energy in the range of 29–300 amu.
Compound identification was based on mass spectra matching using the standard NIST 98 library and the retention index (LRI) of authentic reference standards.
Statistical analyses
One-way ANOVA was performed to evaluate significant differences among yeast strains and among different sampling points for each strain (significance levels p < 0.05). A principal component analysis (PCA) was carried out as an unsupervised method in order to ascertain the degree of differentiation between samples and which compounds were involved. ANOVA and PCA were performed using the Statistica (version 7.0) software package (Statsoft, Tulsa, USA).
Results and discussion
Fermentation kinetics and sugar consumption
Fermentations progress was monitored by measuring changes in OD660 and sugar consumption. In relation to yeast population, despite the fact that both yeasts strains were inoculated to reach the same final population, statistically significant differences were observed between S. cerevisiae G263 and L. thermotolerans G234 strain population during the fermentation process (Table 1). L. thermotolerans showed significant higher population than S. cerevisiae strain. In both strains, number of cells per mL significantly increased during the first 72 h of the assay, to keep more or less constant from T144 sampling point onwards.
Table 1.
Sugar consumption (%) and yeast population (cell/mL) in fermentation assays (results are average and standard deviations of two fermentations conducted by S. cerevisiae (G263) strain and L. thermotolerans (G234) strain
| T0 | T24 | T48 | T72 | T144 | T192 | ||
|---|---|---|---|---|---|---|---|
| S. cerevisiae | % sugar consumption | 0 | 9.1 ± 1.6a,A | 9.3 ± 1.2a,A | 13.4 ± 1.3a,A | 28.2 ± 1.4a,B | 32.7 ± 0.1b,B |
| L. thermotolerans | 0 | 9.3 ± 0.1a,A | 10.6 ± 2.5a,A | 15.0 ± 0.9a,B | 28.4 ± 0.1a,C | 30.9 ± 0.1a,C | |
| S. cerevisiae | cell/mL x 107 | 0.6 | 2.2 ± 0.1a,A | 5.6 ± 0.0a,B | 11.1 ± 0.3a,C | 21.2 ± 0.6a,D | 22.8 ± 0.0a,D |
| L. thermotolerans | 0.6 | 4.8 ± 0.3b,A | 8.3 ± 0.5b,B | 13.5 ± 0.0b,C | 26.7 ± 0.6b,D | 28.6 ± 0.6b,E |
Similar small letter in the same column indicates, for each parameter, no significant statistically differences (p < 0.05) between both yeast strains
Similar capital letter in the same row indicates no significant differences among sampling points for each yeast strain
Traditionally, non-Saccharomyces yeasts were described as weaker fermentative and less ethanol tolerant than S. cerevisiae strains (Fleet and Heard 1993); the latter together with added SO2 toxicity contribute to explain their early disappearance during the fermentation. Recently, Jolly et al. (2014) have reviewed other effects to explain this, as the low oxygen level, especially for L. thermotolerans.
Regarding sugar consumption, due to the high initial sugar content of the must, none of the strains was able to consume total fermentable sugars (Table 1). No statistically significant differences in sugar consumption between both strains was observed until the last sampling point (T192); however L. thermotolerans exhibited a slightly faster sugar consumption than S. cerevisiae during first 72 h. Finally, percentage of sugar consumption by S. cerevisiae was 32.7%. This was in agreement with results reported by López de Lerma et al. (2012) for Saccharomyces strains in partially fermented Pedro Ximénez sun-dried grape musts. For the non-Saccharomyces strain sugar consumption was slightly lower (30.9%).
In this context, it should be taken into account that L. thermotolerans was isolated during the partial fermentation of sun-dried high sugar content Pedro Ximénez grape must, and afterwards tested for its ability to ferment high sugar content media with successful results. Thus, we consider that its high adaptation at such specific media, gave this autochthonous strain a competitive edge, as already described by Cray et al. (2013) for other indigenous non-Saccharomyces strains.
Production of volatile compounds during fermentation assays
HSSE-PDMS extraction method was observed to be useful for determining volatile composition in different foodstuffs. In this work, HSSE-PDMS non-invasive method was observed to be adequate for monitoring the changes of volatile compounds during the alcoholic fermentation. With this technique, the evolution of 141 volatile compounds throughout alcoholic fermentations could be monitored. Eighty-four of them were positively identified and twenty-eight tentatively identified (TI) (Tables 2, 3).
Table 2.
Evolution of volatile compounds monitored online along alcoholic fermentation carried out by non-Saccharomyces yeast strain
| Volatile compounds | IDd | LRI | Peak area ± sde | |||||
|---|---|---|---|---|---|---|---|---|
| MT0 | NST24 | NST48 | NST72 | NST144 | NST192 | |||
| Acetals | ||||||||
| Acetaldehyde diethylacetal | A | 876 | nd | 253 ± 33a,b | 2488 ± 265a,b,c | 3971 ± 565b,c | 11,171 ± 1250a,b,c | 10,594 ± 1152b,c |
| 2,4,5-Trimethyl-1,3-dioxolane | C | 911 | 764 ± 74 | 514 ± 52 | 230 ± 26a,b,c | 286 ± 22b,c | 253 ± 19b,c | 270 ± 21b,c |
| Acetaldehyde ethyl amyl acetal | C | 1069 | nd | nd | 291 ± 36a,b,c | 557 ± 4a,b,c | 3817 ± 375a,b,c | 3572 ± 120b,c |
| Total of acetals | 764 | 767 | 3010a,b,c | 4814b,c | 15,240a,b,c | 14,473b,c | ||
| Acids | ||||||||
| Acetic acid | A | 1444 | 7194 ± 1073 | 6464 ± 726 | 5564 ± 768 | 4921 ± 363 | 3703 ± 378b,c | 3247 ± 390b,c |
| Propanoic acid | A | 1536 | 598 ± 52 | 796 ± 89 | 603 ± 60 | 560 ± 66 | 485 ± 68 | 620 ± 37 |
| Isovaleric acidg | A | 1670 | 491 ± 68 | 495 ± 53 | 393.9 ± 0.9b | 413 ± 21b | 257 ± 35a,b,c | 284 ± 15b |
| Pentanoic acidg | A | 1739 | 562 ± 76 | 463 ± 30 | 263 ± 35a,c | 241 ± 33c | 237 ± 31c | 197 ± 17c |
| Hexanoic acid | A | 1847 | 3871 ± 526 | 2931 ± 164 | 1718 ± 236a,b,c | 1662 ± 208b,c | 1306 ± 180b,c | 1215 ± 24b,c |
| Heptanoic acidg | A | 1958 | 666 ± 85 | 749 ± 101 | 558 ± 35b | 609 ± 78 | 480 ± 36 | 550 ± 54 |
| Octanoic acid | A | 2066 | 1171 ± 165 | 2421 ± 331a | 2750 ± 51b,c | 1632 ± 235a,b | 1041 ± 112b | 1337 ± 180b |
| Nonanoic acidh | A | 2176 | 788 ± 108 | 1362 ± 180 | 1743 ± 255c | 988 ± 87 | 611 ± 71a | 1266 ± 174a |
| Decanoic acid | A | 2283 | 237 ± 26 | 1721 ± 204a,b | 2182 ± 176c | 1860 ± 28b,c | 612 ± 69a,b,c | 628 ± 87b,c |
| Total of acids | 15,577 | 17,400 | 15,775b | 12,886b | 8733a,b,c | 9344b,c | ||
| Alcohols | ||||||||
| Ethanolf,h | A | 922 | 3441 ± 355 | 3905 ± 556 | 6258 ± 14a,c | 6099 ± 268c | 8078 ± 135a,c | 8531 ± 598c |
| 1-Propanol | A | 1017 | 1590 ± 114 | 2717 ± 347a | 7523 ± 943a,c | 5372 ± 251b,c | 6498 ± 141a,b,c | 6794 ± 512c |
| Isobutanolh | A | 1077 | 885 ± 97 | 1138 ± 75 | 2110 ± 152a,b,c | 1804 ± 104b,c | 2041 ± 125b,c | 2754 ± 236b,c |
| 1-Butanolh | A | 1134 | 1099 ± 36 | 968 ± 34 | 821 ± 17a,b,c | 770 ± 23b,c | 1209 ± 18a,b | 1445 ± 14a,b,c |
| 2-Methyl-1-butanolh | A | 1201 | 9851 ± 1237 | 22,981 ± 2052a,b | 33,800 ± 889a,c | 44,924 ± 2545a,c | 53,950 ± 2587c | 52,117 ± 3299c |
| 3-Methyl-1-butanolf,h | A | 1206 | 444 ± 45 | 799 ± 85a | 1818 ± 231a,c | 1598 ± 11b,c | 3320 ± 64a,b,c | 3595 ± 28a,b,c |
| 1-Pentanolg | A | 1245 | 3084 ± 171 | 2159 ± 319 | 1029 ± 31a,b,c | 865 ± 77c | 605 ± 30a,b,c | 422 ± 53c |
