Abstract
Background
In Japan, the clinical efficacy of erlotinib monotherapy in epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer was demonstrated in the phase II JO22903 trial, which reported a median progression-free survival of 11.8 months. Here we report final overall survival data from JO22903.
Methods
JO22903 (JapicCTI-101085) was a single-arm, multicenter, phase II, open-label, non-randomized study of first-line erlotinib monotherapy in EGFR mutation-positive non-small-cell lung cancer. Eligible patients (≥20 years) with stage IIIB/IV or recurrent non-small-cell lung cancer and confirmed activating mutations of EGFR (exon 19 deletion or L858R point mutation in exon 21) received oral erlotinib 150 mg/day until disease progression or unacceptable toxicity. The primary endpoints were progression-free survival and safety; overall survival was a secondary endpoint.
Results
At the final analysis, 102 patients were included in the modified intent-to-treat population and 103 in the safety population. Median follow-up was 32.3 months. Median overall survival was 36.3 months (95 % confidence interval 29.4–not reached). Subgroup analyses of overall survival suggested that the presence of brain metastases was a negative prognostic factor (median overall survival 22.7 months, 95 % confidence interval 19.6–29.4). The impact on overall survival of using versus not using EGFR tyrosine kinase inhibitors in any line of treatment following disease progression was unclear (median 32.8 versus 36.3 months, respectively). No new safety issues were observed.
Conclusion
In this survival update, single-agent erlotinib achieved a median overall survival of more than 3 years in patients with EGFR mutation-positive non-small-cell lung cancer.
Keywords: Erlotinib, EGFR mutations, Non-small-cell lung cancer (NSCLC), First line, Japanese patients, Overall survival
Introduction
In non-small-cell lung cancer (NSCLC), platinum doublet chemotherapy followed by second-line docetaxel monotherapy [1] or pemetrexed maintenance therapy following first-line platinum doublet chemotherapy [2] prolongs survival outcomes for patients with non-squamous NSCLC. Based on the efficacy of these treatments, it has been anticipated that they will improve long-term survival of patients with epidermal growth factor receptor (EGFR) mutation-positive NSCLC after the administration of EGFR tyrosine kinase inhibitors (TKIs).
The treatment of NSCLC has changed considerably in recent years. Following the discovery of the pivotal oncogenic role of EGFR in unselected NSCLC [3, 4], the subsequent development of EGFR TKIs provided new therapeutic options for the treatment of this disease. Greater understanding of tumor biology has since led to the discovery that tumors with sensitizing EGFR mutations, particularly the somatic mutations in EGFR exons 19 and 21, respond favorably to EGFR TKIs compared with chemotherapy [5]. To reflect this, EGFR TKIs are recommended in clinical treatment guidelines for NSCLC.
Currently, gefitinib, erlotinib and afatinib are the only EGFR TKIs approved (US Food and Drug Administration, EU and Japan) for the treatment of EGFR mutation-positive NSCLC [6, 7]. These approvals were supported by data from several phase III clinical trials, which consistently reported that EGFR TKIs demonstrate significant progression-free survival (PFS) benefits compared with standard chemotherapy [8]. Median PFS with first-line gefitinib in EGFR mutation-positive NSCLC ranged between 9.6 and 10.4 months in the pan-Asian IPASS study of gefitinib versus carboplatin/paclitaxel [9], the Japanese NEJ002 study of gefitinib versus carboplatin-paclitaxel [10], and the WJTOG3405 study of gefitinib versus cisplatin/docetaxel [11]. However, despite similar PFS results with gefitinib in these studies, median OS was not consistent; the IPASS study reported a median OS of 21.6 months with gefitinib [9], whereas a longer median OS of 27.7 months was published in the NEJ002 study [10] and a median OS of 34.8 months was reported with gefitinib in the Japanese WJTOG3405 study [11].
Median OS with erlotinib in EGFR mutation-positive NSCLC was 22.7 months in the phase III OPTIMAL study of erlotinib versus gemcitabine plus carboplatin [12], and 22.9 months in the phase III EURTAC study of erlotinib versus chemotherapy [13]. However, as these two studies were conducted outside of Japan, the median OS with erlotinib in Japanese patients with EGFR mutation-positive NSCLC is currently unknown. PFS for the single-agent erlotinib arm of the Japanese phase II JO25567 study was 9.7 months [14], which was similar to the 11.8 months median PFS (primary endpoint) reported for the phase II Japanese JO22903 study [15]. Here, we report final OS data with erlotinib monotherapy in the JO22903 study and present exploratory analyses of OS with respect to EGFR mutation subtype. We also evaluated whether OS was impacted by the use of post-progression therapy.
