Abstract
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a relatively new class of injectable drugs used in the treatment of type 2 diabetes (T2D). This retrospective database study evaluated real-world treatment patterns of T2D patients initiating GLP-1 RAs in Belgium (BE), France (FR), Germany (DE), The Netherlands (NL) and Sweden (SE).
Methods
Adult T2D patients initiating exenatide twice daily (exBID), exenatide once weekly (exQW), liraglutide (LIRA) or lixisenatide (LIXI) during 2013 were identified using the QuintilesIMS (QuintilesIMS, Durham, NC, and Danbury, CT, USA) longitudinal retail pharmacy databases (LRx; BE/FR/DE/NL) and national health register data (SE). Therapy initiation date was termed ‘index date.’ Eligible patients had ≥180-day pre- and variable follow-up (minimum ≥360 days post-index). Baseline patient and treatment characteristics were assessed. Treatment modification and persistence were evaluated over the 1-year follow-up. Kaplan-Meier (KM) survival curves evaluated stopping of the index therapy (first of discontinuation or switch) over the available follow-up.
Results
A total of 4339 exBID, 1499 exQW, 20,955 LIRA and 1751 LIXI patients were included in the analysis (45.1–61.9% female; mean age range 57.1–62.9 years). Mean follow-up ranged from 17.7 to 30.7 months. Across countries/databases, the proportion experiencing a treatment modification at 1-year ranged from 84.1 to 93.8% for exBID, 53.3–73.4% for exQW and 59.5–80.5% for LIRA patients. The proportion of LIXI patients with treatment modification was 55.0% in Belgium (N = 20) and 96.9% in Germany (LIXI taken off the German market in April 2014). In KM analyses, LIRA patients had the lowest proportion stopping therapy, while exBID patients had the highest proportion stopping therapy, across databases, with the exception of LIXI patents.
Conclusion
Treatment patterns varied among GLP-1 RA patients, and persistence was generally highest among LIRA and lowest among exBID across countries. Longer term data would be useful, given the recent approval of several GLP-1 RA therapies.
Funding: Eli Lilly and Co., Indianapolis, IN, USA.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-016-0224-5) contains supplementary material, which is available to authorized users.
Keywords: Exenatide BID, Exenatide QW, Diabetes mellitus, Glucagon-like peptide 1/analogs and derivatives, Glucagon-like peptide 1/therapeutic use, Type 2/drug therapy, Liraglutide, Lixisenatide, Persistence, Retrospective studies, Treatment patterns
Introduction
The International Diabetes Federation estimates that in 2015 there were 59.8 million adults with diabetes in Europe (EU), representing 9.1% of adults, including 23.5 million cases that are undiagnosed [1]. Up to 91% of adults with diabetes in high-income countries have type 2 diabetes (T2D) [1]. This has substantial cost implications to healthcare systems and society, with approximately 12% of global health expenditures spent on diabetes in 2015 [1]. Most patients with T2D will require drug therapy with an antihyperglycemic agent to help in regulating glucose control through reductions in hepatic glucose production, stimulation of insulin release, regulation of insulin and glucagon secretion, or improvement of insulin sensitivity [2].
Metformin, an oral antihyperglycemic medication (OAM), is recommended for most as the optimal drug for initial monotherapy [2]. Over time, combination therapy is needed, and the updated 2015 joint position statement released by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommends one of six treatment classes combined with metformin: an OAM including a sulfonylurea, thiazolidinediones (TZD), dipeptidyl peptidase-4 (DPP-4) inhibitor or sodium–glucose cotransporter 2 (SGLT2) inhibitor, or an injectable antihyperglycemic agent including a glucagon-like peptide-1 receptor agonist (GLP-1 RA) or basal insulin [2]. Progressive beta-cell dysfunction prevents many patients from maintaining adequate glycemic control with OAMs over the long term, and many patients will eventually also require injectable glucose-lowering therapies [3, 4].
Glucagon-like peptide-1 receptor agonists are a relatively new class of injectable drugs used in the treatment of T2D. GLP-1 RAs mimic endogenous GLP-1, stimulating insulin release from the pancreas, suppressing glucagon secretion, slowing gastric emptying and increasing satiety [2]. There are several GLP-1 RAs that have been approved by the European Medicines Agency (EMA). Exenatide twice-daily (BID; Byetta®, AstraZeneca) was first in class and approved by the EMA in 2006, followed by liraglutide (Victoza®, Novo Nordisk) in 2009 and exenatide once weekly (Bydureon®, AstraZeneca) in 2011 [5–7]. GLP-1 RAs recently approved in Europe include lixisenatide (Lyxumia®, Sanofi) in February 2013 (not yet approved by the Food and Drug Administration in the USA), albiglutide (Eperzan®, GlaxoSmithKline) in April 2014 and dulaglutide (Trulicity®, Eli Lilly) in December 2014 [8–10].