| 1-Hexanolg | A | 1351 | 42,527 ± 2847 | 32,803 ± 1103a | 14,269 ± 1384a,c | 13,318 ± 237b,c | 6181 ± 240a,c | 4765 ± 37a,b,c |
| cis-2-Hexen-1-olg | C | 1401 | 3560 ± 351 | nd | nd | nd | nd | nd |
| 1-Octen-3-olg | A | 1445 | 26,032 ± 1262 | 16,571 ± 2338a | 8669 ± 1266c | 8671 ± 532b,c | 3809 ± 19a,b,c | 2701 ± 167a,c |
| 1-Heptanolg | A | 1456 | 8412 ± 84 | 7850 ± 833 | 4027 ± 437a,c | 3554 ± 172b,c | 1380 ± 33a,c | 1059 ± 6a,c |
| 6-Methyl-5-hepten-2-ol | C | 1462 | nd | 651 ± 76a,b | 1917 ± 95a,b,c | 2111 ± 29b,c | 1627.6 ± 1.7a,b,c | 1236 ± 63a,b,c |
| 2-Ethyl-1-hexanolg | A | 1488 | 1198 ± 176 | 1147 ± 156 | 899 ± 125 | 768 ± 96 | 451 ± 31a,c | 454 ± 57c |
| 2-Hepten-1-olg | C | 1509 | 1338 ± 37 | nd | nd | nd | nd | nd |
| 1-Octanolg | A | 1558 | 4780 ± 233 | 3514 ± 430 | 2034 ± 134a,b,c | 1735 ± 46b,c | 621 ± 15a,b,c | 490 ± 50b,c |
| cis-2-Octen-1-olg | B1 | 1614 | 2070 ± 77 | 1004 ± 128a | 215 ± 22a,b,c | 332 ± 47c | nq | nq |
| Furfuryl alcohol | A | 1659 | 3040 ± 414 | 3789 ± 430 | 3123 ± 466 | 2759 ± 125 | 2646 ± 199 | 3276 ± 408 |
| 1-Nonanolg | A | 1663 | 1998 ± 187 | 1509 ± 49 | 274 ± 16a,b,c | nd | nd | nd |
| 3-Methylthio-1-propanolh | B2 | 1723 | nd | nd | 245 ± 31a,b,c | 213 ± 18b,c | 197 ± 25b,c | nq |
| 4-Ethylbenzyl alcohol | C | 1762 | nd | nd | nd | nd | nd | nd |
| 1-Decanol | A | 1764 | nd | nd | nd | nd | nd | nd |
| Benzyl alcoholg | A | 1883 | 693 ± 46 | 579 ± 24 | 356 ± 15a,c | 313.8 ± 1.0c | 254 ± 8a,b,c | 230 ± 5b,c |
| 2-Phenylethanolf,h | A | 1920 | 188 ± 12 | 206,2 ± 1.7 | 918 ± 133a,c | 706 ± 11b,c | 1885 ± 34a,b,c | 1994 ± 181b,c |
| Total of alcoholsf | 5195 | 5904 | 9833a,c | 9292c | 14,068a,b,c | 14,898b,c | ||
| Aldehydes | ||||||||
| 3-Methyl-butanalg | C | 890 | 3903 ± 577 | nd | nd | nd | nd | nd |
| Hexanalg | A | 1040 | 2483 ± 331 | nd | nd | nd | nd | nd |
| Heptanalg | C | 1149 | 343 ± 6 | nd | nd | nd | nd | nd |
| trans-2-Heptenalg | B3 | 1306 | 3289 ± 282 | nd | nd | nd | nd | nd |
| Nonanalg | A | 1375 | 1514 ± 104 | nd | nd | nd | nd | nd |
| 2-Furfuraldehydeg | A | 1448 | 18,968 ± 1883 | 12,614 ± 1413 | 5590 ± 826a,c | 4272 ± 589c | 4149 ± 597c | 4919 ± 13c |
| trans–trans-2,4-Heptadienalg | C | 1483 | 3156.4 ± 2.3 | nd | nd | nd | nd | nd |
| Benzaldehydeg | A | 1508 | 5507 ± 243 | 378 ± 13a | 289 ± 35c | 253 ± 32c | 254 ± 19c | 267 ± 7c |
| trans-2-Nonenalg | B4 | 1525 | 924 ± 114 | nd | nd | nd | nd | nd |
| 5-Methyl-2-furfuraldehydeg | A | 1563 | 861 ± 9 | 844 ± 93 | 745 ± 101b | 407 ± 53c | 402 ± 49c | 404 ± 51c |
| Cinnamaldehydeg | C | 1574 | 309 ± 42 | 405 ± 4 | 263.6 ± 1.3a | 292 ± 37 | 246 ± 33 | 252 ± 22b |
| trans-2-Decenalg | B5 | 1634 | 961 ± 64 | nd | nd | nd | nd | nd |
| Safranalg | C | 1635 | 791 ± 84 | 369 ± 26a | nd | nd | nd | nd |
| trans–trans-2,4-Nonadienalg | B5 | 1696 | 725 ± 77 | nd | nd | nd | nd | nd |
| trans–trans-2,4-Decadienalg | B5 | 1804 | 5168 ± 266 | nd | nd | nd | nd | nd |
| 5-Hydroxymethylfurfural | A | 2489 | 673 ± 39 | 2462 ± 317a,b | 1216 ± 159a,b,c | 515 ± 71a | 564 ± 70b | 642 ± 83 |
| Total of aldehydes | 49,657 | 16,703a | 8104a,c | 5739c | 5616c | 6485b,c | ||
| Acetic esters | ||||||||
| Ethyl acetateh | A | 871 | 9404 ± 1315 | 8741 ± 687 | 18,255 ± 105a,b,c | 23,923 ± 317a,c | 65,748 ± 1463a,b,c | 71,432 ± 1161a,b,c |
| Propyl acetate | A | 934 | nd | nd | 160 ± 11a,b,c | 195 ± 8b,c | 434 ± 27a,b,c | 504 ± 9c |
| Isobutyl acetateh | A | 971 | 166 ± 17 | 248.5 ± 1.2a | 1420 ± 80a,b,c | 2037 ± 33a,b,c | 7025 ± 177a,b,c | 6958 ± 19b,a |
| Isoamyl acetatef | A | 1081 | 8.2 ± 0.7 | 27.7 ± 1.3a,b | 233 ± 7a,b,c | 342 ± 18a,b,c | 1004.8 ± 0.5a,b,c | 992 ± 10c |
| Amyl acetate | A | 1136 | nd | nd | nd | 223 ± 31a,b,c | nd | nd |
| Hexyl acetate | A | 1252 | 398 ± 39 | 578 ± 74b | 633 ± 75b | 797 ± 4b,c | 474 ± 6a,b | 262 ± 16a,b,c |
| Heptyl acetate | B3 | 1356 | nd | nd | nd | nd | nd | nd |
| Octyl acetate | A | 1464 | nd | nd | nd | nd | nd | nd |
| Nonyl acetate | B3 | 1565 | nd | nd | nd | nd | nd | nd |
| Decyl acetate | B3 | 1672 | nd | nd | nd | nd | nd | nd |
| Benzyl acetate | A | 1718 | nd | nd | nd | nd | nd | nd |
| 2-Phenylethanol acetate | A | 1806 | 517 ± 68 | 196 ± 8a,b | 820 ± 110a,b | 789 ± 106b | 2507 ± 47a,b,c | 2577 ± 330b,c |
| Nerolidol acetate | C | 2257 | nd | nd | nd | nd | nd | nd |
| Total of acetic esters | 11,306 | 12,539b | 44,614a,b,c | 62,164a,b,c | 176,670a,b,c | 180,925b,c | ||
| Ethyl esters | ||||||||
| Ethyl propanoateh | A | 924 | 182 ± 23 | 236 ± 27 | 1274 ± 167a,b,c | 2617 ± 134a,b,c | 8655 ± 142a,b,c | 6812 ± 307a,b,c |
| Ethyl 2-methylpropanoateh | A | 928 | nd | nd | 179.9 ± 2.2a,b,c | 184 ± 9b,c | 426 ± 17a,b,c | 437 ± 17b,c |
| Ethyl butyrate | A | 997 | 256 ± 31 | 270 ± 37b | 887 ± 21a,b,c | 1427 ± 21a,b,c | 6209 ± 207a,b,c | 7807 ± 282a,c |
| Ethyl 2-methylbutyrateg | A | 1012 | 198 ± 10 | nd | nd | nd | nd | nd |
| Ethyl valerateh | A | 1092 | 259 ± 38 | 186 ± 23 | 268 ± 9a,b | 445 ± 21a,b,c | 1667 ± 6a,c | 1570 ± 188c |
| Ethyl hexanoatef | A | 1210 | 28.3 ± 1.0 | 37 ± 3 | 179 ± 8a,b,c | 333 ± 11a,b,c | 435 ± 31a,b,c | 363 ± 14b,c |
| Ethyl heptanoate | A | 1315 | 705 ± 83 | 1246 ± 110a | 5344 ± 60a,b,c | 7207 ± 230a,b,c | 6873 ± 199b,c | 4701 ± 434a,b,c |
| Ethyl 2-hexenoateh | B6 | 1325 | nd | nd | nd | nd | 361 ± 16a,b,c | 408.5 ± 1.2c |
| Ethyl octanoatef | A | 1435 | 29 ± 4 | 159 ± 7a | 738 ± 74a,b,c | 522.4 ± 0.3b,c | 490 ± 36b,c | 447 ± 61b,c |
| Ethyl 7-octenoate | A | 1473 | nd | nd | 315 ± 23a,c | 376 ± 6b,c | 393 ± 25b,c | 515 ± 72b,c |
| Ethyl nonanoate | A | 1525 | 1673 ± 133 | 2494 ± 254b | 4305 ± 194a,b,c | 3945 ± 283b,c | 2482 ± 196a,b,c | 2135 ± 310b |
| Ethyl decanoatef | A | 1641 | 7.4 ± 0.8 | 278 ± 18a,b | 509 ± 37a,b,c | 560 ± 39b,c | 436 ± 34b,c | 536 ± 73b,c |
| Ethyl 9-decenoate | B6 | 1686 | nd | nd | 3149 ± 432a,c | 2216 ± 15b,c | 1835 ± 234b,c | 3174 ± 413b,c |
| Ethyl undecanoate | A | 1730 | nq | 177 ± 3a,b | nq | 177 ± 20a,b,c | nq | nq |
| Ethyl phenylacetateh | A | 1774 | 221 ± 14 | 173 ± 8b | 620 ± 89a,b,c | 342.6 ± 2.2a,b,c | 628 ± 38a,b,c | 584 ± 80b,c |
| Ethyl dodecanoatef | A | 1838 | 1.90 ± 0.21 | 3.32 ± 0.22a,b | 18.5 ± 2.1a,b,c | 50.58 ± 0.18a,b,c | 87 ± 3a,b,c | 77 ± 11b,c |
| Ethyl tetradecanoateh | A | 2041 | nq | nq | 402 ± 54a,c | 812 ± 28a,c | 2077 ± 182a,c | 2074 ± 104b,c |
| Ethyl hexadecanoate | A | 2250 | nq | nq | nq | 281 ± 3a,b | 253 ± 23b,c | 287 ± 42b,c |
| Total of ethyl esters | 10,169 | 52,538a,b | 161,211a,b,c | 166,612b,c | 176,679b,c | 172,858b,c | ||
| Isoamyl esters | ||||||||
| Isoamyl propionateh | C | 1155 | nd | nd | 197 ± 18a,c | 307 ± 20a,c | 1506 ± 14a,b,c | 1349 ± 61b,c |
| Isoamyl hexanoate | A | 1450 | nd | nd | nd | nd | nd | nd |
| Isoamyl octanoate | A | 1654 | nd | nd | 1897 ± 204a,b,c | 2246 ± 158b,c | 2443 ± 179b,c | 1620 ± 215b,c |
| Isoamyl decanoate | B7 | 1854 | nd | nd | 1018 ± 126a,c | 1564 ± 53a,c | 1963 ± 27a,b,c | 2057 ± 307b,c |
| Total of isoamyl esters | – | – | 3112a,b,c | 4117b,c | 5911a,b,c | 5026b,c | ||
| Methyl esters | ||||||||
| Methyl hexanoate | A | 1151 | nd | 162.0 ± 0.3a,b | 162.3 ± 1.5b,c | 215 ± 8a,b,c | nq | nq |