Patients and methods
Study design and patients
JO22903 (JapicCTI-101085) was a phase II, single-arm, multicenter, open-label, non-randomized study of first-line erlotinib monotherapy for the treatment of EGFR mutation-positive NSCLC. Full study design information has been previously published [15]. Briefly, the study was conducted at 25 centers in Japan. Patients were aged ≥20 years with stage IIIB/IV or recurrent NSCLC, with no prior chemotherapy, Eastern Cooperative Oncology Group performance status of 0 or 1, and tumors harboring confirmed activating mutations of EGFR (exon 19 deletions or L858R point mutations in exon 21). Patients were excluded if they had symptomatic brain metastases or if they had co-existence or history of interstitial lung disease (ILD). After discontinuation of the protocol treatment, patients were treated at the investigators discretion.
JO22903 was carried out in accordance with the Declaration of Helsinki and also the Japanese Good Clinical Practice Guidelines. All patients provided written informed consent for study participation. The study protocol was approved by the local ethics committees.
Procedures
Full treatment procedures have been published previously [15]. Briefly, patients received oral erlotinib 150 mg/day until disease progression (PD) or unacceptable toxicity. Treatment was interrupted if ILD was suspected; for patients with confirmed ILD diagnosis, erlotinib was discontinued immediately. In cases of gastrointestinal perforation or any grade 4 adverse events (AEs), erlotinib was discontinued. Patients were screened for EGFR mutations in a local or central laboratory; EGFR mutation status was determined using Scorpion ARMS as described previously [15]. Lung and abdominal scans [computed tomography (CT)/magnetic resonance imaging (MRI)] were mandatory at baseline and during treatment until PD. Brain scans were mandatory at baseline (CT/MRI).
Assessments
Tumor response was assessed by an independent review committee (IRC) using Response Evaluable Criteria in Solid Tumours (RECIST) version 1.0. The analysis of safety parameters was descriptive; safety was assessed according to the Medical Dictionary for Regulatory Activities (version 14.0) preferred terms and tabulated by grade. All patients who received at least one dose of study treatment were included in the safety population. A modified intent-to-treat (ITT) population was used for the efficacy analysis, which included all patients from the safety population without major protocol violations.
Study endpoints
The co-primary endpoints were PFS in the modified ITT population as assessed by IRC according to RECIST version 1.0, and safety. Secondary endpoints included OS and overall response rate.
Statistical analyses
Kaplan–Meier methodology was used to estimate median and 95 % confidence intervals (CI) for OS, and hazard ratios (HR) were estimated by the use of a Cox model. CI limits were calculated according to the Greenwood method.
Results
Patients
Patients were enrolled between April 2010 and October 2010. Median follow-up was 32.2 months. At the time of this final analysis, 103 patients with confirmed EGFR mutations were included in the study. The safety population comprised all 103 patients whilst the modified ITT population comprised 102 patients; one patient was excluded due to a major protocol violation (receipt of incorrect study medication) after enrolment.
Baseline patient characteristics have been previously published [15]. Briefly, the majority of patients were female (n = 70), with stage IV disease (n = 74), adenocarcinoma histology (n = 102), and were never-smokers (n = 59).
