Abstract
Purpose
The Drug Burden Index (DBI) is a non-invasive method to quantify patients’ anticholinergic and sedative drug burden from their prescriptions. This systematic review aimed to summarise the evidence on the associations between the DBI and clinical outcomes and methodological quality of studies.
Methods
A search in PubMed and Embase (search terms: ‘drug’, ‘burden’, and ‘index’) was performed and experts were contacted. We excluded publications that did not report empirical results or clinical outcomes. Methodological quality was assessed using the Newcastle-Ottawa Scale. Potential omissions of relevant clinical outcomes and populations were studied.
Results
Of the 2998 identified publications, 21 were eligible. Overall, methodological quality of studies was good. In all but one study, adjustment was made for prevalent co-morbidity. The DBI was examined in diverse older individuals, i.e. both males and females from different settings and countries. However, no studies were conducted in other relevant patient groups, e.g. psychiatric patients. Exposure to anticholinergic and sedative drugs was thoroughly ascertained, though the specific calculation of the DBI differed across studies. Outcomes were assessed from medical records, record linkage or validated objective tests or questionnaires. Many studies found associations between the DBI and outcomes including hospitalisation, physical and cognitive function. Cognitive function and quality of life were understudied and the number and scope of longitudinal studies was limited.
Conclusions
An accumulating body of evidence supports the validity of the DBI. Longitudinal studies of cognitive function and quality of life and in other patient groups, e.g. psychiatric patients, are warranted.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-016-2162-6) contains supplementary material, which is available to authorized users.
Keywords: Antimuscarinic agents; Hypnotics and sedatives; Polypharmacy; Older adults, frailty; Inappropriate prescribing
Introduction
Drugs with anticholinergic and sedative properties are prescribed in over a quarter of older patients [1, 2] despite their likelihood to increase patients’ physical and cognitive impairment [3–5]. In 2007, Hilmer and colleagues published the Drug Burden Index (DBI) [6], a linear additive model that quantifies cumulative anticholinergic and sedative drug load for patients with polypharmacy:
where D is the daily dose of an individual drug and δ usually represents the minimum recommended daily dose of that individual drug. The sigma sign (∑) indicates that the DBI is the sum score of prescribed drugs with probable anticholinergic and sedative properties for each patient. Compared to other methods of estimating patients’ anticholinergic and sedative drug load such as serum anticholinergic activity (SAA) [7] and related scales such as the Anticholinergic Risk Scale (ARS) [8] and the Anticholinergic Drug Scale (ADS) [9], the DBI has two advantages. First, it is non-invasive as it is calculated from drug prescriptions. Unlike SAA, the DBI does not require blood withdrawal. Second, the DBI takes the dosage of each anticholinergic and sedative drug into account whereas the ARS and ADS scales do not. Thus, these two advantages clearly favour the DBI over other measures as a tool for the routine screening of anticholinergic and sedative burden in the process of deprescribing of anticholinergic and sedative drugs by pharmacists and physicians [10–12].
A growing body of studies conducted in Australia, North America, and Europe have examined associations between the DBI and clinical outcomes. Recently, Kouladjian et al. [13] discussed these findings in a comprehensive overview of studies, thereby providing insight into the clinical and theoretical applications of the DBI. There is increasing interest in methodological issues about how to estimate exposure to anticholinergic and sedative drugs. [14–17] Two recent systematic reviews evaluated the scales that are currently used to quantify the anticholinergic and sedative drug burden as well as associated clinical outcomes. [18, 19] These systematic reviews found drugs with anticholinergic properties to increase the risks of cognitive impairment, falls, functional outcomes and all-cause mortality in older adults.
The current systematic review aims to assess the scope and quality of studies about the DBI focusing on methodology, patient populations and outcomes. In doing so, the current systematic review aims to complement these previous systematic reviews. Such an assessment is needed to reflect on current knowledge about the associations between patients’ DBI values and clinical outcomes, to provide advice on methodological requirements for future studies and to identify knowledge gaps about clinically relevant outcomes.
Material and methods
Data sources and search strategy
We conducted a search and extraction according to the PRISMA statement [20] in September 2015. We searched for all English publications about original studies of the DBI since its launch in 2007 with the search term: Drug [All Fields] AND Burden [All Fields] AND Index [All Fields] AND [“2007/01/01”[PDAT] : “2015/09/30”[PDAT]]) in PubMed and Embase and in the reference lists of initially found publications. Thus, the search was not limited to studies conducted in older adults. Authors whose publications were not electronically available were contacted and requested to provide their publications. Lastly, we contacted experts from relevant publications to identify other studies.
Study screening and selection
Publications were included if they reported empirical results and associations between the DBI and clinical outcomes. Publications were excluded if they were not available either electronically or from the authors. Publications were not excluded if the DBI calculation was based on only anticholinergic or sedative drugs.
Data extraction and synthesis
Eligibility, methodological quality and study outcomes were extracted by HW and reviewed by HVDM. In case of disagreements between HW and HVDM, final decisions were made by KT. For studies in which separate DBIs were calculated for anticholinergic and sedative drugs, results of both DBIs were considered. If studies reported both a standard overall DBI calculated for anticholinergic and sedative drugs and DBIs calculated separately for anticholinergic and sedative drugs, only results of the standard overall DBI were considered.