| Methyl octanoate | A | 1371 | 234 ± 32 | 477 ± 66a | 428 ± 60b | 285 ± 7b | nq | nq |
| Methyl decanoate | A | 1581 | nd | 908 ± 101a,b | 308 ± 40a,b,c | 247.82 ± 0.15b,c | nq | nq |
| Methyl salicylate | A | 1762 | 244 ± 14 | 175 ± 22b | nq | nq | 194 ± 24a,b | 200 ± 27b |
| Total of methyl esters | 479 | 1723a,b | 898a,b,c | 748b,c | 194a,b,c | 200b,c | ||
| Others esters | ||||||||
| Propyl hexanoate | B8 | 1299 | nd | nd | nq | nq | nq | nq |
| Propyl octanoate | A | 1509 | nd | nd | nd | nd | nd | nd |
| Propyl decanoate | B9 | 1712 | nd | nd | nd | nd | nd | nd |
| Isobutyl hexanoate | B9 | 1335 | nd | nd | nd | nd | nq | nq |
| Isobutyl octanoate | A | 1542 | nd | nd | nd | nd | nd | nd |
| Isobutyl decanoate | B9 | 1746 | nd | nd | nd | 203 ± 28a,c | 171 ± 11b,c | 184 ± 15b,c |
| Total of other ester | – | – | – | 203a,b,c | 171b,c | 184c | ||
| Ketones | ||||||||
| 2-Pentanoneg | A | 939 | 473 ± 23 | 262 ± 10a | nd | nd | nd | nd |
| 3-Penten-2-oneg | C | 1094 | 1242 ± 140 | nd | nd | nd | nd | nd |
| 2-Heptanoneg | C | 1152 | 744 ± 14 | 291 ± 24a,b | nq | nq | nq | nq |
| 2-Pentylfurang | B3 | 1196 | 29,133 ± 3173 | 29,364 ± 439 | 17,699 ± 2009a,c | 16,158 ± 2314c | 1404 ± 45a,c | 484 ± 14a,c |
| 3-Octanoneg | B3 | 1233 | 1512 ± 143 | 1740 ± 144 | 854 ± 102a,b,c | 940 ± 91b,c | 484 ± 8a,b,c | 367 ± 38b,c |
| 2-Octanoneg | B3 | 1266 | 4291 ± 458 | 1618 ± 223a,b | 492 ± 69a,b,c | 567 ± 25c | 273 ± 17a,c | 250 ± 33c |
| trans-2,2-Pentenyl-furang | C | 1271 | 1516 ± 134 | 1378 ± 38 | 460 ± 55a,c | 637 ± 81c | nd | nd |
| Acetoinf | A | 1273 | 711 ± 88 | 721 ± 83 | 1210 ± 10a,b,c | 815 ± 39a,b | 816 ± 12b | 702 ± 25a |
| 1-Hydroxy-2-propanone | A | 1286 | 2265 ± 296 | 4120 ± 533 | 2373 ± 291 | 2686 ± 76 | 2310 ± 144 | 2459 ± 48 |
| 2-Hexylfurang | C | 1303 | 389 ± 42 | 394 ± 17b | 274 ± 39 | 341 ± 30 | nq | nq |
| 6-Methyl-5-hepten-2-oneg | A | 1319 | 4095 ± 195 | 3988 ± 45 | 2230 ± 283a,b,c | 2326 ± 143b,c | 742 ± 6a,b,c | 464 ± 11a,b,c |
| 1-Hydroxy-2-butanone | B3 | 1363 | 1097 ± 150 | 1442 ± 155 | 1096 ± 62 | 1583 ± 21a,b,c | 1449 ± 104 | 1467 ± 200 |
| 2-Nonanoneh | A | 1374 | 1657 ± 225 | 1072 ± 151b | 2216 ± 92a,b | 2054 ± 66b | 1535 ± 86a | 1464 ± 84b |
| 2-Acetilfurang | A | 1493 | 881 ± 16 | 1123 ± 131 | 721 ± 95 | 579 ± 70c | 487 ± 32c | 580 ± 54c |
| Dihydro-3(2H)-thiophenoneh | C | 1515 | nd | nd | 281 ± 38a,b,c | 268 ± 35b,c | nd | nd |
| 6-Methyl-3,5-heptadiene-2-oneg | B3 | 1588 | 11,941 ± 59 | 6343 ± 470a | 2377 ± 6a,b,c | 1714 ± 208a,b,c | 454 ± 35a,b,c | 271 ± 14a,b,c |
| Acetofenoneh | A | 1641 | 654 ± 77 | 711 ± 106 | 490 ± 67 | 434 ± 45 | 348 ± 49b,c | 297 ± 41b,c |
| 1,2-Cyclopentanedione | C | 1775 | 2224 ± 270 | 3415 ± 260a | 2645 ± 381 | 2238 ± 107 | 2029 ± 179 | 2763 ± 358 |
| Cycloteneh | B10 | 1841 | 317 ± 47 | 397 ± 49 | 352 ± 47 | 312 ± 20 | 261 ± 14 | 403 ± 55b |
| Total of ketones | 135,497 | 129,723 | 155,601b | 114,387a,b | 93,391a,b,c | 81,515a,c | ||
| Lactones | ||||||||
| γ-Butyrolactoneg | A | 1625 | 2903 ± 35 | 2666 ± 252 | 1752 ± 31a,c | 1603 ± 58c | 1352 ± 10a,c | 1483 ± 70b,c |
| γ-Nonalactonag | B3 | 2039 | 1506 ± 217 | 1966 ± 62b | 1404 ± 209 | 1404 ± 33 | 1020 ± 20a | 838 ± 20a,b,c |
| Total of lactones | 4409 | 4632 | 3156a,c | 3007c | 2372a,c | 2321c | ||
| C 13-Norisoprenoids | ||||||||
| TDN | A | 1727 | 793 ± 88 | 740 ± 70 | 737 ± 22b | 891 ± 17a | 708 ± 42a,b | 439 ± 60a,b,c |
| β-Damascenoneg | A | 1813 | 1595 ± 59 | 1125 ± 115a | 790 ± 27b,c | 863 ± 17c | 562 ± 25a,c | 633 ± 26c |
| β-Iononeg | A | 1941 | 174 ± 22 | 129 ± 14b | nq | nq | nd | nd |
| Total of C13-Norisoprenoids | 2562 | 1994 | 1527b,c | 1754a,c | 1270a,b,c | 1073b,c | ||
| Terpenes | ||||||||
| 1R-α-Pineneg | C | 976 | 1502 ± 210 | 983 ± 25b | 780 ± 97b,c | 987 ± 131b | 350 ± 35a,b,c | 219 ± 12a,b,c |
| Roseoxideh | C | 1119 | nd | nd | nd | 525 ± 62a,b,c | 916 ± 41a,b,c | 671 ± 16a,b,c |
| Myrtenal | C | 1123 | 663 ± 69 | 620 ± 44 | 774 ± 76 | 1075 ± 96c | 568 ± 29a | 252 ± 29a,c |
| Limonene | A | 1149 | 426 ± 52 | 332 ± 22 | 798 ± 42a,c | 355 ± 7a | nq | nq |
| Cymeneg | B11 | 1237 | 604 ± 35 | 491 ± 30 | 280 ± 40a,c | 298 ± 41c | nd | nd |
| trans-Linalool oxideg | B12 | 1468 | 219 ± 31 | nq | nd | nd | nd | nd |
| 2-Borneneg | C | 1517 | 846 ± 32 | 619 ± 67a | 417 ± 33c | 388 ± 50c | 275 ± 9c | 186 ± 21a,c |
| Linaloolg | A | 1542 | 439 ± 35 | 246 ± 12a | 186.6 ± 2.1a,b,c | 181 ± 7b,c | nq | nq |
| Citronellol | A | 1767 | nd | nd | 271 ± 33a,b,c | nd | 286 ± 33a,b,c | 296 ± 36b,c |
| α-Calacoreneg | B13 | 1901 | 307 ± 24 | nq | nq | nd | nd | nd |
| Nerolidol | A | 2037 | nd | nd | nd | nd | nq | nq |
| n.i. (m/z 69, 93, 121) | – | 1743 | nd | nd | nd | nd | nd | nd |
| Total of Terpenes | 5005 | 3291a | 3508b,c | 3809b,c | 2396a,b,c | 1624a,b,c | ||
| Volatile phenols | ||||||||
| Guaiacolg | A | 1858 | 273 ± 31 | nq | nq | nq | nq | nq |
| 4-Vinylguaiacol | A | 2203 | nq | nq | nq | nq | nd | nd |
| Coumarang | C | 2406 | 238 ± 32 | 194 ± 28 | 170 ± 21 | nq | nq | nq |
| Total of volatile phenols | 511 | 194a | 170b | – | – | – | ||
| Others compounds | ||||||||
| 2-Methylpyrazineg | A | 1256 | 343 ± 46 | 415 ± 6 | 283 ± 32a | 255 ± 31 | nq | nq |
| Indoleh | B14 | 2436 | 375 ± 56 | 455 ± 20b | 714 ± 100 | 1073 ± 40a,c | 470 ± 21a,b | 205 ± 7a,b |
| Unidentified compounds | ||||||||
| n.i. (m/z 59, 43)h | – | 1328 | nq | nq | 320 ± 22a,b,c | 466 ± 5a,c | 804 ± 13a,c | 743 ± 78c |
| n.i. (m/z 67, 85, 151)g | – | 1395 | 1332 ± 97 | 1076 ± 16 | 590 ± 85a,b,c | 790 ± 70b,c | 314 ± 9a,b,c | 163 ± 18a,b,c |
| n.i. (m/z 55,88, 101) | – | 1887 | nd | nd | nd | nd | nd | nd |
| n.i. (m/z 126, 73)g | – | 2106 | 4188 ± 85 | 394 ± 49a,b | nq | 382 ± 37a,c | 628 ± 56a,b,c | 403.8 ± 1.3a,b,c |
ID: reliability of identification: A, mass spectrum and LRI agreed with standards; B, mass spectrum agreed with mass spectral data base and LRI agreed with the literature data; C, mass spectrum agreed with mass spectral data base
nd: peak not detected or lower than detection limit (a signal-to-noise ratio higher than or equal to 3); nq: lower than quantification limit (a signal-to-noise ratio higher than or equal to 10)
aThere is significant different (p < 0.05) with previous sample
bThere is significant different (p < 0.05) with wine produced by Saccharomyces cerevisiae strain
cThere is significant different (p < 0.05) with substrate, only for samples from 48 to 192 h
dLiterature reference agreed with experimental LRI data:(1) Weckerle et al. (2001), (2) Miranda-Lopez et al. (1992), (3) National Center for Biotechnology Information (2015), (4) Schnermann and Schieberle (1997), (5) Rychlik et al. (1998), (6) Ferrari et al. (2004), (7) Riu-Aumatell et al. (2006), (8) Girard and Lau (1995), (9) Sun et al. (2013), (10) Natali et al. (2006), (11) Olivera et al. (2007), (12) Losco et al. (2007), (13) Bicchi et al. (2003), (14) Shiratsuchi et al. (1994) (complete reference provided as supplementary material)
eValue of peak area and sd have been divided per 1000
fValue of peak area and sd have been divided per 100,000
gVariable highly correlated with substrate and T24 (non-Saccharomyces and Saccharomyces)
hVariable highly correlated with samples from T48 to T192 Saccharomyces
Table 3.