Efficacy analyses
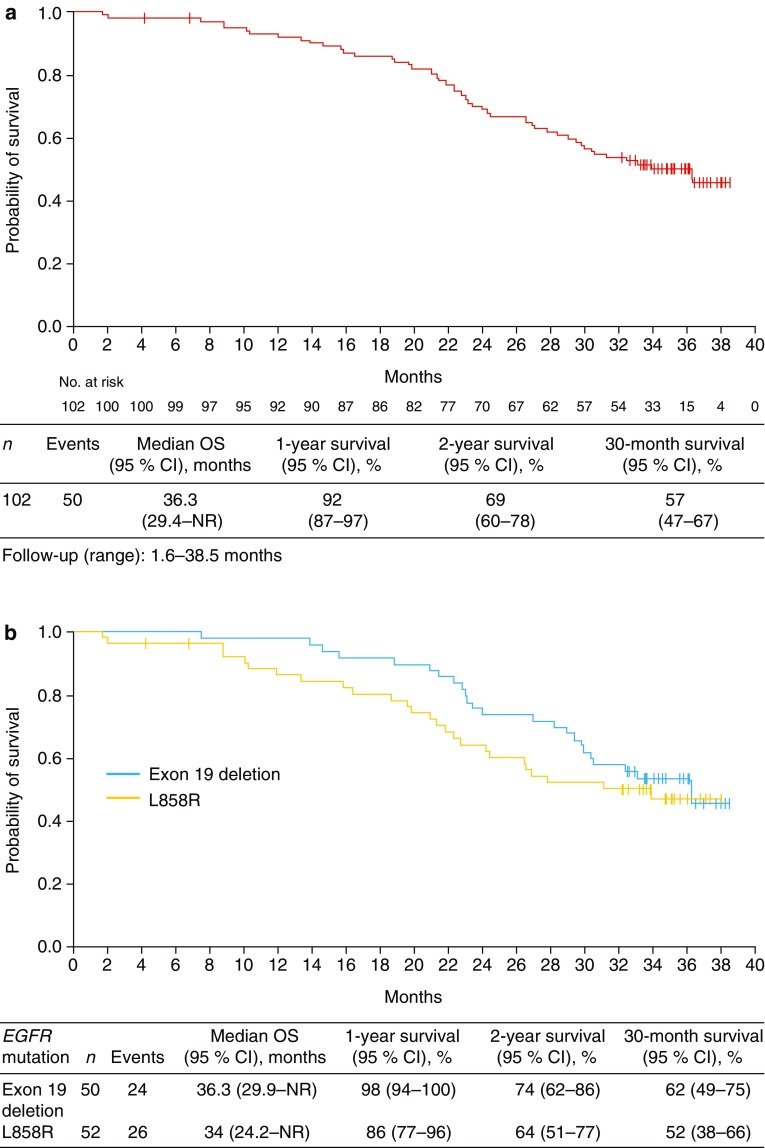
In the modified ITT population at the updated data cut-off, median OS with first-line erlotinib was 36.3 months (95 % CI: 29.4–not reached [NR]) based on the occurrence of 50 events. The 1-year survival rate was 92 % (95 % CI 87–97), the 2-year survival rate was 69 % (95 % CI 60–78) and the 30-month survival rate was 57 % (95 % CI 47–67) (Fig. 1a). Univariate subgroup analyses showed shorter OS in patients with brain metastases at baseline (median OS 22.7 months, 95 % CI 19.6–29.4) and in those with a T790M EGFR mutation (median OS 20.0 months, 95 % CI 15.8–24.2) (Table 1). When analyzed by the specific type of EGFR mutation, median OS was 36.3 months (95 % CI 29.9–NR) versus 34.0 months (95 % CI 24.2–NR), respectively, for patients with exon 19 deletion versus exon 21 L858R point mutation (HR 0.77, 95 % CI 0.44–1.35, p = 0.3662) (Fig. 1b).
Fig. 1.
Overall survival with a erlotinib monotherapy in the modified ITT population and b by EGFR mutation type
Table 1.