Glucagon-like peptide-1 receptor agonists vary in the magnitude of their effect in reducing HbA1c, effect on weight loss and adverse event profiles. Additionally, dosing schedules (daily dose, injection frequencies, etc.) are variable. For example, the initial dose of exenatide BID is 5 µg injected under the skin (subcutaneously) twice daily, within 60 min before the two main meals of the day for at least 1 month [5]. The dose can be increased to 10 µg twice daily thereafter. Liraglutide is administered once daily independent of meals and should be initiated with a dose of 0.6 mg for the first week, followed by a dose increase to 1.2 mg [6]. If the 1.2 mg dose does not result in acceptable glycemic control, the dose may be increased to 1.8 mg after at least 1 week, as some patients are expected to benefit from the increase in dose. Lixisenatide is also administered once per day, within an hour of any meal, with a starting dose in the 2 weeks following initiation of 10 µg and with a subsequent fixed maintenance dose of 20 µg [8]. Exenatide once weekly (exenatide QW) is administered independent of meals at a dose of 2.0 mg [7]. Albiglutide and dulaglutide are also administered once per week, independent of meals [9, 10]. While the ADA/EASD recommend GLP-1 RA therapy as early as second-line therapy, some EU healthcare authorities, such as those in the UK, The Netherlands and Belgium, generally recommend GLP-1 RAs as a third-line therapy, in some cases as a condition of reimbursement and often restricted to certain populations (obese, unable to use insulin, etc.) [11–13].
Only a few prior studies have compared treatment patterns or variable dosing among exenatide BID, liraglutide and/or exenatide QW in the EU and in the US [14–20]. Little is known about current real-world treatment patterns among GLP-1 RA therapy users given the recent introduction of several GLP-1 RA therapies or average patient dosing given variability in dosing for exenatide BID and liraglutide. The primary objective of this analysis was to evaluate current persistence and treatment patterns among patients with T2D newly initiating the GLP-1 RA therapy class (exenatide BID, exenatide QW, liraglutide or lixisenatide; the only approved GLP-1 RAs during the selection window of this study) using available databases containing prescription data in Belgium, France, Germany and The Netherlands and diagnoses and prescription data in Sweden. Secondary objectives included evaluating the average daily dose (ADD) of the GLP-1 RA therapy.
Methods
A retrospective cohort analysis was conducted using five available databases in five European countries of interest: Belgium, France, Germany, The Netherlands and Sweden. Data from these countries were utilized in a prior analysis by the authors, and these countries were selected to provide a broad representation of several European countries [16]. Research ethics approval was received from the regional Ethics Review Board in Stockholm to conduct the Swedish analysis. Ethics approval was not required in the other countries.
Data Sources
Retail Pharmacy (LRx)
The QuintilesIMS (QuintileIMS, Durham, NC, and Danbury, CT, USA) longitudinal retail pharmacy data (henceforth referred to as LRx) were used in Belgium, France, Germany and The Netherlands. LRx contains prescription data [EphMRA Anatomical Classification (ATC) code, quantity dispensed, prescriber specialty, etc.] and limited demographic data [e.g., age (unavailable for analysis in Belgium) and gender (partially available in Germany and unavailable in France)]. The representativeness of the databases based upon current population and pharmacy coverage from 2013 to 2015 is as follows: ~33% Belgium (33% of all prescriptions), 32% France (32% of all pharmacies), ~60% Germany (60% of German statutory health insurance prescriptions) and 75% The Netherlands (75% of all prescriptions). Pharmacy coverage is as follows: >1600 pharmacies in Belgium, 7052 pharmacies in France, 12,300 pharmacies in Germany and 2400 pharmacies in The Netherlands and is representative of both large and small cities and most if not all regions.
Sweden
Patient-level de-identified national data from three registries were linked for the analysis in Sweden: the Drug Register, the Patient Register and the Mortality Register. The Swedish Drug Register provides national, patient level data on all prescription drugs dispensed at all pharmacies from the Swedish National Pharmacy Corp. [World Health Organization (WHO) ATC code]. The Swedish Patient Register includes clinical data—i.e., medical diagnosis codes [International Statistical Classification of Diseases (ICD) ICD-10 format], but not laboratory values—as well as information on healthcare utilization and associated costs from both in- and out-patient specialist care (unavailable from the primary care setting). The Swedish Mortality Register was used to identify patient death and provide full visibility into patient follow-up.
Patient Selection
Patients were first identified based on a prescription for the therapy of interest (exenatide BID, exenatide QW, liraglutide or lixisenatide) within the selection window of 1 January 2013 to 31 December 2013. Important to note, liraglutide (Saxenda®, Novo Nordisk), indicated for weight management for adults who are obese or overweight and approved in the EU in March 2015, was not included in the study [21]. The first prescription for a therapy of interest within the selection window was termed the index drug, and the date was termed the index date. Patients were followed through the end of continuous eligibility [CE, i.e., visibility (composite of patient activity in the database and stability of pharmacy reporting in LRx; patient activity without death in Sweden)] up to the end of the available study data (Belgium, Germany, The Netherlands: 31 October 2015; France: 31 August 2015; Sweden: 31 December 2014).
Adult patients (≥18 years on the index date) were identified as eligible if they met the following inclusion/exclusion criteria: (1) evidence of T2D (and no evidence of T1D) in the 180-day pre-index period; (2) ≥180 days CE pre-index (the 6-month pre-index or baseline period) and (3) ≥360 days CE post-index (minimum 1-year post-index or follow-up period) within the database; (4) naïve to the GLP-1 RA therapy class with no prescription for any GLP-1 RA in the 180-day pre-index period; (5) not initiating any other injectable antihyperglycemic therapy on the index date other than the index therapy; (6) non-missing age or gender [exceptions for Belgium (age unavailable for analysis) and Germany and France (gender partially available and unavailable, respectively)].