Evolution of volatile compounds monitored online along alcoholic fermentation carried out by Saccharomyces yeast strain
| Volatile compounds | ID | LRI | Peak area ± sdc | ||||
|---|---|---|---|---|---|---|---|
| ST24 | ST48 | ST72 | ST144 | ST192 | |||
| Acetals | |||||||
| Acetaldehyde diethylacetalf | A | 876 | nd | 6193 ± 219a,b | 10,607 ± 1467b | 54,828 ± 3506a,b | 42,198 ± 1594a,b |
| 2,4,5-Trimethyl-1,3-dioxolanef | C | 911 | 614 ± 4 | 491 ± 13b | 956 ± 56a | 6902 ± 757a,b | 8819 ± 683b |
| Acetaldehyde ethyl amyl acetalf | C | 1069 | nd | 601 ± 3a,b | 1449 ± 189a,b | 8091 ± 89a,b | 5651 ± 369a,b |
| Total of acetals | 614 | 7285a,b | 13,012a,b | 69,821a,b | 56,668a,b | ||
| Acids | |||||||
| Acetic acidf | A | 1444 | 7022 ± 906 | 6905 ± 888 | 5396 ± 775 | 11,031 ± 1146a | 9543 ± 1354 |
| Propanoic acidf | A | 1536 | 742 ± 96 | 656 ± 80 | 648 ± 94 | 831 ± 102 | 683 ± 5 |
| Isovaleric acide | A | 1670 | 769 ± 90 | 648 ± 56 | 567.1 ± 1.0 | 665 ± 43 | 527 ± 16 |
| Pentanoic acide | A | 1739 | 496 ± 66 | 297.6 ± 1.0b | 293 ± 10b | 286 ± 5b | 255 ± 28b |
| Hexanoic acidf | A | 1847 | 3129 ± 446 | 3136 ± 263 | 4392 ± 176a | 6094 ± 561 | 5551 ± 374 |
| Heptanoic acide | A | 1958 | 687 ± 93 | 386 ± 39 | 564 ± 77 | 389 ± 31b | 565 ± 81 |
| Octanoic acidf | A | 2066 | 2042 ± 291 | 8433 ± 449b | 11,450 ± 392a,b | 10,697 ± 1544b | 11,299 ± 242b |
| Nonanoic acid | A | 2176 | 844 ± 117 | 1219.83 ± 0.19b | 907 ± 123 | 662 ± 89 | 1449 ± 203a |
| Decanoic acidf | A | 2283 | 995 ± 110a | 2655 ± 113a,b | 4961 ± 111a,b | 5065 ± 679b | 5026 ± 75b |
| Total of acids | 16,753a | 24,336a,b | 29,179a,b | 35,719a,b | 34,897b | ||
| Alcohols | |||||||
| Ethanold | A | 922 | 3215 ± 30 | 6537 ± 605b | 6785 ± 365b | 8696 ± 305a,b | 8212 ± 76b |
| 1-Propanolf | A | 1017 | 1675 ± 29 | 7867 ± 122a,b | 7959 ± 123b | 10,132 ± 303a,b | 8346 ± 866b |
| Isobutanol | A | 1077 | 891 ± 58 | 1213 ± 81 | 1287 ± 33b | 1346 ± 178 | 1025 ± 53 |
| 1-Butanol | A | 1134 | 988 ± 20 | 1549 ± 68b | 1250.8 ± 1.1a,b | 825 ± 92a | 622 ± 71b |
| 2-Methyl-1-butanol | A | 1201 | 15,576 ± 632a | 34,803 ± 1522a,b | 37,770 ± 1883b | 50,969 ± 1812a,b | 45,303 ± 2586b |
| 3-Methyl-1-butanold | A | 1206 | 871 ± 58a | 1636 ± 72a,b | 1421 ± 23b | 1387 ± 41b | 1272 ± 42b |
| 1-Pentanole | A | 1245 | 2045 ± 40a | 866 ± 39a,b | 792 ± 17b | 417 ± 3a,b | 381.8 ± 2.3a,b |
| 1-Hexanole | A | 1351 | 34,943 ± 296 | 11,934 ± 715b | 9992 ± 315b | 6106 ± 25a,b | 4225 ± 31a,b |
| cis-2-Hexen-1-ole | C | 1401 | nd | nd | nd | nd | nd |
| 1-Octen-3-ole | A | 1445 | 17,768 ± 597a | 7084 ± 198a,b | 6010 ± 175a,b | 3094 ± 86a,b | 2227 ± 115a,b |
| 1-Heptanole | A | 1456 | 8549 ± 652 | 3555 ± 68b | 2610 ± 77a,b | 1417 ± 25a,b | 1201 ± 50a,b |
| 6-Methyl-5-hepten-2-ol | C | 1462 | nd | 658.7 ± 1.4a,b | 815 ± 7a,b | 899 ± 67b | 667 ± 60b |
| 2-Ethyl-1-hexanole | A | 1488 | 1228 ± 120 | 600 ± 30c | 578 ± 77c | 640 ± 75 | 637 ± 86 |
| 2-Hepten-1-ole | C | 1509 | nd | nd | nd | nd | nd |
| 1-Octanole | A | 1558 | 3700 ± 397 | 3413 ± 60b | 3317 ± 106b | 1710 ± 186a,b | 1186 ± 23b |
| cis-2-Octen-1-ole | B | 1614 | 1455 ± 165a | 427 ± 20a,b | 303 ± 15a,b | nd | nd |
| Furfuryl alcohol | A | 1659 | 4425 ± 628 | 3372 ± 386 | 3262 ± 444 | 3348 ± 482 | 2838 ± 277 |
| 1-Nonanole | A | 1663 | 1742 ± 223 | 672 ± 71b | 735 ± 110b | 331 ± 49a,b | 326 ± 4b |
| 3-Methylthio-1-propanol | B | 1723 | nd | nd | nd | nd | nd |
| 4-Ethylbenzyl alcoholf | C | 1762 | nd | 310 ± 8a,b | 556 ± 49a,b | 363 ± 3a,b | 254 ± 12a,b |
| 1-Decanolf | A | 1764 | nd | 607 ± 35a,b | 1083 ± 15a,b | 765 ± 54a,b | 432 ± 19a,b |
| Benzyl alcohole | A | 1883 | 501 ± 45 | 347 ± 4b | 318 ± 4b | 208 ± 3a,b | 186 ± 11b |
| 2-Phenylethanold | A | 1920 | 227 ± 31 | 1261 ± 78b | 919 ± 13a,b | 946 ± 47b | 1067 ± 6b |
| Total of alcoholsd | 5268 | 10,227b | 9911b | 11,855a,b | 11,249b | ||
| Aldehydes | |||||||
| 3-Methyl-butanale | C | 890 | nd | nd | nd | nd | nd |
| Hexanale | A | 1040 | nd | nd | nd | nd | nd |
| Heptanale | C | 1149 | nd | nd | nd | nd | nd |
| trans-2-Heptenale | B | 1306 | nd | nd | nd | nd | nd |
| Nonanale | A | 1375 | nd | nd | nd | nd | nd |
| 2-Furfuraldehydee | A | 1448 | 14,496 ± 2084 | 6664 ± 755b | 5260 ± 704b | 4553 ± 544b | 4245 ± 420b |
| trans–trans-2,4-Heptadienale | C | 1483 | 1286 ± 41a | nd | nd | nd | nd |
| Benzaldehydee | A | 1508 | 542 ± 74a | 233 ± 5a,b | 260 ± 27b | 285 ± 35b | 274 ± 35b |
| trans-2-Nonenale | B | 1525 | nd | nd | nd | nd | nd |
| 5-Methyl-2-furfuraldehydee | A | 1563 | 652 ± 93 | 407 ± 8b | 399 ± 56b | 529 ± 75b | 314 ± 26b |
| Cinnamaldehydee | C | 1574 | 448 ± 66 | 253 ± 34 | 266 ± 33 | 173 ± 3b | 175 ± 14b |
| trans-2-Decenale | B | 1634 | nd | nd | nd | nd | nd |
| Safranale | C | 1635 | nd | nd | nd | nd | nd |
| trans–trans-2,4-Nonadienale | B | 1696 | nd | nd | nd | nd | nd |
| trans–trans-2,4-Decadienale | B | 1804 | nd | nd | nd | nd | nd |
| 5-Hydroxymethylfurfural | A | 2489 | 909 ± 106 | 672.9 ± 0.7 | 805 ± 103 | 1154 ± 45a,b | 465 ± 57a |
| Total of aldehydes | 18,333a | 8230a,b | 6990b | 6693b | 5473b | ||
| Acetic esters | |||||||
| Ethyl acetate | A | 871 | 8359 ± 519 | 16,234 ± 104b | 23,209 ± 519a,b | 41, 238 ± 4762a,b | 37, 890 ± 2344b |
| Propyl acetatef | A | 934 | nd | 467 ± 40a,b | 610 ± 27b | 756 ± 88b | 565 ± 60b |
| Isobutyl acetate | A | 971 | 237 ± 20 | 2644 ± 203b | 3872 ± 41a,b | 3847 ± 410b | 2320 ± 254a,b |
| Isoamyl acetated,f | A | 1081 | 48.2 ± 0.6a | 1151 ± 73a,b | 1608 ± 81a,b | 1705 ± 199b | 1153 ± 62b |
| Amyl acetatef | A | 1136 | nd | 1969 ± 98a,b | 3095 ± 45a,b | 2368 ± 167a,b | 1149 ± 101a,b |
| Hexyl acetatef | A | 1252 | 4806 ± 36a | 53,965 ± 3425a,b | 78,336 ± 485a,b | 46,441 ± 2127a,b | 23,961 ± 826a,b |
| Heptyl acetate | B | 1356 | 1212 ± 30a,b | 9352 ± 405a,b | 13,844 ± 142a,b | 6651 ± 32a,b | 3247 ± 47a,b |
| Octyl acetatef | A | 1464 | nd | 5540 ± 185a,b | 11,319 ± 287a,b | 7289 ± 26a,b | 4205 ± 4a,b |
| Nonyl acetatef | B | 1565 | nd | 1204 ± 23a,b | 2644 ± 130a,b | 1558 ± 164a,b | 1036 ± 112b |
| Decyl acetatef | B | 1672 | nd | 556 ± 41a,b | 1716 ± 126a,b | 2414 ± 115a,b | 2281 ± 83b |
| Benzyl acetate | A | 1718 | nd | 230 ± 3a,b | 215 ± 3a,b | 164.2 ± 2.2a,b | nq |
| 2-Phenylethanol acetatef | A | 1806 | 1000 ± 128a | 89,451 ± 2541a,b | 61,507 ± 5304a,b | 50,422 ± 872b | 51,838 ± 461b |
| Nerolidol acetatef | C | 2257 | nd | nd | 298 ± 25a,b | 691 ± 97a,b | 1023 ± 48a,b |
| Total of acetic esters | 20,433a | 296,712a,b | 361,498a,b | 334,354b | 244,861a,b | ||
| Ethyl esters | |||||||
| Ethyl propanoate | A | 924 | 209 ± 5 | 362 ± 6b | 626 ± 34a,b | 860 ± 74b | 866 ± 37b |
| Ethyl 2-methylpropanoate | A | 928 | nd | nd | nd | nd | nd |
| Ethyl butyratef | A | 997 | 426 ± 20a | 2423 ± 3a,b | 5636 ± 91a,b | 12,466 ± 1600a,b | 11,076 ± 1394b |
| Ethyl 2-methylbutyratee | A | 1012 | nd | nd | nd | nd | nd |
| Ethyl valerate | A | 1092 | 230,4 ± 1,3 | 352 ± 10 | 642 ± 16a,b | 1664 ± 178a,b | 1887 ± 109b |
| Ethyl hexanoated,f | A | 1210 | 41.86 ± 0.14a | 1603 ± 112a,b | 2989 ± 114a,b | 4317 ± 182a,b | 3620 ± 156b |
| Ethyl heptanoatef | A | 1315 | 943 ± 49 | 12,828 ± 507a,b | 13,765 ± 280b | 15,435 ± 220a,b | 16,275 ± 276b |
| Ethyl 2-hexenoate | B | 1325 | nd | nd | nd | 226 ± 4b | 394 ± 10a,b |
| Ethyl octanoated,f | A | 1435 | 136,3 ± 2,2a | 4567 ± 121a,b | 8601 ± 893a,b | 14,101 ± 183a,b | 15,920 ± 19a,b |
| Ethyl 7-octenoatef | A | 1473 | nd | 642 ± 30b | 779 ± 6a,b | 1339 ± 26a,b | 2431 ± 158a,b |
| Ethyl nonanoatef | A | 1525 | 1065 ± 126a | 5573 ± 53a,b | 10,019 ± 63a,b | 13,327 ± 595a,b | 15,005 ± 87b |
| Ethyl decanoated,f | A | 1641 | 24.5 ± 1.8a | 1353 ± 52a,b | 3708 ± 589a,b | 9668 ± 32a,b | 10,261 ± 295b |