Subgroup analysis of median overall survival
| Characteristics | n | Events | Median OS (months) | 95 % CI |
|---|---|---|---|---|
| Gender | ||||
| Female | 69 | 33 | 36.3 | 29.4–NR |
| Male | 33 | 17 | 34.0 | 23.4–NR |
| Age | ||||
| <75 years | 88 | 43 | 36.3 | 28.3–NR |
| ≥75 years | 14 | 7 | 31.2 | 18.6–NR |
| Stage | ||||
| IIIB/IV | 77 | 43 | 31.2 | 26.5–NR |
| Recurrence | 25 | 7 | NR | 28.3–NR |
| Smoking status | ||||
| Yes | 44 | 24 | 31.2 | 23.4–NR |
| No | 58 | 26 | 36.3 | 29.8–NR |
| EGFR mutation status | ||||
| Exon 19 deletion | 50 | 24 | 36.3 | 29.8–NR |
| L858R | 50 | 24 | 34.0 | 22.7–NR |
| L858R + T790M | 2 | 2 | 20.0 | 15.8–24.2 |
| Brain metastases | ||||
| Yes | 21 | 16 | 22.7 | 19.6–29.4 |
| No | 81 | 34 | NR | 32.4–NR |
CI confidence interval, EGFR epidermal growth factor receptor, OS overall survival, NR not reached
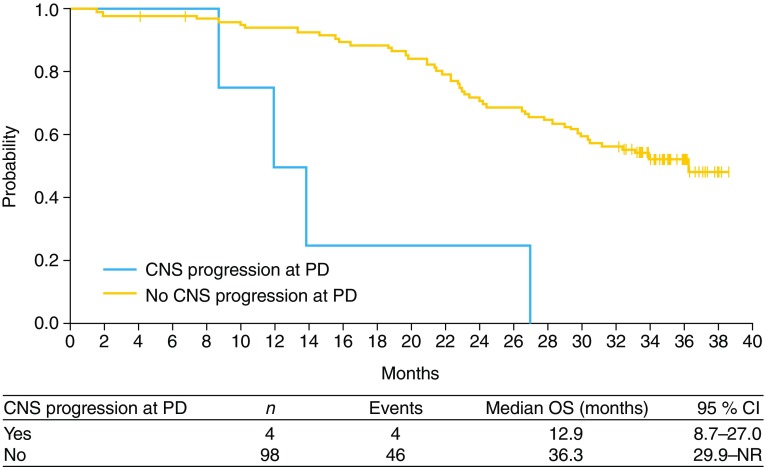
Four patients had PD with central nervous system (CNS) progression (Table 2; Fig. 2). Median OS was shorter in patients with CNS PD compared with those without (12.9 months [95 % CI 8.7–27.0] versus 36.3 months [95 % CI 22.9–NR]).
Table 2.
Characteristics of patients who had CNS progression in erlotinib treatment
| Number | Age (years) | Gender | ECOG PS | EGFR mutation | Baseline CNS metastases | Erlotinib dose at PD (mg) | PFS (days) | OS (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | 1 | 19 del | No | 100 | 106 | 420 |
| 2 | 55 | M | 1 | 19 del | Yes | 150 | 335 | 823 |
| 3 | 55 | F | 0 | L858R | No | 150 | 168 | 363 |
| 4 | 73 | F | 1 | L858R | Yes | 150 | 80 | 266 |
CNS central nervous system, del deletion, ECOG PS Eastern Cooperative Oncology Group performance status, EGFR epidermal growth factor receptor, F female, M male, OS overall survival, PD progressive disease, PFS progression-free survival
Fig. 2.
Overall survival by CNS progression
Post-progression therapy
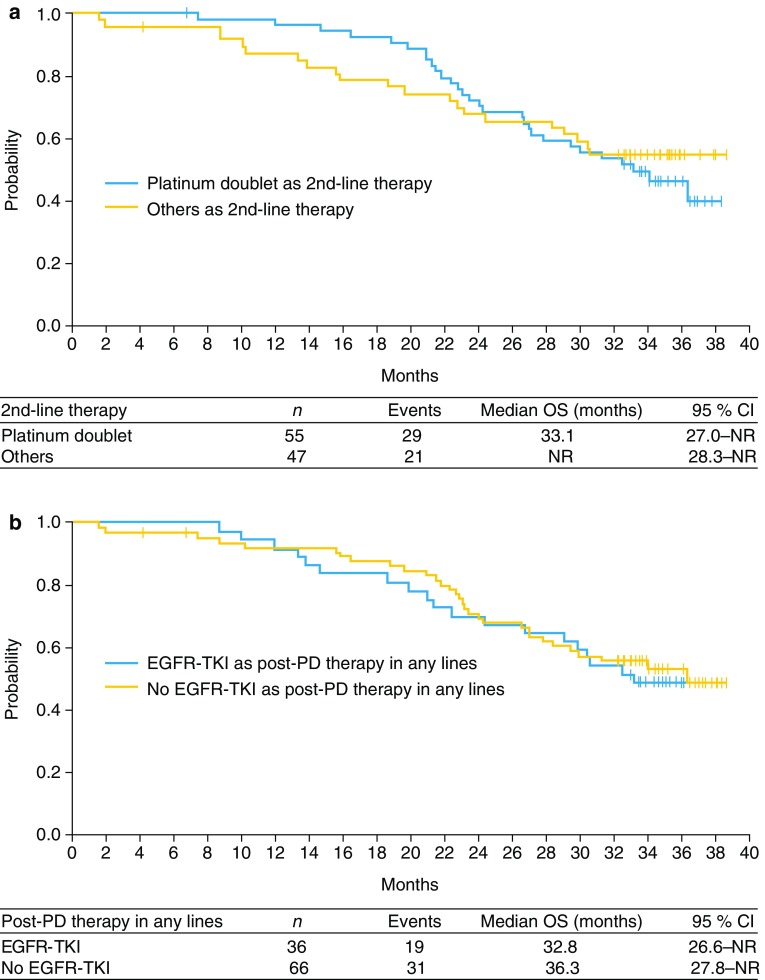
Following PD, the majority of patients went on to receive either platinum doublet chemotherapy, with or without bevacizumab (n = 60), further EGFR TKIs (n = 35), or single-agent chemotherapy (n = 39) (Table 3). In terms of second-line therapy, median OS was similar in patients who were treated with platinum doublet chemotherapy or other types of therapy [median OS 33.1 months (95 % CI 27.0–NR) versus NR, respectively; Fig. 3a]. The use of further EGFR TKIs in any line of treatment following PD also had no apparent impact on OS compared with not using an EGFR TKI as post-PD therapy in any line [median OS 32.8 months (95 % CI 26.6–NR) versus 36.3 months (95 % CI 27.8–NR), respectively; Fig. 3b].