All eligible studies were rated for their methodological quality using the Newcastle-Ottawa Scale [NOS] [21]. The NOS awards stars with regard to selection of participants, i.e. representativeness (1 star), and selection of participants not exposed to anticholinergic and sedative drugs (controls) (1 star), ascertainment of anticholinergic and sedative exposure (1 star), comparability of participants with high and low DBI values, i.e. by taking the most important confounding factor (1 star) and additional confounding factors (1 star) into account, and outcomes, i.e. whether these were assessed in a blind manner (1 star). Furthermore, for longitudinal studies, whether incidence of outcomes was assessed or whether their absence at baseline was verified or adjusted for (1 star), and adequacy of follow-up, i.e. whether length of follow-up was adequate (1 star), and whether the amount of attrition, and presence of differential attrition or selective loss to follow-up, e.g. more loss to follow-up among patients with higher baseline frailty, was assessed and acceptable (1 star). Furthermore, we also assessed how often relevant clinical outcomes were studied and potential omissions of relevant clinical outcomes, as well as the specific patient populations that were studied.
Results
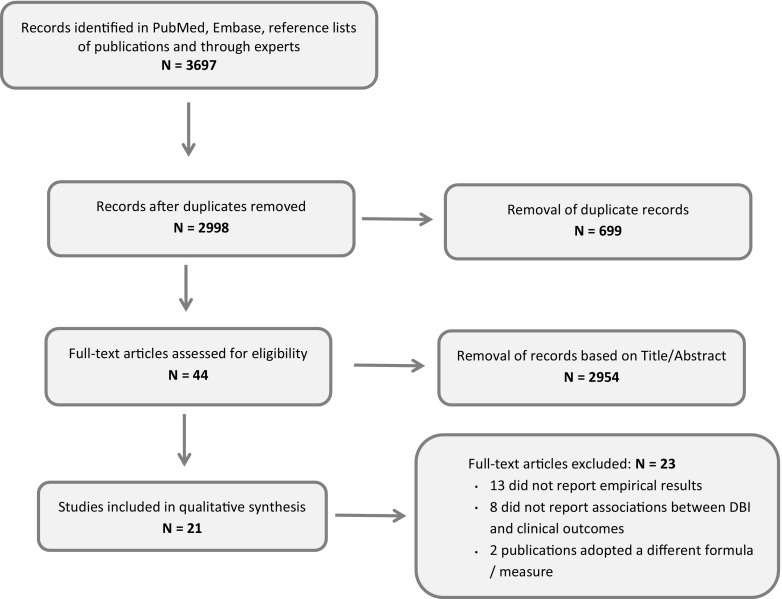
The search resulted in 2998 publications. After exclusion of 2954 publications based on title and abstract, and excluding 23 publications after reviewing their full texts, 21 eligible publications were included in the review (see flowchart in Fig. 1). Experts confirmed there were no other eligible publications. Owing to the substantial heterogeneity with regard to clinical outcomes, classification of DBI values, and study design, we decided that a qualitative synthesis of the literature was more suitable than a quantitative meta-analysis. Key characteristics of eligible publications are presented in Table 1.
Fig. 1.
Flowchart of identification and assessment of eligibility of DBI publications
Table 1.
Characteristics of eligible publications of cross-sectional and longitudinal studies (N = 21)
| Publication 1st author et al. year) | Sample size | Data source and country | Setting | Participants’ characteristics | ||
|---|---|---|---|---|---|---|
| % women | Age | Number of medicines | ||||
| Cross-sectional studies | ||||||
| Best et al. 2013 [22] | 329 | I, Australia | Hospital wards | 62 | 85 [±7] | 8 [±4] |
| Bosboom et al. 2012 [23] | 226 | II, Australia | RACF | 75 | 86 [±8] | 10 [±4] |
| Cao et al. 2008 [24] | 932 | III, USA | Community | 100 | 78 [71–86]a | – |
| Gnjidic et al. 2012a [25] | 700 | IV, Finland | Community | 69 | 81 [±5] | 5 [±3] |
| Gnjidic et al. 2009 [26] | 1705 | V, Australia | Community | 0 | 77 [±6] | 4 [±3] |
| Gnjidic et al. 2012b [27] | 115 | VI, Australia | Community | 73 | 82 [±6] | 5 [±3] |
| Gnjidic et al. 2012c [28] | 987 | V, Australia | Community | 0 | 77 [±6] | 4 [±3] |
| Hilmer et al. 2007 [6] | 3075 | VIII, USA | Community | 52 | 74 [±3] | 3 [±3] |
| Lowry et al. 2012 [29] | 362 | X, UK | Geriatric wards | 59 | 84 [±7] | 7 [5–9]a |
| Mangoni et al. 2013 [30] | 71 | XI, Holland | Hospital wards | 70 | 84 [±6] | 4 [±3] |
| Longitudinal studies | ||||||
| Dauphinot et al. 2014 [31] | 337 | XIV, France | Geriatric wards | 65 | 85 [±7] | 7 [±4] |
| Gnjidic et al. 2012d [32] | 1662 | V, Australia | Community | 0 | 77 [±5] | 4 [±3] |
| Gnjidic et al. 2014 [33] | 33,206 | VII, Finland | Community | 67 | 79 [±9] | – |
| Hilmer et al. 2009 [34] | 2172 | VIII, USA | Community | 53 | 73 [±3] | – |
| Kashyap et al. 2014 [35] | 102 | IX, Canada | Community | 84 | 72 [±7] | 7 [±4] |
| Lönnroos et al. 2012 [36] | 339 | IV, Finland | Community | 68 | 81 [±5] | 5 [±3] |
| Nishtala et al. 2014 [37] | 537,387 | XII, New Zealand | Community | 55 | 75 [±7 ] | 6 [±4] |
| Salahudeen et al. 2015 [38] | 537,387 | XII, New Zealand | Community | 55 | 75 [±8] | 6 [±4] |
| Wilson et al. 2010 [39] | 602 | XIII, Australia | RACF | 71 | 86 [±6] | 6 [±3] |
| Wilson et al. 2011 [40] | 602 | XIII, Australia | RACF | 71 | 86 [±6] | 6 [±3] |
| Wilson et al. 2012 [41] | 602 | XIII, Australia | RACF | 71 | 86 [±6] | 6 [±3] |