For the LRx analyses, the requirement for evidence of T2D (and no evidence of T1D) was determined by ≥1 OAM class used in the pre-index period, as only prescription data were available (and no diagnoses). In Sweden, where diagnoses were available, evidence of T2D was required as either: (1) diagnosis codes of diabetes (ICD-10 CM: E10–E14) in the 180 days pre-index up to 60 days post-index or (2) at least ≥1 OAM class and no diagnosis of polycystic ovarian syndrome (ICD-10 CM: E28.2) in the pre-index period. Patients were excluded if they had evidence of T1D, which was evaluated among patients with a diagnosis of E10 in the pre-index period, and identified if all of the following criteria were met: (1) no E11 diagnosis (T2D), (2) no OAM use, (3) insulin use in the pre-index period and (4) 40 years of age or younger at the first E10 diagnosis. Patients were also excluded if they had pregnancy diagnoses (ICD-10 CM: O00–O9A0) in the pre-index period.
Measures and Analysis
Baseline demographic characteristics (age and gender where available) and prescriber specialty associated with the index therapy were assessed as well as non-index antihyperglycemic therapy classes used in the pre-index period. Concomitant antihyperglycemic therapy use on the index date was also assessed. A non-index antihyperglycemic therapy class was defined as concomitant if the time between a prescription for a therapy class in the pre- and post-index period was less than 120 days, with overlap on the index date, or if the therapy class was filled on the index date.
Missing prescription quantity data were present in The Netherlands only, but not for the other databases. For the analysis in The Netherlands, patients with missing prescription quantity data were excluded from the subsequent ADD and treatment modification analyses [5 liraglutide patients (0.4% of the total liraglutide patients)].
Experience of a first treatment modification was assessed during the 1-year post-index period. Treatment modifications included discontinuation, switch, augmentation, off-label up-titration and off-label down-titration of the index GLP-1 therapy, assessed following previously published methods [15, 16]. A detailed description of the methods and definitions utilized may be found in our prior publication [16]. Discontinuation was defined as a gap in a series of successive index therapy prescriptions ≥2× the expected duration of the first prescription. Switching was defined as a new non-index antihyperglycemic prescription within 30 days before or after discontinuation of the patient’s index treatment, while augmentation was defined as a new non-index antihyperglycemic prescription, started more than 30 days before the end of follow-up or the index discontinuation date. Off-label up-titration was identified as any dose increase outside of label recommendations (daily dose >20 µg for exenatide BID; two consecutive prescriptions with daily dose >1.8 mg for liraglutide). Off-label down-titration was defined as two consecutive prescriptions with doses lower than the index dose. On-label up-titration was assessed as a separate outcome, defined as any dose increase based on label recommendations (two consecutive prescriptions with ADD of 20 µg for exenatide BID; two consecutive prescriptions with ADD ≥1.2 mg up to 1.8 mg for liraglutide).
Persistence (i.e., continuation of the index therapy) was evaluated during the 1-year post-index period. Patients were considered persistent until evidence of discontinuation or switch. A stop outcome was defined as the occurrence of either discontinuation or switch (whichever came first).
The ADD of the index therapy was assessed for all patients while persistent. Daily dose was calculated by dividing the total amount or units of drug prescribed by the number of days between two consecutive prescriptions. ADD was evaluated by calendar month intervals for patients with an index therapy prescription within that month. Average ADDs over calendar months were summarized to provide both a yearly ADD and an overall ADD. An average weekly dose (AWD) was calculated for exenatide QW by multiplying the daily dose by 7. For yearly and overall ADD/AWD calculations, calendar months with fewer than 30 patients were trimmed. Further details on data cleaning for the ADD calculations can be found in our prior publication [16].
Descriptive summary statistics were used to describe frequency and percentage distributions for categorical variables while continuous and count variables were described using the mean, standard deviation and median. Time to stop the index therapy over the variable follow-up was assessed using Kaplan-Meier (KM) analysis. No formal statistical tests were performed to compare outcomes between index therapy cohorts. However, for the KM analysis, the log-rank test was conducted to examine differences among all included index therapies for each country/data set. Statistical and descriptive analyses were performed using SAS version 9.2 or higher (Cary, NC, USA).
Results
Patient Sample
After application of the inclusion/exclusion criteria, the final sample consisted of 4339 exenatide BID patients (80 Belgium; 1884 France; 2261 Germany; 44 The Netherlands; 70 Sweden), 1499 exenatide QW patients (1035 Germany; 122 The Netherlands; 342 Sweden), 20,955 liraglutide patients (666 Belgium; 8606 France; 6916 Germany; 1324 The Netherlands; 3443 Sweden) and 1751 lixisenatide patients (20 Belgium; 1731 Germany).
Please see Table S1 in the supplementary material for baseline demographic characteristics and antihyperglycemic therapy use of the study sample. Across index therapy cohorts and databases, patients were mostly in the 50–64 age group (41.8–59.1%) with mean age ranging from 57.1 to 62.9 years. Approximately half or more of patients were female (45.1–61.4%). LRx patients had mean follow-up of approximately 2 years or more (20.0–30.7 months); mean follow-up in Sweden was shorter at 18 months.
On average, patients used 1.6–3.0 antihyperglycemic therapy classes in the 180-day pre-index period (with a median of 2 classes for most index therapy cohorts). Biguanides (i.e., metformin) were the most common antihyperglycemic therapy class used in the 180-day pre-index period for all cohorts across databases (54.0–88.5%). Sulfonylureas were the second most commonly used antidiabetic class in the pre-index period in Belgium, France and The Netherlands (43.7–74.5%). In Germany, the second most commonly used antidiabetic class was DPP-4/biguanides for exenatide QW (30.0%) and liraglutide (21.9%) patients, while long-acting insulin was most commonly used for exenatide BID (26.2%) and lixisenatide (39.2%) patients. In Sweden, DPP-4s were the second most commonly used antidiabetic class in the pre-index period for exenatide QW (28.6%) patients, while intermediate-acting insulin was second most commonly used for exenatide BID (28.5%) and liraglutide (22.9%) patients.