Table 3.
Therapies given upon disease progression (eight patients were receiving study treatment at data collection. Information was unavailable for ten patients)
| Therapy (n) | Second-line therapy | All lines of treatment |
|---|---|---|
| Platinum doublet | 46 | 60 |
| Without bevacizumab | 31 | 41 |
| With bevacizumab | 15 | 21 |
| EGFR TKI | 30 | 35 |
| Erlotinib | 21 | 25 |
| Gefitinib | 8 | 14 |
| Erlotinib + tivantinib | 1 | 1 |
| Erlotinib + pemetrexed | 0 | 1 |
| Gefitinib + pemetrexed | 0 | 1 |
| Single-agent chemotherapy | 7 | 39 |
| Docetaxel + bevacizumab | 3 | 4 |
| Pemetrexed | 3 | 15 |
| Docetaxel | 1 | 24 |
| Pemetrexed + bevacizumab | 0 | 1 |
| Platinum doublet + EGFR TKI | 1 | 2 |
| With erlotinib | 1 | 2 |
| Others | 0 | 16 |
EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor
Fig. 3.
Overall survival by post-PD therapy with a second-line platinum doublet chemotherapy and b EGFR TKI in any line
Safety
The safety profile of erlotinib did not change at this data update (Table 4) and was as previously reported [15]. The most common all grade treatment-related AEs were rash (82.5 %) and diarrhea (79.6 %), and the most common grade ≥3 treatment-related AEs were rash (14.6 %) and an increase in alanine aminotransferase (8.7 %).
Table 4.
Treatment-related adverse events, all grades (≥30 %) and grade ≥3 (≥5 %)
| All grades (≥30 %) | Grade ≥3 (≥5 %) | |
|---|---|---|
| n (%) | n (%) | |
| Rash | 85 (82.5) | 15 (14.6) |
| Diarrhea | 82 (79.6) | 0 (0.0) |
| Dry skin | 82 (79.6) | 0 (0.0) |
| Paronychia | 69 (67.0) | 0 (0.0) |
| Stomatitis | 65 (63.1) | 0 (0.0) |
| Pruritus | 67 (65.0) | 0 (0.0) |
| Decreased appetite | 35 (34.0) | 0 (0.0) |
| ALT increased | 33 (32.0) | 15 (14.6) |
ALT alanine aminotransaminase
Discussion
EGFR TKIs are the standard of care for the first-line treatment of EGFR mutation-positive NSCLC [6, 7]. In Japan, the phase II, single-arm JO22903 study demonstrated efficacy of erlotinib monotherapy in EGFR mutation-positive NSCLC, with a reported median PFS of 11.8 months [15]. In this updated analysis of the JO22903 study, the 30-month OS rate was 57 % (95 % CI 47–67) and median OS was 36.3 months (95 % CI 29.4–NR). These findings represent a more favorable OS than observed in previous studies of first-line erlotinib in EGFR mutation-positive NSCLC outside Japan (median OS range 22.9–26.3 months [12, 13, 16]), and are in line with results from prospective studies of other EGFR TKIs in Japanese populations (median OS range 27.7–34.8 months [10, 11]). Recently, a median OS of 46.9 months was reported for Japanese patients who received afatinib in the LUX-Lung 3 study [17], which was longer than that observed in the entire study population [18]. Across these studies, the median PFS values observed in Japanese and global populations were very similar, at approximately 1 year [10–13, 16–18]. Thus, it seems that the current treatment landscape in Japan may be contributing to a longer OS compared with non-Japanese populations, and that OS in Japanese populations can reasonably be expected to reach beyond 3 years.