Data sources: I, Concord Repatriation General Hospital Sydney New South Wales Australia; II, DIRECT Study Beer C et al. Trials 2010; 11: 63; III, Medicare beneficiaries Baltimore City and Baltimore Country, Maryland; IV, GeMS study Rikala et al., Drugs Aging. 2010; 27:337–49; V, CHAMP, Cumming et al., Int J Epidemiol 2008; 38: 374–8; VI, Self-care retirement villages in Sydney, Australia; VII, Linkage of Finnish National Prescription and Special Reimbursement Registers with Finnish Hospital Discharge Register; VIII, Health ABC study community-resident Medicare recipients, Pittsburgh, Pennsylvania and Memphis, Tennessee; IX, Patients of incontinence clinics, Montreal and Sherbrooke areas of Quebec, Canada; X, Two acute geriatric medicine units from Aberdeen Royal Infirmary and Woodend Hospital, NHS Grampian, Aberdeen, Scotland, United Kingdom; XI, Academic Medical Centre, Amsterdam, the Netherlands; XII, Pharmaceutical Claims Data Mart [Pharms] of the Pharmaceutical Management Agency [PHARMAC] and data from the Ministry of Health of New Zealand; XIII, Multicentre cluster-randomised controlled trial of RACFs residents in the Northern Sydney Central Coast Health [NSCCH] service area, Australia; XIV Consecutive sample of hospitalised patients of three geriatric hospitals, University Hospital of Lyon, France
RACF residential aged care facility
aMedian value with interquartile range
Selection of participants and populations studied
Ratings of the methodological quality of eligible studies are presented in Online Resource 1. All studies focused on older geriatric patients as the mean age of study populations ranged from 72 to 86 years. Furthermore, sampling in several studies was found to be restricted to a single urban area [25, 27, 31, 36], to participants from RCTs [23, 39–41], to either female [24] or male participants [26, 28, 32] or to a single or two recruitment sites [22, 29, 30]. Other studies were more representative of geriatric patients [6, 34, 35], very large [33], or even studied a national sample of older individuals [37, 38]. Overall representativeness of studies was good as individual studies included both male and female participants were conducted in different settings, i.e. hospital wards, residential aged care facilities or community-dwelling older people. Studies were also conducted in various countries although these were predominantly countries from Australasia, Europe and North America (Table 1). Individuals who were not exposed to anticholinergic and sedative drug load were consistently drawn from the same population as exposed individuals.
Ascertainment of anticholinergic and sedative exposure
Exposure to drugs was thoroughly ascertained in the majority of studies, thus providing a substantial base for the DBI calculation. Drug exposure was assessed through participants’ self-report during a structured interview and a verification of participants’ answers through inspection of prescription forms and packages by qualified assessors [6, 24–28, 32, 34–36], through dispensing data on medication [33, 37, 38] or from clinical records [22, 23, 29–31, 39–41]. In some studies, the DBI was calculated for anticholinergic and/or sedative drugs separately [24, 30, 35].
However, some caveats were also observed. Although the grounds were mentioned for classifying drugs as being anticholinergic or sedative, none of the lists of DBI drugs has been published. Furthermore, studies conducted in the USA used the minimum recommended daily dose as approved by the US Food and Drug Administration [6] while studies outside the USA used other national reference sources to estimate the δ or the minimum daily dose. Possible differences between studies and the influence of such differences on associations between patients’ DBI values and clinical outcomes could not be assessed. One study examined the relationship between the DBI and SAA but found no significant relationship [30]. Other studies compared the DBI with other anticholinergic scales [35] or the Beers criteria [27].
Comparability of participants with high and low DBI values
In all but one study, [35] adjustment was made for prevalent co-morbidity. In all studies, age was adjusted for in relevant analyses and studies that included men and women also adjusted for sex. Several of these studies also adjusted for cognitive impairment, or presence of dementia [22, 23, 25–27, 29, 30, 36, 39, 40] and depressive or other neuropsychiatric symptoms including sleep problems [23, 24, 26–28, 34, 39, 40]. If cognitive function was the outcome, analyses had most of the time been adjusted for age [6, 24, 28, 39] and educational level [6, 24, 28], which are important determinants of cognitive function. Five studies adjusted for prescribed drugs other than those included in the DBI calculation [22, 24, 29, 40, 41]. In one study, patients and controls were matched for age, sex and region of residence [33].
Outcomes
Outcomes were usually assessed through record linkage, e.g. national prescription or reimbursement registers and hospital discharge registers [30, 33, 36–38], medical records and clinical notes [22, 31, 40, 41] or through objective tests (see below).