Mean number of concomitant antidiabetic therapy classes used on the index date ranged from 1.0 to 2.0 classes. Median number of co-occurring antidiabetic therapy classes used on the index date varied, and all index therapy cohorts in Germany and Sweden had a median of 1, while most index therapy cohorts in Belgium, France and The Netherlands had a median of 2. Across countries/data sets, biguanides were most frequently co-occurring on the index date and used by more than half (53.5–83.9%). Sulfonylureas were the second most commonly co-occurring antidiabetic therapies on the index date for most index therapy cohorts in Belgium, France and The Netherlands (45.0–66.2%). In Germany and Sweden, after biguanides, most index therapy cohorts did not have a co-occurring antidiabetic therapy on the index date (15.7–24.5%).
For the overall GLP-1 RA cohorts across countries/databases, a general practitioner (GP) was the most common prescribing physician specialty associated with the index prescription in Belgium (47.5%), France (72.8%) and Sweden (50.1%). An internist was most common in Germany (52.7%) and The Netherlands (71.1%). For the most part, specialty did not vary by index therapy cohort, with the exception of Germany, where the proportion of patients with an internist and proportion with a GP were equal for exenatide QW patients (46.3% and 46.2%, respectively).
Treatment Patterns
At 1 year post-index, the proportion persistent for liraglutide patients ranged from 29.0% (Belgium) to 60.8% (The Netherlands) and for exenatide BID patients ranged from 17.5% (Belgium) to 44.4% (France) (Table 1). The proportion persistent for exenatide QW ranged from 32.8% (Germany) to 50.8% (The Netherlands). The proportion persistent for lixisenatide was 50.0% in Belgium and 4.2% in Germany (lixisenatide was taken off the market in April 2014 in Germany) [22].
Table 1.
Treatment modifications and persistence on the index therapy
| Exenatide BID | Liraglutide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE (N = 80) | FR (N = 1884) | DE (N = 2261) | NL (N = 44) | SE (N = 70) | BE (N = 666) | FR (N = 8606) | DE (N = 6916) | NL (N = 1324) | SE (N = 3443) | |
| AT 1 year post-index | ||||||||||
| Persistent (%) | 17.5 | 44.4 | 29.0 | 34.1 | 31.4 | 29.0 | 51.5 | 43.1 | 60.8 | 59.0 |
| Stopped (%) | 82.5 | 56.7 | 71.0 | 65.9 | 68.6 | 71.0 | 49.2 | 56.9 | 39.2 | 41.0 |
| Persistence days, all patients | ||||||||||
| Mean | 152.0 | 232.0 | 191.7 | 207.8 | 206.2 | 201.1 | 253.0 | 230.2 | 275.3 | 272.5 |
| SD | 127.3 | 132.9 | 130.4 | 131.8 | 134.1 | 132.7 | 126.9 | 132.3 | 122.2 | 123.4 |
| Median | 111 | 273 | 165 | 159 | 199 | 192 | 360 | 263 | 360 | 360 |
| 95% CI LL | 123.6 | 226.0 | 186.3 | 167.7 | 174.2 | 191.0 | 250.3 | 227.1 | 268.7 | 268.4 |
| 95% CI UL | 180.3 | 238.0 | 197.1 | 247.9 | 238.1 | 211.2 | 255.6 | 233.3 | 281.9 | 276.6 |
| First treatment modification | ||||||||||
| No treatment modification (%) | 6.3 | 13.3 | 12.5 | 15.9 | s | 19.5 | 21.9 | 26.1 | 40.5 | 40.3 |