Although patients with brain metastases have a poor prognosis, which is reflected by the shorter median OS for this subgroup, the findings of this present analysis suggest that erlotinib could be considered effective for patients with brain metastases, as only four patients had CNS progression. This finding is consistent with the phase II ASPIRATION study in Asian patients, which reported that just 4.3 % of patients treated with post-PD erlotinib had new brain lesions [19]. This role for EGFR TKIs has also been observed in populations not restricted to Japanese or Asian patients [20–22]. A case series of 15 patients with NSCLC with EGFR mutations and CNS metastases who received cerebrospinal fluid concentration (CSF) examinations during EGFR TKI treatment provides further evidence to support this conclusion. In this case series, CNS response rate was 57 % with a favorable penetration rate of erlotinib in the CSF [23]. The penetration rate of erlotinib may be dependent on its affinity for p-glycoprotein, which pumps drugs out of the CNS. These findings suggest that erlotinib has a favorable pharmacokinetic profile as a treatment option for patients with brain metastases.
Patients with an exon 19 deletion appeared to have longer OS in our analysis than those with exon 21 L858R EGFR mutation-positive NSCLC. This is similar to the results of a meta-analysis of seven trials (n = 1649), which concluded that patients with an exon 19 deletion had better efficacy outcomes than patients with exon 21 L858R EGFR mutation-positive NSCLC, regardless of which EGFR TKI they received [24]. These data suggest that patients with exon 19 deletion and exon 21 L858R EGFR mutation are clinically distinct populations that should be evaluated further.
In the present study, there was no apparent difference in OS according to subsequent treatments. Median OS was similar for patients who received EGFR TKIs as post-PD therapy (n = 36), which were mainly continuous erlotinib administration following RECIST PD (n = 21) (Table 3), and for those who did not. In contrast to our findings, in a retrospective study of patients with activating EGFR mutations (n = 123) who were treated with EGFR TKIs, OS showed a trend in favor of continuing versus discontinuing EGFR TKI treatment following RECIST PD (33.0 versus 21.2 months, respectively; p = 0.054) [25]. Furthermore, a retrospective clinical modeling study that evaluated the usefulness of EGFR TKI failure pattern for selecting subsequent management, suggested that the efficacy of EGFR TKI continuation differed between patients with gradual progression, local progression, and dramatic progression [26]. Thus, one hypothesis for the inconsistency between studies is the difference in the EGFR TKI failure pattern. Meanwhile, in the present study, various EGFR TKIs were used as post-PD therapy (i.e., erlotinib beyond progression, erlotinib re-challenge after another treatment, and other therapies), which should be noted as one of the limitations. As effective post-PD therapy options are important for patients with disease recurrence, any benefit of EGFR TKI re-administration or continuation after PD requires further study.
At this updated analysis, no new safety signals for erlotinib were observed; single-agent erlotinib was well tolerated and had an acceptable and manageable safety profile in EGFR mutation-positive NSCLC. The safety profile of erlotinib was also in line with previous studies of first-line erlotinib [13], with the most common AEs being rash and diarrhea.
In conclusion, single-agent erlotinib resulted in a median OS of 36.3 months in the first-line treatment of EGFR mutation-positive NSCLC. Subgroup analyses of OS suggested that the presence of brain metastases was a negative prognostic factor, as these patients had shorter median OS compared with other subgroups. No further differences in OS between specific EGFR subgroups were observed. Although many patients went on to receive additional EGFR TKI therapy following progression, there was no significant difference in median OS for patients who received EGFR TKI as post-PD therapy compared with those who did not. The findings of this single-arm study should be validated in randomized controlled trials.
Acknowledgments
The authors would like to thank all participating physicians, registered patients, Tomomi Shimura for data analysis and Gardiner-Caldwell Communications for medical writing assistance. Medical writing assistance was funded by Chugai Pharmaceutical Co. Ltd.