| Ethyl 9-decenoatef | B | 1686 | nd | 14,756 ± 415a,b | 36,581 ± 4997a,b | 67,374 ± 15a,b | 107,580 ± 2435a,b |
| Ethyl undecanoatef | A | 1730 | nq | 479 ± 9a,b | 886 ± 88a,b | 2397 ± 59a,b | 2402 ± 35b |
| Ethyl phenylacetate | A | 1774 | nq | 319 ± 26a,b | 246 ± 8 | 282 ± 12b | 310 ± 11b |
| Ethyl dodecanoated,f | A | 1838 | 1.69 ± 0.23 | 99 ± 4b | 325 ± 44a,b | 1627 ± 23a,b | 1657 ± 79b |
| Ethyl tetradecanoate | A | 2041 | nq | 256 ± 20a,b | 737 ± 71a,b | 2957 ± 399a,b | 3302 ± 129b |
| Ethyl hexadecanoatef | A | 2250 | nq | nq | 411.2 ± 1.7a,b | 1330 ± 179a,b | 1717 ± 63b |
| Total of ethyl esters | 23,309a | 800,155a,b | 1632,542a,b | 3091,015a,b | 3308,983a,b | ||
| Isoamyl esters | |||||||
| Isoamyl propionate | C | 1155 | nd | 220 ± 21a,b | 349 ± 7a,b | 554 ± 20a,b | 454.9 ± 0.3a,b |
| Isoamyl hexanoatef | A | 1450 | nd | 2439 ± 90a,b | 6615 ± 724a,b | 11,509 ± 392a,b | 8916 ± 76a,b |
| Isoamyl octanoatef | A | 1654 | nd | 7176 ± 184a,b | 15,667 ± 1742a,b | 32,624 ± 816a,b | 31,538 ± 616b |
| Isoamyl decanoatef | B | 1854 | nd | 700 ± 72a,b | 2282 ± 324a,b | 10,581 ± 572a,b | 15,170 ± 904a,b |
| Total of isoamyl esters | – | 10,535a,b | 24,913a,b | 55,268a,b | 56,079b | ||
| Methyl Esters | |||||||
| Methyl hexanoatef | A | 1151 | 204 ± 3a | 797 ± 33a,b | 1392 ± 42a,b | 983 ± 106a,b | 526 ± 12a,b |
| Methyl octanoatef | A | 1371 | 623 ± 50a | 2932 ± 124a,b | 3304 ± 127b | 2691 ± 76a,b | 2023 ± 73a,b |
| Methyl decanoatef | A | 1581 | 164 ± 3a | 981 ± 77a,v | 2066 ± 261a,b | 2260 ± 143b | 1617 ± 12a,b |
| Methyl salicylatef | A | 1762 | nq | nq | nq | 297 ± 13a | 374 ± 24b |
| Total of methyl esters | 991a | 4711a,b | 6761a,b | 6231v | 4540a,b | ||
| Others esters | |||||||
| Propyl hexanoatef | B | 1299 | nd | 425 ± 13a,b | 627 ± 31a,b | 836 ± 33a,b | 625 ± 7a,b |
| Propyl octanoatef | A | 1509 | nd | 406 ± 5a,b | 778 ± 85a,b | 1301 ± 45a,b | 1148 ± 50b |
| Propyl decanoatef | B | 1712 | nd | nq | 257 ± 38a,b | 858 ± 32a,b | 750 ± 41b |
| Isobutyl hexanoatef | B | 1335 | nd | 388 ± 15a,b | 662 ± 5a,b | 745 ± 73b | 465 ± 7a,b |
| Isobutyl octanoatef | A | 1542 | nd | 899 ± 19a,b | 1690 ± 171a,b | 3624 ± 136a,b | 3091 ± 74a,b |
| Isobutyl decanoate | B | 1746 | nd | nd | 277 ± 12a,b | 1364 ± 111a,b | 1570 ± 64b |
| Total of other ester | – | 2188a,b | 4291a,b | 8728a,b | 7650a,b | ||
| Ketones | |||||||
| 2-Pentanonee | A | 939 | 385 ± 45 | nd | nd | nd | nd |
| 3-Penten-2-onee | C | 1094 | nd | nd | nd | nd | nd |
| 2-Heptanonee | C | 1152 | 691 ± 44 | 234 ± 6b | 221 ± 14b | 205 ± 7b | nd |
| 2-Pentylfurane | B | 1196 | 29,825 ± 275 | 14,917 ± 489b | 13,853 ± 1139b | 1546 ± 71a,b | 571 ± 34a,b |
| 3-Octanonee | B | 1233 | 1845 ± 76 | 489 ± 7b | 540 ± 69b | 225 ± 1a,b | nq |
| 2-Octanonee | B | 1266 | 4118 ± 234 | 748.6 ± 1.4b | 564 ± 49a,b | 228 ± 14a,b | 180.7 ± 2.1a,b |
| trans-2,2-Pentenyl-furane | C | 1271 | 1456 ± 40 | 421 ± 3b | 392 ± 12b | nd | nd |
| Acetoind | A | 1273 | 650 ± 38 | 1522 ± 90b | 1664 ± 40b | 1477 ± 115b | 654.2 ± 0.6a |
| 1-Hydroxy-2-propanone | A | 1286 | 3346 ± 493 | 2970 ± 420 | 3160 ± 446 | 2883 ± 150 | 2782 ± 124 |
| 2-Hexylfurane | C | 1303 | 312.37 ± 0.17 | 355 ± 22 | 324 ± 6 | nq | nq |
| 6-Methyl-5-hepten-2-onee | A | 1319 | 4016 ± 71 | 5999 ± 292b | 5618 ± 205b | 3493 ± 152a | 2645 ± 259b |
| 1-Hydroxy-2-butanone | B | 1363 | 1442 ± 209 | 1339 ± 190 | 1572 ± 208 | 1271 ± 18 | 1427 ± 111 |
| 2-Nonanone | A | 1374 | 1628 ± 40 | 841 ± 27b | 793 ± 72b | 1517 ± 12a | 1023 ± 47a |
| 2-Acetilfurane | A | 1493 | 1033 ± 137 | 610 ± 9b | 637 ± 80 | 576 ± 79b | 427 ± 58b |
| Dihydro-3(2H)-thiophenone | C | 1515 | nd | nd | nd | nd | nd |
| 6-Methyl-3,5-heptadiene-2-onee | B | 1588 | 6926 ± 913a | 1677 ± 43a,b | 697 ± 65a,b | nq | nq |
| Acetofenone | A | 1641 | 750 ± 66 | 431 ± 10 | 356 ± 34b | nd | nd |
| 1,2-Cyclopentanedione | C | 1775 | 2838 ± 357 | 2861 ± 319 | 2912 ± 345 | 3460 ± 479 | 2619 ± 335 |
| Cyclotene | B | 1841 | 440 ± 47 | 369 ± 23 | 373 ± 49 | 321 ± 36 | 215 ± 3 |
| Total of ketones | 126,097 | 186,469b | 198,450b | 163,455 | 77,256a,b | ||
| Lactones | |||||||
| γ-Butyrolactonee | A | 1625 | 2743 ± 33a | 1837 ± 26a,b | 1607 ± 124b | 1344 ± 79b | 1223 ± 6b |
| γ-Nonalactonae | B | 2039 | 1208 ± 180 | 1502 ± 38 | 1432 ± 95 | 1025 ± 124 | 999 ± 20 |
| Total of lactones | 3951 | 3339b | 3039a,b | 2370a,b | 2222b | ||
| C 13-Norisoprenoids | |||||||
| TDNf | A | 1727 | 718 ± 54 | 887 ± 25 | 1006 ± 88 | 1067.4 ± 0.3 | 1017 ± 37 |
| β-Damascenonee | A | 1813 | 1071 ± 88a | 906 ± 3a,b | 819 ± 7a,b | 588 ± 39a,b | 600 ± 69b |
| β-Iononee | A | 1941 | nq | nq | nq | nd | nd |
| Total of C13-Norisoprenoids | 1789a | 1793b | 1825b | 1656b | 1616b | ||
| Terpenes | |||||||
| 1R-α-Pinenee | C | 976 | 1572 ± 190 | 1311 ± 74 | 1512 ± 22 | 878 ± 61a | 395 ± 32a,b |
| Roseoxide | C | 1119 | nd | nd | nd | 316 ± 26b | 331 ± 22b |
| Myrtenal | C | 1123 | 486 ± 19 | 893 ± 4b | 1148 ± 74a,b | 583 ± 14a | 330 ± 18a,b |
| Limonene | A | 1149 | 379 ± 23 | 880 ± 18b | 354 ± 8a | nq | nq |
| Cymenee | B | 1237 | 563 ± 26 | 317 ± 5b | 315 ± 9b | nd | nd |
| trans-Linalool oxidee | B | 1468 | nq | nq | nd | nd | nd |
| 2-Bornenee | C | 1517 | 616 ± 25a | 392 ± 5a,b | 364 ± 24b | 233 ± 32a,b | 189 ± 4b |
| Linaloole | A | 1542 | 219 ± 28a | 227 ± 5a,b | 238 ± 11 | nd | nd |
| Citronellolh | A | 1767 | nd | 488 ± 15a,b | 535 ± 31b | 615 ± 58b | 673 ± 41b |
| α-Calacorenee | B | 1901 | nd | nd | nd | nd | nd |
| Nerolidolf | A | 2037 | nd | nd | nd | 370 ± 40a,b | 403 ± 26b |
| n.i. (m/z 69, 93, 121)f | – | 1743 | nd | 1029 ± 48a,b | 1326 ± 109b | 830 ± 99a,b | 693 ± 68b |
| Total of terpenes | 3833a | 5536a,b | 5793b | 3825a,b | 3015a,b | ||
| Volatile phenols | |||||||
| Guaiacole | A | 1858 | nq | nq | nq | nq | nq |
| 4-Vinylguaiacolf | A | 2203 | nq | 274 ± 32a,b | 465 ± 47a,b | 890 ± 7a,b | 1976 ± 110a,b |
| Coumarane | C | 2406 | 174 ± 10 | 187 ± 7 | 214 ± 29 | 176 ± 11 | 229 ± 6a |
| Total of volatile phenols | 174 | 460a | 679a | 1066a,b | 2204a,b | ||
| Others compounds | |||||||
| 2-Methylpyrazinee | A | 1256 | 619 ± 75a | 327 ± 7a | 233 ± 15a | nq | nq |
| Indole | B | 2436 | 182.2 ± 0.9a | 806 ± 29a,b | 1196 ± 34a,b | 316 ± 26a | nq |
| Unidentified compounds | |||||||
| n.i. (m/z 59, 43) | – | 1328 | nq | 461 ± 14a,b | 456 ± 25b | 720 ± 35a,b | 506 ± 11a,b |
| n.i. (m/z 67, 85, 151)e | – | 1395 | 1180 ± 38 | 1249 ± 19 | 1403 ± 13a | 755 ± 5a,b | 419 ± 24a,b |
| n.i. (m/z 55,88, 101)f | – | 1887 | nd | 192 ± 3a,b | 774 ± 97a,b | 4071 ± 330a,b | 5952 ± 17a,b |
| n.i. (m/z 126, 73)e | – | 2106 | 788 ± 49a | nq | 317 ± 34a,b | nq | nq |
ID: reliability of identification: A, mass spectrum and LRI agreed with standards; B, mass spectrum agreed with mass spectral data base and LRI agreed with the literature data; C, mass spectrum agreed with mass spectral data base
nd: peak not detected or lower than detection limit (a signal-to-noise ratio higher than or equal to 3); nq: lower than quantification limit (a signal-to-noise ratio higher than or equal to 10)
aThere is significant different (p < 0.05) with previous sample
bThere is significant different (p < 0.05) with substrate, only for samples from 48 to 192 h
cValue of peak area and sd have been divided per 1000
dValue of peak area and sd have been divided per 100,000
eVariable highly correlated with substrate and T24 (non-Saccharomyces and Saccharomyces)
fVariable highly correlated with samples from T48 to T192 Saccharomyces
The extraction method was highly reproducible, among the 11 extractions performed in duplicate only in 6 of them, RSDs next to 15% were obtained for just 12–16 volatile compounds, that is, 9–11% of compounds determined. These compounds were primary acids followed by ketones and aldehydes.