| With a first treatment modification (%) | 93.8 | 86.7 | 87.5 | 84.1 | 90.0 | 80.5 | 78.1 | 73.9 | 59.5 | 59.7 |
| First treatment modification type, among patients with a first treatment modification | ||||||||||
| Off-label up-titration | 17.3 | 39.3 | 13.4 | 24.3 | 27.0 | 4.5 | 20.3 | 1.7 | 11.1 | 8.8 |
| Off-label down-titration | 5.3 | 19.7 | 5.6 | 2.7 | s | 13.1 | 33.7 | 10.8 | 19.2 | 16.4 |
| Discontinuation | 64.0 | 37.2 | 64.2 | 56.8 | 49.2 | 70.7 | 40.6 | 64.1 | 51.1 | 54.6 |
| Switch | 5.3 | 0.6 | 6.2 | 5.4 | s | 6.0 | 1.1 | 6.0 | 6.0 | 5.6 |
| Augmentation | 8.0 | 3.3 | 10.7 | 10.8 | s | 5.8 | 4.4 | 17.5 | 12.6 | 14.6 |
| % on-label up-titration | 21.3 | 62.0 | 20.2 | 13.6 | 18.6 | 54.8 | 83.9 | 44.3 | 70.4 | 65.6 |
| Exenatide QW | Lixisenatide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DE (N = 1035) | NL (N = 122) | SE (N = 342) | BE (N = 20) | DE (N = 1731) | ||||||
| AT 1 year post-index | ||||||||||
| Persistent (%) | 32.8 | 50.8 | 42.7 | 50.0 | 4.2 | |||||
| Stopped (%) | 67.3 | 49.2 | 57.3 | 50.0 | 95.8 | |||||
| Persistence days, all patients | ||||||||||
| Mean | 194.7 | 246.8 | 227.0 | 246.0 | 151.4 | |||||
| SD | 136.7 | 134.8 | 135.0 | 149.6 | 107.9 | |||||
| Median | 165 | 360 | 270 | 353 | 129 | |||||
| 95% CI LL | 186.3 | 222.6 | 212.6 | 176.0 | 146.3 | |||||
| 95% CI UL | 203.0 | 271.0 | 241.4 | 316.0 | 156.5 | |||||
| First treatment modification | ||||||||||
| No treatment modification (%) | 26.6 | 46.7 | 38.6 | 45.0 | 3.1 | |||||
| With a first treatment modification (%) | 73.4 | 53.3 | 61.4 | 55.0 | 96.9 | |||||
| First treatment modification type, among patients with a first treatment modification | ||||||||||
| Off-label up-titration | ||||||||||
| Off-label down-titration | ||||||||||
| Discontinuation | 76.6 | 69.2 | 73.3 | 81.8 | 73.8 | |||||
| Switch | 10.9 | 20.0 | 14.3 | 9.1 | 14.2 | |||||
| Augmentation | 12.5 | 10.8 | 12.4 | 9.1 | 12.0 | |||||
| % on-label up-titration | ||||||||||
s Data suppressed in Sweden because of patient count less than 10 in compliance with Swedish privacy legislation. BE Belgium, DE Germany, FR France, NL The Netherlands, SE Sweden, CI confidence interval, LL lower limit, UL upper limit
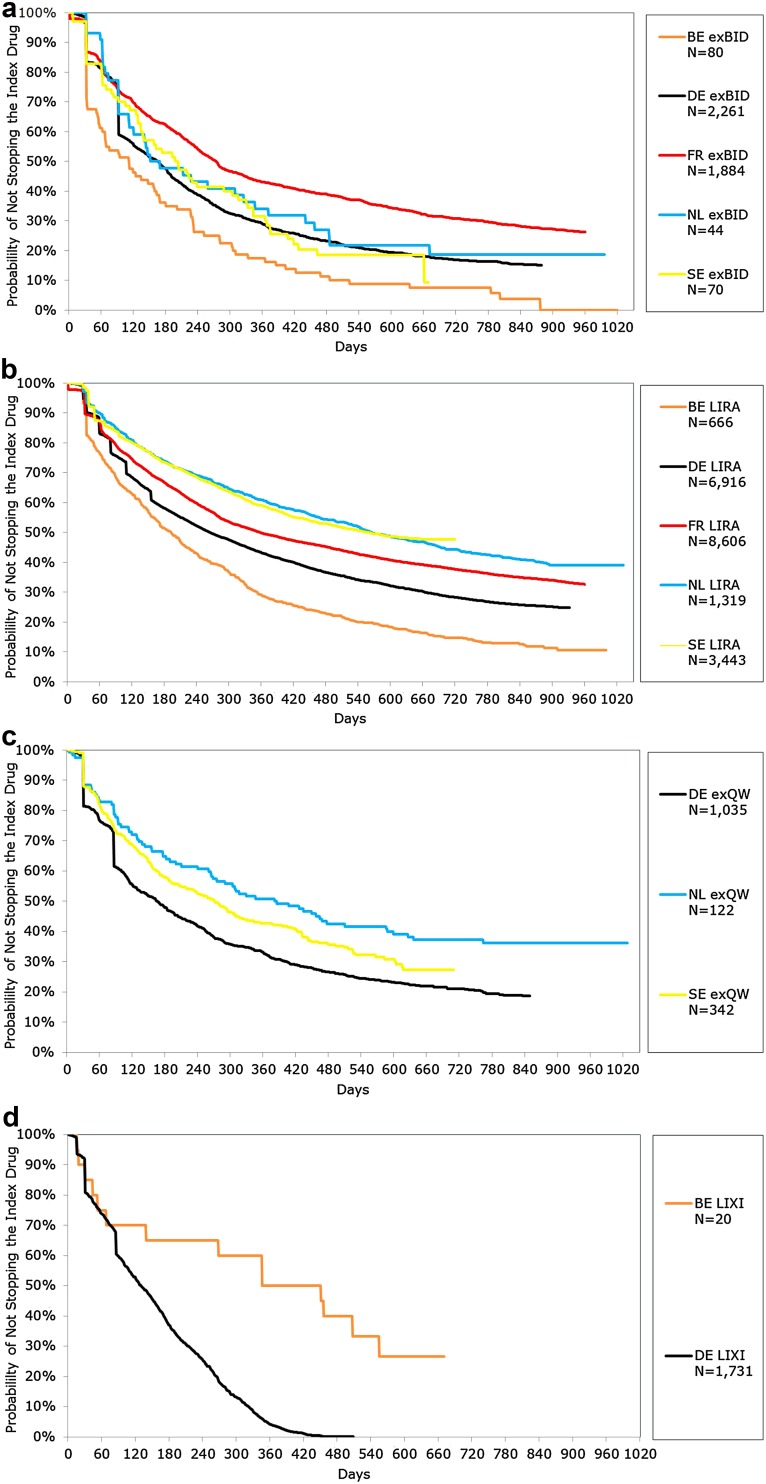
KM results for time to stop (discontinuation or switch) over the variable follow-up by index therapy cohort can be found in Fig. 1a–d. Across countries/data sets, for most time points, the proportion stopping was lowest among liraglutide patients and highest among exenatide BID patients at all time points, again with the exception of lixisenatide patients in Belgium and Germany. Median time to stop ranged from 111 days (Belgium) to 273 days (France) for exenatide BID, 193 days (Belgium) to 566 days (The Netherlands) for liraglutide and 164 days (Germany) to 385 days (The Netherlands) for exenatide QW, and for lixisenatide, it was 347 days in Belgium and 129 days in Germany. Across data sets/countries, the log-rank test resulted in a p value <0.001 between the available index therapies.