Tables 2 and 3 present the associations found in different studies between the DBI and various clinical outcomes. Across different studies, the DBI was either studied as a continuous or categorised measure.
Table 2.
Associations between the Drug Burden Index [DBI] and mortality, healthcare utilisation and falls
| Outcome category | S/NS | Outcome | DBI categorisation | Statistic |
|---|---|---|---|---|
| Mortality | ||||
| Dauphinot et al., 2014 [31] | NS | In-hospital mortality | DBI DDD increase | HR, 1.9 [95% CI: 0.8–4.4]a |
| Gnjidic et al., 2014 [33] | S | Mortality AD patients | Continuous | HR: 1.21 [95% CI: 1.09–1.33] |
| S | Mortality non-AD patients | Continuous | HR: 1.37 [95% CI: 1.20–1.56] | |
| Nishtala et al., 2014 [37] | S | Mortality | DBI [>0] | HR: 1.29 [95% CI: 1.25–1.33] |
| Wilson et al., 2012 [41] | NS | Mortality | DBI [0–1] | HR: 1.13 [95% CI: 0.82–1.57] |
| NS | Mortality | DBI [≥1] | HR: 1.19 [95% CI: 0.82–1.74] | |
| Mangoni et al., 2013 [30] | S | 1-year mortality | Anticholinergics | HR: 3.2 [95% CI: 1.1–9.4]a |
| Hospitalisation and GP visits | ||||
| Best et al., 2013 [22] | NS | Delirium related | DBI 0–1 | OR: 1.43 [95% CI: 0.79–2.62] |
| S | Delirium related | DBI [≥1] | OR: 2.95 [95% CI: 1.34–6.51] | |
| NS | Fall related | DBI 0–1 | OR: 1.30 [95% CI: 0.74–2.28] | |
| NS | Fall related | DBI [≥1] | OR: 1.52 [95% CI: 0.70–3.30] | |
| NS | Length of stay | DBI 0–1 | OR: 0.98 [95% CI: 0.59–1.63] | |
| NS | Length of stay | DBI [≥1] | OR: 0.74 [95% CI: 0.37–1.49] | |
| Gnjidic et al., 2014 [33] | NS | Length of stay AD patients | Continuous | IRR: 1.06 [95% CI: 0.99–1.12] |
| S | Length of stay non-AD patients | Continuous | IRR: 1.35 [95% CI: 1.24–1.46] | |
| S | No. admissions AD patients | Continuous | IRR: 1.22 [95% CI: 1.17–1.27] | |
| S | No. admissions non-AD patients | Continuous | IRR: 1.36 [95% CI: 1.28–1.43] | |
| Lowry et al., 2012 [29] | S | Length of stay | Continuous | HR: 1.23 [95% CI: 1.06–1.42] |
| Lönnroos et al., 2012 [36] | NS | Days per person-year | DBI 0–1 | RR: 1.10 [95% CI: 0.53–2.28] |
| NS | Days per person-year | DBI ≥ 1 | RR: 0.80 [95% CI: 0.29–2.22] | |
| Nishtala et al., 2014 [37] | S | Fall-related hospitalisation | DBI > 0 | IRR: 1.56 [95% CI: 1.48–1.65] |
| S | GP visits | DBI > 0 | IRR: 1.13 [95% CI: 1.12–1.13] | |
| Salahudeen et al., 2015 [38] | S | Hospital admission | Continuous | IRR: 1.36 [95% CI: 1.31–1.42] |
| S | Fall related | Continuous | IRR: 1.59 [95% CI: 1.46–1.74] | |
| S | Length of stay | Continuous | IRR: 1.50 [95% CI:1.44–1.56] | |
| S | GP visits | Continuous | IRR: 1.26 [95% CI:1.25–1.27] | |
| Falls | ||||
| Dauphinot et al., 2014 [31] | S | During hospital stay | DBI WHO increase | HR: 2.85 [95% CI: 1.14–7.12] |
| Wilson et al., 2011 [40] | S | 12-month study period | DBI 0–1 | IRR: 1.61 [95% CI: 1.17–2.23] |
| S | 12-month study period | DBI ≥ 1 | IRR: 1.90 [95% CI: 1.30–2.78] | |
S significant, NS not significant, HR hazard ratio, OR odds ratio, IRR incidence rate ratio, RR relative risk, AD Alzheimer’s disease, DDD defined daily dose, WHO World Health Organisation
aNot adjusted for covariates
Table 3.