Regarding the volatile profile of the substrate stood out alcohols, ketones and aldehydes as chemical groups with high values of total peak area (Tables 2, 3). In comparison with the other sampling points, we observed that the substrate presented the lowest values of total peak area for alcohols, ethyl and acetic esters, and the highest for aldehydes and C13-norisoprenoids. Some compounds were only detected in the substrate such as cis-2-hexen-1-ol, several aldehydes, ethyl 2-methylbutyrate, 3-penten-2-one, trans-linalool oxide, α-calacorene (TI), guaiacol, whilst isoamyl and others esters were not detected in it.
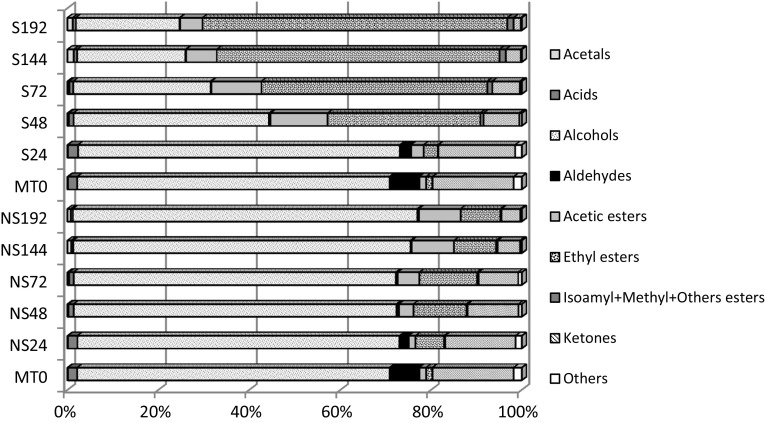
Figure 1, which groups the compounds according to their chemical classes, shows clearly the change in volatile profile throughout fermentation processes studied. The primary change is the importance acquired by ethyl esters during fermentation carried out by Saccharomyces strain, which implied a decrease of the proportion of alcohols and acetates. Whereas fermentation carried out by L. thermotolerans strain did not reveal a pronounced increase in ethyl esters, for this reason, in this case, alcohols continued to be the group of compounds that contributed more to volatile profile. Moreover, the percentage of ketones decreased during both types of fermentations.
Fig. 1.
Contribution (%) of different chemical groups of volatile compounds to the volatile profile of samples(S = S. cerevisiae and NS = L. thermotolerans)
In general, the two strains used in this study provided different volatile profile. Thus, the higher numbers of compounds with peak area values significantly different between strains were observed in the last sampling points (T144 and T192), 83 and 78 respectively. For most of these compounds, the values were higher when fermentation was carried out by S. cerevisiae than L. thermotolerans.
Acetals
The total content of acetals increased along fermentation. This increase was higher for S. cerevisiae than L. thermotolerans strain, reaching significant different values at 48 h after inoculation. Three different acetals were determined. Acetaldehyde diethyl acetal and acetaldehyde ethyl amyl acetal increased in both fermentation processes reaching to maximum area values at 144 h after inoculation. Saccharomyces strain produced considerable increased being the highest amount for the first compound four times than that produced by the other strain at the final sampling point.
On the other hand, an opposite trend between both strains was observed for 2,4,5-trimethyl-1,3-dioxolane that decreased with the use of non-Saccharomyces strain and increased with the Saccharomyces one.
Acids
Regarding acids, after 192 h of fermentation the overall balance was increased for of acidity for wines produced by Saccharomyces strain and a decrease for wines produced by non-Saccharomyces. Although these compounds have unpleasant aromas (Beckner et al. 2015), they are precursors of esters which provided fruity aromas to wines. Saerens et al. (2006) verified that the addition of hexanoic or octanoic acid to the fermentation medium caused a strong increase in the formation of the corresponding ethyl ester.
The evolution of each acid throughout fermentation was very different among compounds and between strains. Pentanoic acid clearly diminished in both cases. However, contrary trends between strains were observed specially for octanoic, decanoic and hexanoic acids. In the case of Saccharomyces strain, the highest increase was for octanoic acid.
Similar results were reported by Gobbi et al. (2013) and Beckner et al. (2015), who found higher volatile acidity and total amount of carboxylic acids in wines produced by S. cerevisiae than those by L. thermotolerans.
Alcohols
During fermentation, an increase in alcohols was observed. As expected, the alcohol that underwent the highest augmentation was ethanol, with the most important change between 24 and 48 h. The use of non-Saccharomyces yeast to produce wine with reduced alcohol content was reported earlier (Contreras et al. 2015; Quiros et al. 2014). Gobbi et al. (2013) reported that L. thermotolerans as little ethanol producers. However, in our study the same rate of ethanol production for both yeasts was observed and there were no significant differences between any of the stage analysed between strains. This agreed with the above stated relation of sugar consumption and the origin of both autochthonous yeast which were isolated during the spontaneous fermentation of sun-dried grape must and, thus, have developed a great adaptation to high osmotic pressure media. In addition to ethanol, among 23 alcohols determined, other 6 alcohols increased, standing out 3-methyl-1-butanol and 2-phenyletanol. Most of these were higher alcohols which were produced by yeast involving degradation of an amino via the Ehrlich pathway (Ugliano and Henschke 2009).
The alcohol global augmentation were significantly higher when the fermentation was carried out by non-Saccharomyces strain (significant different at T144 and T192). It seemed to be due to the higher increase of 3-methyl-1-butanol in this process. Moreover, some authors have reported higher production of 2-phenyletanol by L. thermotolerans (Gobby et al. 2013), in our case, it was observed in last fermentation stages (6 and 8 days), where the peak areas were two time higher in wine produced by aforesaid strain.
On the contrary, some alcohols decreased, especially, 1-hexenol and 1-octen-3-ol. The decrease was more pronounced between 24 and 48 h.
Aldehydes
Regarding total sum of aldehydes, the values followed a similar trend in both types of fermentations, decreasing significantly until 48 h.
Most aldehydes reached relative peak area under detection limits at 24 h from inoculation. Only furanic aldehydes, cinnamaldehyde and benzaldehyde presented quantifiable values at all sampling points throughout the fermentation process.
Acetic esters
The acetic esters are compounds where the acyl group is derived from acetate (in the form of acetyl-CoA), and the alcohol group is ethanol or a complex alcohol (Cordente et al. 2012). During alcoholic fermentation, these are synthesised by different alcohol acetyltransferases (Ugliano and Henschke 2009).
In present study, these compounds increased especially during fermentation by Saccaromyces strain. The changes were significant until 72 h from inoculation, after that, a decrease was observed. Non-Saccharomyces strain showed less pronounced increase which continued until the sampling point of 144 h, thus a good correlation between relative area values and the time was observed (0.949). Overall, acetic esters content in all the stages were significant higher for Saccharomyces strain. The difference observed between strains may be probably due to the high values of relative area accounted for compounds such as hexyl and 2-phenylethanol acetate and to the six acetic esters that were formed by Saccharomyces strain only. Among all the acetic esters determined, the highest increase was accounted by isoamyl acetate for both strains, the most relevant acetates of the wines.
Most acetic esters have pleasant fruity and flower aromas (Lilly et al. 2006), however, ethyl acetate provides solvent and glue odour (Callejón et al. 2008). This compound presented area values significantly higher after 144 and 192 h of fermentation by L. thermotolerans, as observed by Gobbi et al. (2013). Although non-Sacharomyces strain produced higher amount of 2-phenylethanol (approximately two-fold), the values of the corresponding acetate reached area values 20 times higher in wines obtained by Saccharomyces at the final stages of alcoholic fermentation.
Ethyl esters
Ethyl esters are formed by ethanol and an acyl group derived from activated medium-chain fatty acids (Cordente et al. 2012). During Saccharomyces cerevisiae fermentation, the formation of the ethyl esters has been attributed to two acyl-CoA:ethanol O-acyltransferase enzymes (Saerens et al. 2008).
As mentioned above, the primary difference between the two yeast strains studied was the different rate of production of ethyl esters. After 48 h from inoculation, the values of total area of ethyl esters were significantly higher for Saccharomyces strain, being more than 15 times higher at the two last sampling points (T144 and T192). Beckner et al. (2015) also observed a considerable difference between the amount of ethyl esters produced by Sacharomyces and Lachancea yeast strains.
Thus, it led us to think that S. cerevisiae probably produced more amount of ethanol than L. thermotolerans but it was in form of ethyl ester, so that no differences were observed in ethanol production between strains.
Moreover, the evolution of these values was different for both yeast strains, during alcoholic fermentation by Lachancea strain a significant increase was observed until 48 h from inoculation. However, Saccharomyces cerevisiae produced ethyl esters continuously throughout the fermentation, for that, the correlation coefficient between total area of ethyl esters and the fermentation time was 0.954. The increase observed between each sampling point were statistically significant.
Thus, the values of peak area were higher for Sacharomyces yeast for the most of these compounds except to ethyl propanoate or ethyl 2-methylpropanoate.
During alcoholic fermentation carried out by S. cerevisiae, the highest increase was observed for ethyl octanoate and ethyl decanoate. Other remarkable increments were observed for ethyl hexanoate, ethyl dodecanoate and ethyl 9-decanoate.
In the case of L. thermotolerans, the ethyl decanoate was the ester that showed a greater increase during the fermentation, but in a much lesser extent than in fermentation by S. cerevisiae.
Since most of determined esters in this study have fruity aromas, probably wines produced using Saccharomyces strain may have more fruity aroma than those produced with Lachancea strain.
Others esters
In this study, we have also determined others esters formed by alcohols such as methanol, isoamyl alcohol, propanol and isobutanol, previously reported in wines (Beckner et al. 2015; Suklje et al. 2016). Different behaviour with respect to these compounds was also observed between both yeast strains tested. The total areas of these esters were significantly higher for S. cerevisiae than for L. thermotolerans in all sampling points from 48 h. On the contrary, isoamyl esters did not increase to a greater extent during fermentation carried out by Lachancea, methyl esters became non detectable in most of cases and, the only isobutyl ester determined was isobutyl decanoate.
Within this group of esters, S. cerevisiae caused the most considerable increase in isoamyl esters, being the total areas changed significant from 48 to 144 h. Moreover, for S. cerevisiae, the formation of esters derived from octanoic acid was more clearly over the others (isoamyl, methyl, propyl and isobutyl octanoate).