Fig. 1.
Kaplan-Meier analyses for time to stop: a exBID; b LIRA; c exQW; d LIXI. BE Belgium, exBID exenatide twice daily, exQW exenatide once weekly, FR France; DE Germany, LIRA liraglutide, LIXI lixisenatide, NL The Netherlands, SE Sweden
Treatment modifications at 1 year post-index can be found in Table 1 by index therapy cohort. Most exenatide BID patients experienced treatment modification, ranging from 84.1% (The Netherlands) to 93.8% (Belgium). More than half of liraglutide patients experienced treatment modification, ranging from 59.5% (Sweden) to 80.5% (Belgium). Proportion of exenatide QW patients with treatment modification ranged from 53.3% (The Netherlands) to 73.4% (Germany). Proportion of lixisenatide patients with treatment modification was 55.0% in Belgium and 96.9% in Germany. For most cohorts, among patients with a treatment modification, discontinuation was the most common first treatment modification (37.2– 81.8%).
Average Daily Dose
ADD by calendar year (year of prescription) and overall (over the available follow-up period) is reported in Table 2. Mean (SD) overall ADD for exenatide BID was on the higher end of the approved doses and was 18.55 (1.24) µg in Germany and 18.69 (0.74) µg in France. Mean yearly ADD for exenatide BID was stable in France and increased in Germany. Overall ADD for liraglutide was generally in the middle of the indicated maintenance doses and ranged from 1.41 (0.12) mg in Belgium to 1.68 (0.14) mg in the Netherlands. Mean yearly ADD for liraglutide increased by year across countries/data sets. Overall ADD for exenatide QW ranged from 0.29 (0.01) mg in Germany to 0.30 (0.01) mg in The Netherlands, with a respective average weekly dose of 2.03 (0.07) mg to 2.10 (0.09) mg, close to the expected average weekly dose. Mean yearly ADD for exenatide QW was stable by year across countries/data sets. Mean (SD) overall ADD for lixisenatide was 20.11 (2.33) µg in Germany, close to the approved maintenance dose. Mean yearly ADD increased for lixisenatide from 2013 to 2014 in Germany.
Table 2.
Yearly and overall ADD
| Exenatide BID (µg) | Liraglutide (mg) | Exenatide QW (mg) | Lixisenatide (µg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FR (N = 1884) | DE (N = 2261) | BE (N = 666) | FR (N = 8606) | DE (N = 6916) | NL (N = 1324) | SE (N = 3443) | DE (N = 1035) | NL (N = 122) | SE (N = 342) | DE (N = 1731) | |
| Average daily dose | |||||||||||
| Overall | |||||||||||
| Mean | 18.69 | 18.55 | 1.41 | 1.64 | 1.55 | 1.68 | 1.60 | 0.29 | 0.30 | 0.30 | 20.11 |
| SD | 0.74 | 1.24 | 0.12 | 0.13 | 0.21 | 0.14 | 0.39 | 0.01 | 0.01 | 0.01 | 2.33 |
| Median | 18.64 | 18.26 | 1.37 | 1.65 | 1.51 | 1.70 | 1.49 | 0.29 | 0.30 | 0.30 | 19.80 |
| 2013 | |||||||||||
| Mean | 18.61 | 17.70 | 1.31 | 1.59 | 1.41 | 1.53 | 1.43 | 0.29 | 0.30 | 0.29 | 19.04 |
| SD | 0.22 | 0.75 | 0.05 | 0.03 | 0.03 | 0.08 | 0.05 | 0.01 | 0.02 | 0.01 | 1.26 |
| Median | 18.53 | 17.62 | 1.30 | 1.59 | 1.41 | 1.55 | 1.46 | 0.30 | 0.30 | 0.30 | 19.73 |
| 2014 | |||||||||||
| Mean | 18.80 | 18.60 | 1.43 | 1.66 | 1.54 | 1.72 | 1.76 | 0.29 | 0.30 | 0.30 | 23.30 |
| SD | 0.38 | 0.72 | 0.10 | 0.02 | 0.06 | 0.08 | 0.51 | 0.01 | 0.00 | 0.01 | 1.79 |
| Median | 18.76 | 18.40 | 1.42 | 1.65 | 1.52 | 1.72 | 1.56 | 0.29 | 0.30 | 0.30 | 22.61 |
| 2015 | |||||||||||
| Mean | 18.65 | 19.93 | 1.53 | 1.70 | 1.76 | 1.81 | 0.29 | ||||
| SD | 0.30 | 1.42 | 0.09 | 0.02 | 0.31 | 0.06 | 0.01 | ||||
| Median | 18.64 | 19.72 | 1.50 | 1.70 | 1.65 | 1.80 | 0.29 | ||||
| Overall average weekly dose | |||||||||||
| Mean | 2.03 | 2.10 | 2.08 | ||||||||
| SD | 0.07 | 0.09 | 0.09 | ||||||||
| Median | 2.03 | 2.10 | 2.10 | ||||||||
ADD presented represents a summary of monthly ADD for eligible prescription records. Patient N changes over time, and patients reflect a mix of new initiators and prevalent users over time. Lixisenatide was taken off the market in April 2014 in Germany. Available study data ended December 2014 in Sweden
Yearly/overall data not presented if all component months have patient N <30; monthly ADD calculations trimmed where N <30
AWD calculated as ADD × 7
BE Belgium, DE Germany, FR France, NL The Netherlands, SE Sweden
Discussion
In this real-world analysis in five European countries, treatment patterns varied among new initiators of GLP-1 RAs. For the most part, we observed that exenatide BID patients were most likely to modify or stop therapy, while liraglutide patients were most persistent. We found that treatment modification results for exenatide QW relative to liraglutide varied by data set (similar between exenatide QW and liraglutide patients in Germany and Sweden and lower for exenatide QW patients compared to liraglutide patients in The Netherlands). In Belgium, proportion with a first treatment modification at 1 year post-index was lower for lixisenatide patients compared to liraglutide patients (55.0% and 80.5%); however, the lixisenatide sample was limited (N = 20). The overall ADD of GLP-1 RAs was generally within the label-indicated ranges. The overall ADD for liraglutide was generally in the middle of the indicated maintenance doses (1.2 or 1.8 mg following the second week) and increased by year from initiation. Longer term data would be useful to further clarify practice patterns among the GLP-1s, given the recent launch of several new GLP-1 RAs in the EU. While our study is the first to examine treatment patterns and dosing for lixisenatide specifically, additional research is needed to more broadly understand its use outside of Belgium and Germany.