Associations between the Drug Burden Index [DBI] and physical and cognitive function, and quality of life
| Outcome category | S/NS | Outcome | DBI categorisation | Statistic |
|---|---|---|---|---|
| Physical function and IADL | ||||
| Cao et al., 2008 [24] | S | Mobility difficulty | Anticholinergics | OR: 3.2 [95% CI: 1.5–6.9] |
| S | Slow gait | Anticholinergics | OR: 3.6 [95% CI: 1.6–8.0] | |
| S | Balance difficulty | Anticholinergics | OR: 4.9 [95% CI: 2.0–12.0] | |
| S | Chair stands | Anticholinergics | OR: 4.2 [95% CI: 2.0–8.7] | |
| S | Grip strength | Anticholinergics | OR: 2.4 [95% CI: 1.1–5.3] | |
| S | Upper extremity | Anticholinergics | OR: 2.7 [95% CI: 1.3–5.4] | |
| S | ADL | Anticholinergics | OR: 3.4 [95% CI: 1.7–6.9] | |
| S | Mobility difficulty | Sedatives | OR: 2.4 [95% CI: 1.1–5.3] | |
| NS | Slow gait | Sedatives | OR: 0.9 [95% CI: 0.4–1.9] | |
| NS | Balance difficulty | Sedatives | OR: 1.7 [95% CI: 0.7–4.0] | |
| NS | Chair stands | Sedatives | OR: 1.8 [95% CI: 0.8–3.9] | |
| S | Grip strength | Sedatives | OR: 3.3 [95% CI: 1.5–7.3] | |
| NS | Upper extremity | Sedatives | OR: 2.0 [95% CI: 1.0–4.2] | |
| NS | ADL | Sedatives | OR: 1.2 [95% CI: 0.6–2.2] | |
| Gnjidic et al., 2012a [25] | S | 10-m walking speed | DBI > 0 | B: −0.13 [95% CI: −0.19, −0.08] |
| S | Chair stands | DBI > 0 | B: 1.11 [95% CI: 1.05, 1.16] | |
| S | TUG | DBI > 0 | B: 1.13 [95% CI: 1.07, 1.19] | |
| S | IADL | DBI > 0 | B: −0.61 [95% CI: −0.84, −0.39] | |
| S | ADL | DBI > 0 | B: −3.21 [95% CI: −4.68, −1.75] | |
| NS | Grip strength | DBI > 0 | B: −0.98 [95% CI: −2.05, 0.08] | |
| Gnjidic et al., 2009 [26] | NS | Chair stands | DBI > 0 | B: 0.58 [95% CI: −0.11, 1.27] |
| S | Walking speed | DBI > 0 | B: −0.03 [95% CI: −0.05, −0.00] | |
| S | Narrow walk speed | DBI > 0 | B: −0.03 [95% CI: −0.05, −0.01] | |
| S | Balance difficulty | DBI > 0 | B: −0.11 [95% CI: −0.18, −0.03] | |
| S | Grip strength | DBI > 0 | B: −1.09 [95% CI: −1.90, −0.28] | |
| S | IADL | DBI > 0 | B: 0.18 [95% CI: 0.04, 0.32] | |
| Gnjidic et al., 2012b [27] | S | SPPB | Continuous | B: −1.28 [95% CI: −2.53, −0.04] |
| NS | Grip strength [kg] | Continuous | B: 0.10 [95% CI: −2.54, 2.74] | |
| Hilmer et al., 2009 [34] | S | SPPB | AUCDB | B: −0.08, t value: 2.46, p < .01 |
| S | Gait speed | AUCDB | B: −0.01, t value: −2.86, p = 0.004 | |
| S | Grip strength | AUCDB | B: −0.27, t value: −2.87, p = .004 | |
| Hilmer et al., 2007 [6] | S | Health ABC performance score | Continuous | B: −0.15, t value: −5.73, p < .001 |
| Lowry et al., 2012 [29] | S | Barthel Index | Continuous | OR: 0.71 [95% CI: 0.55–0.91] |
| Wilson et al., 2010 [39] | S | Balance | AUCDB sedatives | OR: 1.57 [95% CI: 1.08–2.27] |
| Gnjidic et al., 2012d [32] | S | Prefrail | DBI > 0 | OR: 1.62 [95% CI: 1.21, 2.15] |
| S | Frail | DBI > 0 | OR: 2.14 [95% CI: 1.25, 3.64] | |
| Cognitive function | ||||
| Cao et al., 2008 [24] | S | MMSE | Anticholinergics | OR: 2.4 [95% CI: 1.1–5.1] |
| NS | MMSE | Sedatives | OR: 1.1 [95% CI: 0.5–2.3] | |
| Gnjidic et al., 2012c [28] | NS | ACE | DBI > 0 | OR: 0.98 [95% CI: 0.66–1.47] |
| NS | TMT | DBI > 0 | OR: 0.71 [95% CI: 0.40–1.24] | |
| NS | Cognitive impairment | DBI > 0 | OR: 1.34 [95% CI: 0.83–2.16] | |
| Hilmer et al., 2007 [6] | S | DSST | Continuous | B: −1.51, t value: −2.50, p = .01 |
| Kashyap et al., 2014 [35] | S | TMT-B | Anticholinergic | OR: 2.2 [95% CI: 1.1–8.06]a |
| S | Delayed recall | Anticholinergic | OR: 4.2 [95% CI: 1.8–15.4] | |
| Quality of life | ||||
| Bosboom et al., 2012 [23] | S | QoL | DBI > 0 | B: −4.07 [95% CI: –7.25, −0.89] |
S significant, NS not significant, ACE Addenbrooke’s Cognitive Examination, ADL activities of daily living, DSST digit symbol substitution test, IADL instrumental activities of daily living, MMSE Mini-Mental Status Examination, QoL quality of life, SPPB Short Physical Performance Battery, TMT Trailmaking Test, TMT-B Trailmaking Test part-B, TUG Time Up and Go test, B unstandardised regression coefficient, OR odds ratio
aNot adjusted for covariates
The majority of associations of the DBI with mortality, hospitalisation, falls, physical function and (instrumental) activities of daily living ([I]ADL), cognitive function and quality of life were statistically significant. Three of the five studies which assessed mortality and five of the six studies assessing hospital admissions found positive associations between the DBI and these outcomes. Higher DBI values were consistently found to be associated with increased fall risk. Impairments of physical function and IADL were examined in nine studies. Most studies consistently showed a higher DBI to be associated with several impairments with regard to mobility, balance difficulty, gait speed, IADL and ADL. Findings were equivocal for grip strength and chair stands. Compared to physical function, cognitive function was less frequently studied. Cognitive function was investigated in four studies using measures of global cognition and executive function, e.g. concentration and planning ability. Tests used were the Mini Mental Status Examination (MMSE) [24], specific tests [6, 39] or more extensive neuropsychological test batteries. [28, 35] Quality of life was also understudied as it was explicitly addressed in one study [23].