Ketones, lactones, C13-norisoprenoids and terpenes
Most of compounds included in this section came from the grapes (Ribéreau-Gayon et al. 2006). They may be present as glycosylated flavourless precursors, such as terpenes and C13-norisoprenoids and they were released by enzymatic hydrolysis during alcoholic fermentation.
Nevertheless, several authors have reported that neither Sacharomyces cerevisiae (Van Rensburg et al. 2005) nor Lachancea thermotolerans (Comitini et al. 2011) seemed to have glycosidase activity.
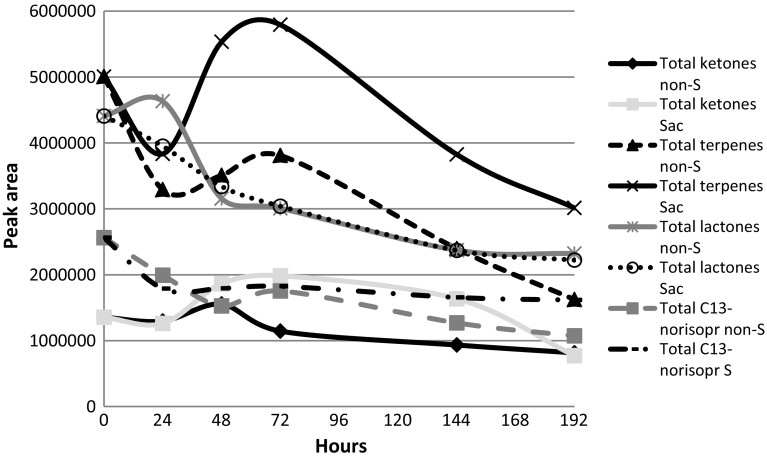
In our assays, the overall changes in total areas of these groups of compounds were significantly decreased between initial and final values (192 h). The evolution of total area for each group was fluctuating for both strains and only a similar trend was observed for terpenes (Fig. 2).
Fig. 2.
Evolution of total area of ketones, terpenes and lactones in fermentation processes with L. thermotolerans and S. cerevisiae: diamond ketone L. thermotolerans, square ketone S. cerevisiae, traingle terpenes L. thermotolerans, cross terpenes S. cerevisiae, asterisks lactones L. thermotolerans, circle lactones S. cerevisiae, grey square C13-norisoprenoids L. thermotolerans, dots C13-norisoprenoids S. cerevisiae
Despite the downward trend of terpenes, we observed that three of them, roseoxide, 3,7-dimethyl-6-octen-1-ol and nerolidol, increased using both yeasts. The first one especially in the case of L. thermotolerans and the last two when fermentations was carried out by S. cerevisae.
Volatile phenols
Regarding volatile phenols, the behaviour of these strains was also different, especially for 4-vinylguaiacol. This compound increased significantly from 48 h onwards when alcoholic fermentation was carried out by Saccharomyces. This yeast can synthesize 4-vinylguicacol during fermentation (Coghe et al. 2004).
Principal component analysis
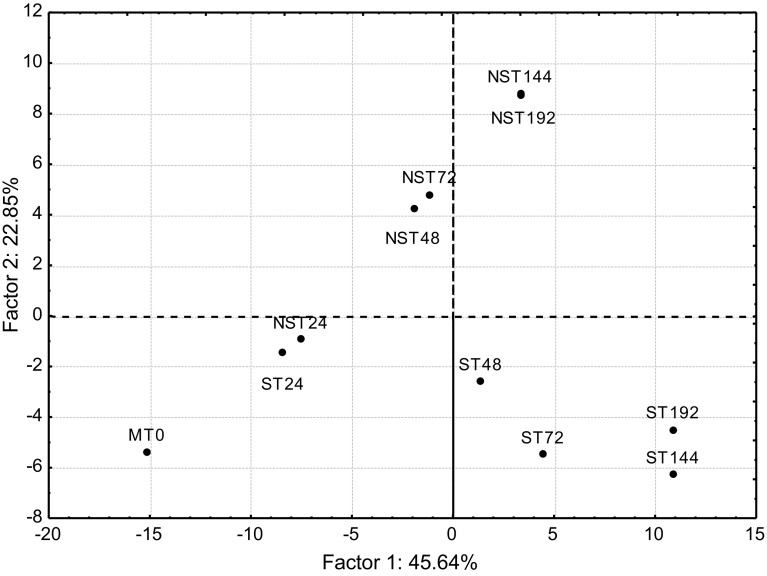
Principal component analysis (PCA) was applied to data. The first three principal components explained 81.76% of cumulative variance. Figure 3 shows how the samples are separated into the plan formed by two first components. In this Figure, it can clearly be seen that the differences in volatile profile of samples produced by the two yeasts are considerably different from 48 h of inoculation. Thus, the initial and at 24 h samples for S. cerevisiae as well as for L. thermotolerans are together in the same quadrant (second one). The PC1 separates these samples from the rest of those obtained using S. cerevisiae, placed all in the third quadrant. Finally, the samples belonging to fermentations carried out by L. thermotolerans, from 48 to 192 h, are separated from S. cerevisiae by PC2. Table 2 showed the variables that are more correlated with these three groups according to their loadings. For instance, initial samples are correlated with most of aldehydes, terpenes and ketones and samples from Saccharomyces fermentations with most of acids and all kind of esters. Moreover, the variables that contributed more to the two first components with their loading values are shown in Table 4.
Fig. 3.
Data scores of all samples plot on the plan made up of the first two principal components (PC1 against PC2)
Table 4.
Variables with high contribution to factor 1 and 2 in PCA, their loading values and sample groups with which these are correlated
| Volatile compounds | Sample group | Loading values | |
|---|---|---|---|
| F1 | F2 | ||
| Isovaleric acid | MT0, NST24 and ST24 | −0.073760 | −0.772098 |
| 1-Pentanolg | MT0, NST24 and ST24 | −0.937031 | −0.296206 |
| 1-Hexanol | MT0, NST24 and ST24 | −0.931785 | −0.297974 |
| 1-Octen-3-ol | MT0, NST24 and ST24 | −0.947265 | −0.289980 |
| 1-Heptanol | MT0, NST24 and ST24 | −0.918735 | −0.279695 |
| 2-Ethyl-1-hexanol | MT0, NST24 and ST24 | −0.833305 | −0.327027 |
| 1-Octanol | MT0, NST24 and ST24 | −0.722133 | −0.633091 |
| cis-2-Octen-1-ol | MT0, NST24 and ST24 | −0.903878 | −0.366522 |
| Benzyl alcohol | MT0, NST24 and ST24 | −0.948099 | −0.270730 |
| 2-Furfuraldehyde | MT0, NST24 and ST24 | −0.881613 | −0.358810 |
| Cinnamaldehyde | MT0, NST24 and ST24 | −0.917130 | −0.040580 |
| 2-Pentanone | MT0, NST24 and ST24 | −0.855324 | −0.324833 |
| 2-Pentylfuran | MT0, NST24 and ST24 | −0.899011 | −0.271896 |
| 3-Octanone | MT0, NST24 and ST24 | −0.915904 | −0.067750 |
| 2-Octanone | MT0, NST24 and ST24 | −0.845447 | −0.325230 |
| trans-2,2-Pentenyl-furan | MT0, NST24 and ST24 | −0.921479 | −0.281489 |
| 6-Methyl-5-hepten-2-one | MT0, NST24 and ST24 | −0.204803 | −0.798575 |
| 6-Methyl-3,5-heptadiene-2-one | MT0, NST24 and ST24 | −0.928428 | −0.290765 |
| γ-Butyrolactone | MT0, NST24 and ST24 | −0.928418 | −0.274416 |
| β-Damascenone | MT0, NST24 and ST24 | −0.909969 | −0.366544 |
| 1R-α-Pinene | MT0, NST24 and ST24 | −0.558796 | −0.619010 |
| Cymene | MT0, NST24 and ST24 | −0.892236 | −0.335194 |
| 2-Bornene | MT0, NST24 and ST24 | −0.944324 | −0.309547 |
| Linalool | MT0, NST24 and ST24 | −0.836326 | −0.387011 |
| Coumaran | MT0, NST24 and ST24 | −0.165912 | −0.885934 |
| n.i. (m/z 67, 85, 151) | MT0, NST24 and ST24 | −0.517886 | −0.685602 |
| Acetaldehyde ethyl amyl acetal | ST48, ST72, ST144 and ST192 | 0.819661 | −0.109366 |
| Acetic acid | ST48, ST72, ST144 and ST192 | 0.252113 | −0.805874 |
| Propanoic acid | ST48, ST72, ST144 and ST192 | 0.094181 | −0.623983 |
| Hexanoic acid | ST48, ST72, ST144 and ST192 | 0.346509 | −0.905755 |
| Octanoic acid | ST48, ST72, ST144 and ST192 | 0.692898 | −0.681746 |
| Decanoic acid | ST48, ST72, ST144 and ST192 | 0.727791 | −0.604349 |
| 1-Propanol | ST48, ST72, ST144 and ST192 | 0.920658 | −0.026868 |
| 4-Ethylbenzyl alcohol | ST48, ST72, ST144 and ST192 | 0.597740 | −0.638649 |
| 1-Decanol | ST48, ST72, ST144 and ST192 | 0.590222 | −0.636173 |
| Propyl acetate | ST48, ST72, ST144 and ST192 | 0.925594 | −0.128528 |
| Isoamyl acetate | ST48, ST72, ST144 and ST192 | 0.874614 | −0.211771 |
| Amyl acetate | ST48, ST72, ST144 and ST192 | 0.586742 | −0.624018 |
| Hexyl acetate | ST48, ST72, ST144 and ST192 | 0.508613 | −0.609353 |
| Heptyl acetate | ST48, ST72, ST144 and ST192 | 0.448069 | −0.582699 |
| Octyl acetate | ST48, ST72, ST144 and ST192 | 0.576516 | −0.627550 |
| Nonyl acetate | ST48, ST72, ST144 and ST192 | 0.574227 | −0.624231 |
| Decyl acetate | ST48, ST72, ST144 and ST192 | 0.746226 | −0.642165 |
| 2-Phenylethanol acetate | ST48, ST72, ST144 and ST192 | 0.570674 | −0.573411 |
| Ethyl butyrate | ST48, ST72, ST144 and ST192 | 0.884764 | −0.133885 |
| Ethyl hexanoate | ST48, ST72, ST144 and ST192 | 0.793592 | −0.597680 |
| Ethyl heptanoate | ST48, ST72, ST144 and ST192 | 0.878184 | −0.376239 |
| Ethyl octanoate | ST48, ST72, ST144 and ST192 | 0.769181 | −0.604613 |
| Ethyl 7-octenoate | ST48, ST72, ST144 and ST192 | 0.819209 | −0.346015 |
| Ethyl undecanoate | ST48, ST72, ST144 and ST192 | 0.737432 | −0.588533 |
| Isoamyl hexanoate | ST48, ST72, ST144 and ST192 | 0.743734 | −0.637847 |
| Methyl hexanoate | ST48, ST72, ST144 and ST192 | 0.541028 | −0.641413 |
| Methyl decanoate | ST48, ST72, ST144 and ST192 | 0.635654 | −0.697817 |
| Propyl hexanoate | ST48, ST72, ST144 and ST192 | 0.727725 | −0.671690 |
| Propyl octanoate | ST48, ST72, ST144 and ST192 | 0.752368 | −0.645882 |
| Isobutyl hexanoate | ST48, ST72, ST144 and ST192 | 0.693044 | −0.670428 |
| Isobutyl octanoate | ST48, ST72, ST144 and ST192 | 0.750106 | −0.624371 |
| TDN | ST48, ST72, ST144 and ST192 | 0.418331 | −0.738714 |
| Citronellol | ST48, ST72, ST144 and ST192 | 0.877780 | −0.324558 |
| n.i. (m/z 69, 93, 121) | ST48, ST72, ST144 and ST192 | 0.581783 | −0.628157 |
| 3-Methylthio-1-propanol | NST48 and NST72 | −0.007238 | 0.652629 |
| Acetofenone | NST48 and NST72 | −0.931971 | 0.104946 |
| Ethanol | NST144 and NST192 | 0.923472 | 0.251117 |
| Isobutanol | NST144 and NST192 | 0.280307 | 0.860025 |
| 2-Methyl-1-butanol | NST144 and NST192 | 0.868649 | 0.436190 |
| 3-Methyl-1-butanol | NST144 and NST192 | 0.444480 | 0.808629 |
| 6-Methyl-5-hepten-2-ol | NST144 and NST192 | 0.357365 | 0.703500 |
| 2-Phenylethanol | NST144 and NST192 | 0.644828 | 0.588573 |
| Ethyl acetate | NST144 and NST192 | 0.617928 | 0.574295 |
| Ethyl propanoate | NST144 and NST192 | 0.264100 | 0.835185 |
| Ethyl 2-methylpropanoate | NST144 and NST192 | 0.166192 | 0.932632 |
| Ethyl phenylacetate | NST144 and NST192 | 0.364038 | 0.729613 |
| Ethyl tetradecanoate | NST144 and NST192 | 0.847124 | 0.055234 |
| Isoamyl propionate | NST144 and NST192 | 0.519286 | 0.655810 |
| Roseoxide | NST144 and NST192 | 0.444037 | 0.694096 |
| n.i. (m/z 59, 43) | NST144 and NST192 | 0.834766 | 0.393159 |
Conclusions
HSSE method allows for monitoring a large number of compounds throughout fermentation. Thus, these results point out the HSSE as useful non-invasive method to study the evolution of volatile compounds during fermentation processes. It could be used to establish the optimal point to stop the fermentation according to volatile profile and moreover, it could be very useful to study the aroma evolution in co-inoculation assays and sequential inoculation, which are of great interest currently.