Our findings mirror those in several other studies that conclude that treatment patterns vary among GLP-1 RA patients [15, 16]. Miller et al., using a German EMR database (2009–2010), found that time to treatment modification was shorter for exenatide BID patients compared to liraglutide patients, similar to our findings of more exenatide BID patients experiencing treatment modification at 1 year post-index compared to liraglutide patients across countries/databases [15]. A prior analysis conducted by the authors of this study, using various data sources across several European countries (including some of the same databases/countries used in the current analysis), found that the proportion of patients that experienced a treatment modification and that stopped the index therapy by 180 days post-index were highest among exenatide BID patients compared to liraglutide or exenatide QW patients [16]. Similar to our analysis, the proportion persistent at 180 days was highest for liraglutide compared to exenatide QW in The Netherlands LRx, Germany LRx and Sweden. Fewer exenatide QW patients experienced treatment modification compared to liraglutide patients in Sweden, while proportions were more similar in The Netherlands LRx and Germany LRx. In our analysis, treatment modification at 1 year post-index was similar between exenatide QW and liraglutide patients in Germany and Sweden, but lower for exenatide QW patients compared to liraglutide patients in The Netherlands. Differences in study findings may be related to different time periods as the prior analysis was conducted soon after exenatide QW’s launch, as well as different follow-up times.
Average daily dose of GLP-1 RAs was generally within the label-indicated ranges, similar to findings from other European studies. Using the LRx database in Germany, Fuchs et al. found a mean daily dose of 1.42 mg including extreme values for liraglutide (2009–10) and a mean daily dose of 1.29 mg excluding extreme values, both lower than the 1.55 mg observed in our study [14]. Differences may in part be related to varying methods (trimming vs. removing extreme values). Miller et al. evaluated ADD for exenatide BID and liraglutide (2009–10) using EMR data in Germany [15]. Mean ADD was 16.7 µg for exenatide BID and 1.43 mg for liraglutide, while in our Germany LRx analysis, we found higher mean ADD (18.55 µg and 1.55 mg, respectively). Compared to the prior analysis conducted by the authors in an earlier study period (2010–2013) and the same databases/countries, we observed in our current analysis that overall ADD has increased over time using a more recent study period (2013–2015) [16]. For example, overall ADD for exenatide BID increased from 17.70 µg in the prior analysis to 18.55 µg in the current study in Germany. This trend was also observed for overall ADD for liraglutide in Belgium (1.30–1.41 mg), Germany (1.40–1.55 mg), The Netherlands (1.61–1.68 mg) and Sweden (1.52–1.60 mg). The observed increase in ADD over time may suggest that prescribing physicians are more comfortable up-titrating exenatide BID and liraglutide per label as they become more familiar with GLP-1 RAs. As a note, we also observed fewer initiators of exenatide BID compared to our prior analysis, suggesting a potential preference of prescribing physicians for use of other GLP-1 RAs such as liraglutide or exenatide QW, which may be related to their more convenient dosing schedules. Additionally, it may be important to understand the dosing associated with GLP-1 RA therapy from the payer perspective. Changes in dosing may result in a less predictable budgetary impact as compared to regimens with fixed dosing.
There are a few limitations to note related to typical database research. Patients included in the LRx databases may not be fully representative of all patients in the respective country, as data are collected only from participating pharmacies. LRx lacks visibility to any prescriptions purchased outside of the participating pharmacies. The lack of medical diagnosis codes in LRx and unavailability of medical diagnosis codes from the primary care setting in Sweden made it difficult to confirm the presence/absence of T1D and/or T2D. LRx lacks the ability to identify patient mortality. Additionally, while a prescription may be prescribed or filled, real-world consumption patterns may differ. Results from retrospective database studies must be interpreted with caution, and in context with results from other studies, because they can only establish associations and not cause-and-effect relationships. We were unable to investigate reasons for treatment modifications (lack of effectiveness, adverse events, etc.) as the data lack this clinical detail. Our sample may be biased toward a healthier population because of our continuous enrollment requirements, which were necessary to ensure adequate visibility into the patients’ clinical history; this may be less of an issue among patients with chronic diseases, such as diabetes. Further, small sample sizes [particularly for exenatide BID in Belgium (N = 80), The Netherlands (N = 44) and Sweden (N = 70) and lixisenatide patients in Belgium (N = 20)] for some cohorts/databases limited comparisons. Lixisenatide was taken off the market in Germany in April 2014, impacting observed treatment patterns.