Longitudinal studies
Eleven studies reported longitudinal results from eight patient cohorts. Incidence of outcomes was consistently taken into consideration, either through studying incidence of falls, frailty, GP visits, hospitalisation, mortality and physical function during a follow-up period [31–33, 36–38, 40, 41], through adjusting in the analyses for baseline physical function [34, 39] or through assessing change in cognitive function [35]. Studies with the shortest follow-ups, up to 12 months [36, 39, 40], had low attrition, being 4 and 13%, whereas those with a longer follow-up had attrition rates that not surprisingly ranged from 20% in a 2-year follow-up study [32] to ~30% in a 6-year follow-up study [34]. Although the ‘lost to follow-up’ rate of the latter study was substantial, it was associated with only minor differential attrition or selective loss to follow-up. An interesting modification of the DBI was the area under the curve for drug burden (AUCDB) or the average drug burden at each point in time multiplied by the time of exposure [34, 39]. This AUCDB enabled researchers to estimate the cumulative long-term exposure to anticholinergic and sedative drugs.
However, at the same time, the scope of the longitudinal studies was rather limited. They included registry data about ultimate outcomes such as mortality [31, 33, 37, 41], hospital admission, [33, 36–38] and falls [31, 40]. The number of prospective cohort studies that examined proximal outcomes, as directly assessed from patients themselves, was limited and addressed a limited number of outcomes of frailty, physical function, e.g. gait speed, grip strength, and balance and cognitive function [32, 34, 35, 39].
Discussion
Overall, the studies of the DBI that have been conducted so far were of good methodological quality. Importantly, in all but one study, analyses were adjusted for co-morbidity. Although it is impossible to adjust for all confounding factors, adjusting for co-morbidity renders it unlikely that positive associations between the DBI and clinical outcomes simply reflected the treatment of multiple diseases or disorders with multiple drugs. The general picture of studies suggested that the DBI was examined in large to very large samples of older individuals who were diverse with respect to gender, residence and mean number of medicines prescribed. Exposure to drugs was in the majority of studies thoroughly ascertained through assessing medicine packages or dispensing data. Some longitudinal studies also adopted the AUCDB an adaptation of the DBI suitable for longitudinal research that takes the time of exposure to anticholinergic and sedative drugs into account. Differences between patients with high and low DBI values were adjusted for in analyses.
A large number of studies found associations between the DBI and relevant clinical outcomes. Impairments of physical function and IADL were most extensively examined. The physical measures provided interesting objective ‘proxy measures’ of fall risk particularly mobility and balance measures. Outcomes were assessed through medical records, validated tests or questionnaires. Longitudinal studies often had adequate follow-ups with attrition being either minor or not differential. Together, these findings support the use of the DBI in both research and clinical practice. In research, the DBI could serve as an important covariate that should be controlled for when examining, e.g. predictors of falling. In clinical practice, the DBI may be useful as a screener of frail patients to identify individuals with high anticholinergic and sedative exposure which might aggravate their physical and cognitive impairment. For example, a 1.5- to 3-fold increased risk of falling was observed for patients with high exposure to anticholinergic and sedative drugs compared to patients with no such exposure (see Table 2). Such patients are likely to be eligible for deprescribing interventions such as medication reviews conducted by pharmacists and general practitioners. In turn, screening with the DBI could be examined in controlled trials.
We have three suggestions about representativeness and selection of populations for further research. First, the DBI has been studied exclusively in older geriatric patient groups. However, the DBI could also be useful for other vulnerable but younger patient groups such as patients with psychiatric disorders and people with severe intellectual disabilities. Like in geriatric patients, these patients struggle with cognitive impairment. Polypharmacy with psychotropic medication is very common in people suffering from schizophrenia [42] and depression [43]. Patients often experience a high burden from the side effects of psychotropic medications [44]. Similar problems are known in people with severe intellectual disabilities [45, 46]. Second, studies of patients from other parts of the world including, e.g. China, the Middle East and South America are also worthwhile to pursue. Third, to further improve the clinical utility of the DBI for older individuals and in other vulnerable patient groups, more knowledge about the clinical implications of a certain DBI score is needed. In particular, whether this also depends on the underlying disease. DBI scores may carry a higher risk for patients who suffer from a degenerative disease such as Alzheimer’s disease, because of the loss of cholinergic neurons and the increased uptake of anticholinergic drugs in the brain due to increased permeability of the blood brain barrier [15].
A recommendation about the DBI calculation is that this should be based on a consensus list of medicines with anticholinergic and sedative properties which will be updated regularly, e.g. Duran et al. [47] have made attempts for a list of medications with anticholinergic properties. Also, the current DBI formula assumes that different drugs contribute linearly to the DBI score regardless of their potency. We suggest exploring the effects of weighing medication with high and low anticholinergic or sedative potency [15]. Finally, a potential source of bias is the high mortality rate in frail older people with high anticholinergic and sedative burden. This makes a careful examination of ‘differential attrition’ or selective loss to follow-up, owing to, e.g. baseline frailty, of even greater importance for cohort studies which enrol frail older people than for cohort studies in general.