In this study, considerable changes in volatile compounds were observed from substrate to final sampling point. The two strains used had a similar capacity to ferment a must with high sugar content. However, they resulted into the wines with different aroma. S. cerevisiae produced higher amount of volatile compounds than L. thermotorelans. Moreover, wines produced by S. cerevisiae strain were richer in esters imparted fruity aroma. This showed that this strain could produce wines with better aromatic and volatile profile than those produced by non-Saccharomyces strain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for financial support of FEDER funds (Project RM2010-00009-C03-03 from INIA-Ministerio de Ciencia e Innovación). We also appreciate the kindness of Bodegas del Pino for facilitating grape must for fermentation trials.
References
- Beckner ME, Carlin S, Jacobson D, Weighill D, Divol B, Conterno L, Du Toit M, Vrhovsek U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: a targeted approach. LWT Food Sci Technol. 2015;64:412–422. doi: 10.1016/j.lwt.2015.05.018. [DOI] [Google Scholar]
- Benito A, Calderón F, Palomero F, Benito S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules. 2015;20:9510–9523. doi: 10.3390/molecules20069510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss PK, Pearce AD, Zhao Y, Nicholson EL, Dennis EG, Jeffery DW. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules. 2015;20:7845–7873. doi: 10.3390/molecules20057845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejón RM, Morales ML, Silva Ferreira AC, Troncoso AM. Defining the typical aroma of Sherry vinegar: sensory and chemical approach. J Agric Food Chem. 2008;56:8086–8095. doi: 10.1021/jf800903n. [DOI] [PubMed] [Google Scholar]
- Callejón RM, Clavijo A, Ortigueira P, Troncoso AM, Paneque P, Morales ML. Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal Chim Acta. 2010;660:68–75. doi: 10.1016/j.aca.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Callejón RM, Margulies B, Hirson GD, Ebeler SE. Dynamic changes in volatiles compounds during fermentation of Cabernet Sauvignon grapes with and without skins. Am J Enol Vitic. 2012;63:301–312. doi: 10.5344/ajev.2012.12009. [DOI] [Google Scholar]
- Clavijo A, Calderón IL, Paneque P. Yeast assessment during alcoholic fermentation inoculated with a natural ‘‘pied de cuve’’ or a commercial yeast strain. World J Microb Biotechnol. 2011;27:1569–1577. doi: 10.1007/s11274-010-0609-y. [DOI] [Google Scholar]
- Coghe S, Benoot K, Delvaux F, Vanderhaegen B, Delvaux FR. Ferulic acid release and 4-vinyl guaiacol formation during brewing and fermentation: indications for feruloyl esterase activity in Saccharomyces cerevisiae. J Agric Food Chem. 2004;52:602–608. doi: 10.1021/jf0346556. [DOI] [PubMed] [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciania M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Concejero B, Hernandez-Orte P, Astrain J, Lacau B, Baron C, Ferreira V. Evolution of polyfunctional mercaptans and their precursors during Merlot alcoholic fermentation. LWT Food Sci Technol. 2016;65:770–776. doi: 10.1016/j.lwt.2015.09.018. [DOI] [Google Scholar]
- Contreras A, Hidalgo C, Schmidt S, Henschke PA, Curtin C, Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int J Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Cordente AG, Curtin CD, Varela C, Pretorius IS. Flavour-active wine yeasts. Appl Microbiol Biotechnol. 2012;96:601–618. doi: 10.1007/s00253-012-4370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray JA, Bell ANW, Bhaganna P, Mswaka AY, Timson DJ, Hallsworth JE. The biology of habitat dominance; can microbes behave as weeds? Microb Biotechnol. 2013;6:453–492. doi: 10.1111/1751-7915.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet GH, Heard GM. Yeast: growth during fermentation. In: Fleet GH, editor. Wine microbiology and biotechnology. Chur: Harwood Academic Publishers; 1993. pp. 27–54. [Google Scholar]
- Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Guillamón JM, Sabate J, Barrio E, Cano J, Querol A. Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch Microbiol. 1998;169:387–392. doi: 10.1007/s002030050587. [DOI] [PubMed] [Google Scholar]
- Jolly NP, Varela C, Pretorius IS. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–237. doi: 10.1111/1567-1364.12111. [DOI] [PubMed] [Google Scholar]
- Lancas FM, Queiroz MEC, Grossi P, Olivares IRB. Recent developments and application of stir bar sorptive extraction. J Sep Sci. 2009;32:313–824. doi: 10.1002/jssc.200800669. [DOI] [PubMed] [Google Scholar]
- Lilly M, Bauer FF, Lambrechts MG, Swiegers JH, Cozzolino D, Pretorius IS. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast. 2006;23:641–659. doi: 10.1002/yea.1382. [DOI] [PubMed] [Google Scholar]
- MAPA (1993) Métodos Oficiales de Análisis. Tomo II. Sec. Gral. Técnica. Ministerio de Agricultura, Pesca y Alimentación, Madrid
- Martinez-Gil AM, Pardo-Garcia AI, Zalacain A, Alonso GL, Salinas MR. Lavandin hydrolat applications to Petit Verdot vineyards and their impact on their wine aroma compounds. Food Res Int. 2013;53:391–402. doi: 10.1016/j.foodres.2013.05.012. [DOI] [Google Scholar]
- Mouret JR, Cadiere A, Aguera E, Rollero S, Ortiz-Julien A, Sablayrolles JM, Dequin S. Dynamics and quantitative analysis of the synthesis of fermentative aromas by an evolved wine strain of Saccharomyces cerevisiae. Yeast. 2015;32:257–269. doi: 10.1002/yea.3028. [DOI] [PubMed] [Google Scholar]
- Querol A, Barrio E, Huerta T, Ramón D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl Environ Microbiol. 1992;58:2948–2953. doi: 10.1128/aem.58.9.2948-2953.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros M, Rojas V, Gonzalez R, Morales P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int J Food Microbiol. 2014;181:85–91. doi: 10.1016/j.ijfoodmicro.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Renault P, Coulon J, de Revel G, Barbe JC, Bely M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int J Food Microbiol. 2015;207:40–48. doi: 10.1016/j.ijfoodmicro.2015.04.037. [DOI] [PubMed] [Google Scholar]
- Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of enology. Chichester: Wiley; 2006. [Google Scholar]
- Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am J Enol Vitic. 2014;65:1–24. doi: 10.5344/ajev.2013.12070. [DOI] [Google Scholar]
- Romano P, Pietrafesa R, Romaniello R, Zambuto M, Calabretti A, Capece A. Impact of yeast starter formulations on the production of volatile compounds during wine fermentation. Yeast. 2015;32:245–256. doi: 10.1002/yea.3034. [DOI] [PubMed] [Google Scholar]
- Saerens SMG, Verstrepen KJ, Van Laere SDM, Voet ARD, Van Dijck P, Delvaux FR, Thevelein JM. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem. 2006;281:4446–4456. doi: 10.1074/jbc.M512028200. [DOI] [PubMed] [Google Scholar]
- Saerens S, Delvaux F, Verstrepen K, Van Dijck P, Thevelein J, Delvaux F. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol. 2008;74:454–461. doi: 10.1128/AEM.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Ferreira AC, Monforte AR, Silva Teixeira C, Martins R, Fairbairn S, Bauer FF. Monitoring alcoholic fermentation: an untargeted approach. J Agric Food Chem. 2014;62:6784–6793. doi: 10.1021/jf502082z. [DOI] [PubMed] [Google Scholar]
- Suárez-Lepe JA, Morata A. New trends in yeast selection for winemaking. Trends Food Sci Technol. 2012;23:39–50. doi: 10.1016/j.tifs.2011.08.005. [DOI] [Google Scholar]
- Suklje K, Antalick G, Buica A, Langlois J, Coetzee ZA, Gouot J, Schmidtke LM, Deloire A. Clonal differences and impact of defoliation on Sauvignon blanc (Vitis vinifera L.) wines: a chemical and sensory investigation. J Sci Food Agric. 2016;96:915–926. doi: 10.1002/jsfa.7165. [DOI] [PubMed] [Google Scholar]
- Synos K, Reynolds AG, Bowen AJ. Effect of yeast strain on aroma compounds in Cabernet Franc icewines. LWT Food Sci Technol. 2015;64:227–235. doi: 10.1016/j.lwt.2015.05.044. [DOI] [Google Scholar]
- Ugliano M, Henschke PA. Yeasts and wine flavour. In: Moreno-Arribas AV, Polo MC, editors. Wine chemistry and biochemistry. New York: Springer; 2009. pp. 313–392. [Google Scholar]
- Van Rensburg P, Stidwell T, Lambrechts MG, Cordero Otero RR, Pretorius IS. Development and assessment of a recombinant Saccharomyces cerevisiae wine yeast producing two aroma-enhancing β-glucosidases encoded by the Saccharomycopsis fibuligera BGL1 and BGL2 genes. Ann Microbiol. 2005;55:33–42. [Google Scholar]
- Vilanova M. Sensory descriptive analysis and consumer acceptability of Godello wines from Valdeorras appellation origen controlee (North West Spain) J Sens Stud. 2006;21:362–372. doi: 10.1111/j.1745-459X.2006.00071.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.