Conclusion
The GLP-1 RA class has grown in the last decade with several agents available for use in the US and Europe and several more in development [23]. This study is one of the first to comprehensively examine recent treatment patterns and ADD of GLP-1 RA therapies, including lixisenatide, across various EU countries and data sets. In this real-world analysis, treatment patterns varied among GLP-1 RA patients in the sample of European countries considered in this study. ADD was within indicated label ranges. Longer term data would be useful to further understand treatment patterns associated with GLP-1 RAs, given the recent approval of several GLP-1 RA therapies and the observed changes in GLP-1 RA treatment patterns and dosing over time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study and article processing charges were financially supported by Eli Lilly and Co., Indianapolis, IN, USA.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
The authors thank Heval Beydogan, Hartmut Richter, Nadia El Mouaddin and Xavier Ansolabehere, employees of QuintilesIMS, who were also involved in data analysis in Sweden, Germany and France, respectively.
Disclosures
Kirsi Norrbacka is an employee of Eli Lilly. Kristina Secnik Boye is an employee of Eli Lilly. Hélène Sapin is an employee of Eli Lilly. Victoria Divino is an employee of QuintilesIMS. Mitch DeKoven is an employee of QuintilesIMS. Farhad Khan is an employee of QuintilesIMS. QuintilesIMS received consulting fees from Lilly for this study.
Compliance with Ethics Guidelines
This study involved a retrospective cohort analysis using five databases, and the analysis does not contain new studies with human or animal subjects performed by any of the authors. Research ethics approval was received from the regional Ethics Review Board in Stockholm in order to conduct the Swedish analysis. Ethics approval was not required in the other countries.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CF47F0603B352418.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels: International Diabetes Federation; 2015. http://www.idf.org/diabetesatlas. Accessed May 10, 2016.
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Lau DC, Teoh H. Impact of current and emerging glucose-lowering drugs on body weight in type 2 diabetes. Can J Diabetes. 2015;39(Suppl 5):S148–S154. doi: 10.1016/j.jcjd.2015.09.090. [DOI] [PubMed] [Google Scholar]
- 4.Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776–785. doi: 10.1089/dia.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byetta: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. Accessed Nov 4, 2016.
- 6.Victoza: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed Nov 4, 2016.
- 7.Bydureon: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. Accessed May 5, 2016.
- 8.Lyxumia: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed May 5, 2016.
- 9.Eperzan: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Accessed Nov 4, 2016.
- 10.Trulicity: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002825/WC500179470.pdf. Accessed May 5, 2016.
- 11.Type 2 diabetes in adults: management (NG28): NICE clinical guidelines. 2015. https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-1837338615493. Accessed May 5, 2016.
- 12.GLP-1-agonisten: MedicijnBalans. 2016. http://medicijnbalans.nl/medicijngroepen/glp-1-agonisten/vergoeding. Accessed Nov 4, 2016.
- 13.Diabetes Mellitus Type 2: Domus Medica. 2015. http://www.domusmedica.be/documentatie/downloads/praktijkdocumenten/richtlijnen/726-diabetes-mellitus-type-2/file.html. Accessed June 6, 2016.
- 14.Fuchs S, Kostev L, Seitz L, Wohlleben M. Ermittlung der tatsächlichen Tagesdosierung von Liraglutid (PDD) unter realen Versorgungsbedingungen im Hinblick auf die Berechnung von Tagestherapiekosten. Diabetologie und Stoffwechsel. 2011;6:P234. [Google Scholar]
- 15.Miller LA, Burudpakdee C, Zagar A, et al. Exenatide BID and liraglutide QD treatment patterns among type 2 diabetes patients in Germany. J Med Econ. 2012;15:746–757. doi: 10.3111/13696998.2012.679756. [DOI] [PubMed] [Google Scholar]
- 16.Divino V, DeKoven M, Hallinan S, et al. Glucagon-like Peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5:499–520. doi: 10.1007/s13300-014-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonell AL, Kiiskinen U, Zammit DC, et al. Estimating the real world daily usage and cost for exenatide twice daily and liraglutide in Germany, the Netherlands, and the UK based on volumes dispensed by pharmacies. Clinicoecon Outcomes Res. 2015;7:95–103. doi: 10.2147/CEOR.S69981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31:1119–1133. doi: 10.1007/s12325-014-0166-0. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier EM, Pawaskar M, Smith PJ, Best JH, Chapman RH. Economic outcomes of exenatide vs liraglutide in type 2 diabetes patients in the United States: results from a retrospective claims database analysis. J Med Econ. 2012;15:1039–1050. doi: 10.3111/13696998.2012.688903. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Xie J, Fernandez Lando L, Kabul S, Swindle RW. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38:149–160. doi: 10.1016/j.clinthera.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Saxenda: EPAR–European Medicines Agency-Europa, European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003780/WC500185786.pdf. Accessed Nov 4, 2016.
- 22.Sanofi Deutschland Fachpresse. Lyxumia® (Lixisenatid) in Deutschland außer Vertrieb. http://www.sanofi.de/l/de/de/layout.jsp?cnt=0085FC03-80A0-4B01-B600-7A435B964A7E. Accessed July 18, 2016.
- 23.Trujillo J, Nuffer W, Ellis S. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.