Further research about the relationship between the DBI and cognitive function is needed. Specifically, for community-dwelling geriatric patients without dementia or early dementia as well as patients with psychiatric disorders, assessment of cognition with a standardised neuropsychological examination as was previously done [35] is needed in addition to an assessment of cognition with the MMSE [24] which has a diminished capacity to detect early cognitive impairment. [48] An extensive neuropsychological test battery allows a more sensitive assessment of a wide range of different cognitive functions. Furthermore, examination how the DBI relates to brain function using functional magnetic resonance imaging (fMRI) would be relevant in this regard. [49] For physical function tests, the opposite may actually be true, as Wilson et al. [39] argued that these tests might be too difficult for residents in RACFs who have advanced functional decline. For people with more advanced physical decline, selection of easier physical tests would be recommended. Moreover, quality of life could also be assessed [50] as a measure of general well-being.
This review had several strengths. The most important strength was our assessment of the methodological quality of studies using a standardised scale. Another strength was that the data extraction was reviewed by a second researcher. A possible limitation of our review was that it was not possible to conduct a quantitative meta-analysis, because of the limited number of studies, the wide array of clinical outcomes, the analysis of the DBI in different ways (i.e. continuous or dichotomous) and the use of country-specific minimum daily doses. Future meta-analyses, preferably individual patient data meta-analyses, should examine whether differences in study findings are associated with methodological differences between studies.
Thus, an accumulating body of evidence supports the validity of the DBI. What lies ahead are steps towards further refinement of the DBI in longitudinal studies aimed at substantiating the present body of evidence using an array of clinical outcomes in geriatric and other relevant patient groups.
Authors’ contributions
Study design: HW KT
Data extraction: HW HVDM KT
Synthesis of findings: HW KT
Manuscript drafting: HW
Critical review of manuscript contents: HVDM KT
Electronic supplementary material
(DOC 386 kb)
Compliance with ethical standards
Funding
This work was supported by the Netherlands Organisation for Health Research and Development ([ZonMw] [grant number 80-83600-98-10176]) and Stichting Stoffels-Hornstra.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42–51. doi: 10.1016/j.amjopharm.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Johnell K, Fastbom J. The use of benzodiazpines and related drugs amongst older people in Sweden: associated factors and concomitant use of other psychotropics. Int J Geriatr Psychiatry. 2009;24(7):731–738. doi: 10.1002/gps.2189. [DOI] [PubMed] [Google Scholar]
- 3.Gray SL, Penninx BW, Blough DK, Artz MB, Guralnik JM, Wallace RB, et al. Benzodiazepine use and physical performance in community-dwelling older women. J Am Geriatr Soc. 2003;51(11):1563–1570. doi: 10.1046/j.1532-5415.2003.51502.x. [DOI] [PubMed] [Google Scholar]
- 4.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60(2):198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 5.Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235–241. doi: 10.1038/sj.clpt.6100035. [DOI] [PubMed] [Google Scholar]
- 6.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–787. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 7.Tune L, Coyle JT. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry. 1980;37(3):293–297. doi: 10.1001/archpsyc.1980.01780160063007. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 9.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer HG, Wouters H, van Hulten R, Pras N, Taxis K. Decreasing the load? Is a multidisciplinary multistep medication review in older people an effective intervention to reduce a patient’s Drug Burden Index? Protocol of a randomised controlled trial. BMJ Open. 2015;5(12):e009213. doi: 10.1136/bmjopen-2015-009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnjidic D, Le Couteur D, Abernethy D, Hilmer S. A pilot randomized clinical trial utilizing the Drug Burden Index to reduce exposure to anticholinergic and sedative medications in older people. Ann Pharmacother [Internet] 2010;44:1725–1732. doi: 10.1345/aph.1P310. [DOI] [PubMed] [Google Scholar]
- 12.Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the ‘oldest old’: a systematic review of the literature. Drugs Aging. 2015;32(10):835–848. doi: 10.1007/s40266-015-0310-9. [DOI] [PubMed] [Google Scholar]
- 13.Kouladjian L, Gnjidic D, Chen TF, Mangoni AA, Hilmer SN. Drug Burden Index in older adults: theoretical and practical issues. Clin Interv Aging. 2014;9:1503–1515. doi: 10.2147/CIA.S66660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pont LG, Nielen JT, McLachlan AJ, Gnjidic D, Chan L, Cumming RG et al. (2015) Measuring anticholinergic drug exposure in older community-dwelling Australian men: a comparison of four different measures. Br J Clin Pharmacol 80(5):1169–1175 [DOI] [PMC free article] [PubMed]
- 15.Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain—how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151–159. doi: 10.1111/bcpt.12140. [DOI] [PubMed] [Google Scholar]
- 16.Pasina L, Djade CD, Lucca U, Nobili A, Tettamanti M, Franchi C, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging. 2013;30(2):103–112. doi: 10.1007/s40266-012-0044-x. [DOI] [PubMed] [Google Scholar]
- 17.Lertxundi U, Domingo-Echaburu S, Hernandez R, Peral J, Medrano J. Expert-based drug lists to measure anticholinergic burden: similar names, different results. Psychogeriatrics. 2013;13(1):17–24. doi: 10.1111/j.1479-8301.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 18.Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–220. doi: 10.1111/bcp.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital/l’Hôpital d’Ottawa. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 22.Best O, Gnjidic D, Hilmer SN, Naganathan V, McLachlan AJ. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43(8):912–918. doi: 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 23.Bosboom PR, Alfonso H, Almeida OP, Beer C. Use of potentially harmful medications and health-related quality of life among people with dementia living in residential aged care facilities. Dement Geriatr Cogn Dis Extra. 2012;2(1):361–371. doi: 10.1159/000342172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, et al. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther. 2008;83(3):422–429. doi: 10.1038/sj.clpt.6100303. [DOI] [PubMed] [Google Scholar]
- 25.Gnjidic D, Bell JS, Hilmer SN, Lonnroos E, Sulkava R, Hartikainen S. Drug Burden Index associated with function in community-dwelling older people in Finland: a cross-sectional study. Ann Med. 2012;44(5):458–467. doi: 10.3109/07853890.2011.573499. [DOI] [PubMed] [Google Scholar]
- 26.Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, et al. Drug Burden Index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68(1):97–105. doi: 10.1111/j.1365-2125.2009.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. Drug burden index and beers criteria: impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol. 2012;52(2):258–265. doi: 10.1177/0091270010395591. [DOI] [PubMed] [Google Scholar]
- 28.Gnjidic D, Le Couteur DG, Naganathan V, Cumming RG, Creasey H, Waite LM, et al. Effects of drug burden index on cognitive function in older men. J Clin Psychopharmacol. 2012;32(2):273–277. doi: 10.1097/JCP.0b013e3182487825. [DOI] [PubMed] [Google Scholar]
- 29.Lowry E, Woodman RJ, Soiza RL, Hilmer SN, Mangoni AA. Drug burden index, physical function, and adverse outcomes in older hospitalized patients. J Clin Pharmacol. 2012;52(10):1584–1591. doi: 10.1177/0091270011421489. [DOI] [PubMed] [Google Scholar]
- 30.Mangoni AA, van Munster BC, Woodman RJ, de Rooij SE. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry. 2013;21(8):785–793. doi: 10.1016/j.jagp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Dauphinot V, Faure R, Omrani S, Goutelle S, Bourguignon L, Krolak-Salmon P, et al. Exposure to anticholinergic and sedative drugs, risk of falls, and mortality: an elderly inpatient, multicenter cohort. J Clin Psychopharmacol. 2014;34(5):565–570. doi: 10.1097/JCP.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 32.Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91(3):521–528. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 33.Gnjidic D, Hilmer SN, Hartikainen S, Tolppanen AM, Taipale H, Koponen M, et al. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer’s disease: a national population cohort study. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilmer SN, Mager DE, Simonsick EM, Ling SM, Windham BG, Harris TB, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12):1142. doi: 10.1016/j.amjmed.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashyap M, Belleville S, Mulsant BH, Hilmer SN, Paquette A, Tu le M, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. J Am Geriatr Soc. 2014;62(2):336–341. doi: 10.1111/jgs.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonnroos E, Gnjidic D, Hilmer SN, Bell JS, Kautiainen H, Sulkava R, et al. Drug Burden Index and hospitalization among community-dwelling older people. Drugs Aging. 2012;29(5):395–404. doi: 10.2165/11631420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–758. doi: 10.1002/pds.3624. [DOI] [PubMed] [Google Scholar]
- 38.Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85–90. doi: 10.1111/jgs.13206. [DOI] [PubMed] [Google Scholar]
- 39.Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and physical function in older people in residential aged care facilities. Age Ageing. 2010;39(4):503–507. doi: 10.1093/ageing/afq053. [DOI] [PubMed] [Google Scholar]
- 40.Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59(5):875–880. doi: 10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilson NM, Hilmer SN, March LM, Chen JS, Gnjidic D, Mason RS, et al. Associations between drug burden index and mortality in older people in residential aged care facilities. Drugs Aging. 2012;29(2):157–165. doi: 10.2165/11598570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Patel MX, Bishara D, Jayakumar S, Zalewska K, Shiers D, Crawford MJ, et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol. 2014;24(4):499–509. doi: 10.1016/j.euroneuro.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Jureidini J, Tonkin A. Overuse of antidepressant drugs for the treatment of depression. CNS Drugs. 2006;20(8):623–632. doi: 10.2165/00023210-200620080-00002. [DOI] [PubMed] [Google Scholar]
- 44.Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353–362. doi: 10.1177/0269881114562090. [DOI] [PubMed] [Google Scholar]
- 45.Tsiouris JA. Pharmacotherapy for aggressive behaviours in persons with intellectual disabilities: treatment or mistreatment? J Intellect Disabil Res. 2010;54(1):1–16. doi: 10.1111/j.1365-2788.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 46.van der Heide DC, van der Putten AA, van den Berg PB, Taxis K, Vlaskamp C. The documentation of health problems in relation to prescribed medication in people with profound intellectual and multiple disabilities. J Intellect Disabil Res. 2009;53(2):161–168. doi: 10.1111/j.1365-2788.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 47.Duran CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69(7):1485–1496. doi: 10.1007/s00228-013-1499-3. [DOI] [PubMed] [Google Scholar]
- 48.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 49.Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, et al. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci U S A. 2002;99(1):455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 386 kb)