Compliance with ethical standards
Conflict of interest
Noboru Yamamoto received honoraria from AstraZeneca, Eli Lilly, Pfizer and Chugai Pharmaceutical. He also received research funding from Daiichi-Sankyo, Kyowa-Kirin, Chugai Pharmaceutical, Eli Lilly, Takeda, Quintiles, Bristol-Myers Squibb, Astellas, Taiho, Pfizer, Novartis and Eisai. Koichi Goto received research funding from Chugai Pharmaceutical. Makoto Nishio received honoraria from Chugai Pharmaceutical, Boehringer Ingelheim and AstraZeneca. He also received research funding from Chugai Pharmaceutical and AstraZeneca. Kenichi Chikamori received honoraria from Chugai Pharmaceutical. He also received research funding from Chugai Pharmaceutical and Bristol-Myers Squibb. Toyoaki Hida received honoraria from Chugai Pharmaceutical, Taiho, AstraZeneca and Boehringer Ingelheim. He also received research funding from Chugai Pharmaceutical, Taiho, AstraZeneca, Boehringer Ingelheim, Clovis Oncology and Astellas. Makoto Maemondo received honoraria from Chugai Pharmaceutical, AstraZeneca and Boehringer Ingelheim. He also received research funding from Chugai Pharmaceutical, AstraZeneca and Boehringer Ingelheim. Nobuyuki Katakami received honoraria and research funding from Chugai Pharmaceutical. Toshiyuki Kozuki received honoraria from Chugai Pharmaceutical, AstraZeneca, Eli Lilly, Pfizer, Kyowa Kirin, Sanofi, Taiho and Roche. He also received research funding from Chugai Pharmaceutical, Bristol-Myers Squibb and Pfizer. Hiroshige Yoshioka received honoraria from Boehringer Ingelheim, Eli Lilly and Chugai Pharmaceutical. He also received research funding from Chugai Pharmaceutical, Novartis, Takeda, Pfizer, Merck-Serono, Eli Lilly and Kyowa Kirin. Takashi Seto received honoraria and lecture fees from Chugai Pharmaceutical. Kosei Tajima is an employee of Chugai Pharmaceutical. Tomohide Tamura received honoraria from Chugai Pharmaceutical, Taiho, Ono, Eli Lilly, Eisai, Yakult Honsha, Boehringer Ingelheim and Bristol-Myers Squibb.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s10147-016-1052-3.
References
- 1.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line–is there a difference? J Clin Oncol. 2013;31:1081–1088. doi: 10.1200/JCO.2012.43.0652. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for non-small-cell lung cancer V7 (2015) http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed Sept 2016
- 7.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl. 3):iii27–iii39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 8.Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19:3337–3352. doi: 10.2174/092986712801215973. [DOI] [PubMed] [Google Scholar]
- 9.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 10.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka H, Mitsudomi T, Morita S et al (2014) Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 32(Suppl.):Abstract 8117
- 12.Zhou C, Wu YL, Liu X et al (2012) Overall survival (OS) results from OPTIMAL (CTONG0802), a phase III trial of erlotinib (E) versus carboplatin plus gemcitabine (GC) as first-line treatment for Chinese patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC). J Clin Oncol 30(Suppl.):Abstract 7520
- 13.Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20:2001–2010. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 14.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 15.Goto K, Nishio M, Yamamoto N, et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC) Lung Cancer. 2013;82:109–114. doi: 10.1016/j.lungcan.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Yoshioka H, Okamoto I, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci. 2015;106:1202–1211. doi: 10.1111/cas.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 19.Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 20.Dempke WC, Edvardsen K, Lu S, et al. Brain metastases in NSCLC—are TKIs changing the treatment strategy? Anticancer Res. 2015;35:5797–5806. [PubMed] [Google Scholar]
- 21.Brower JV, Robins HI. Erlotinib for the treatment of brain metastases in non-small cell lung cancer. Expert Opin Pharmacother. 2016;17:1013–1021. doi: 10.1517/14656566.2016.1165206. [DOI] [PubMed] [Google Scholar]
- 22.Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18:938–944. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- 23.Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi: 10.1007/s00280-012-1929-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33:1958–1965. doi: 10.1200/JCO.2014.58.1736. [DOI] [PubMed] [Google Scholar]
- 25.Auliac JB, Fournier C, Audigier Valette C, et al. Impact of continuing first-line EGFR tyrosine kinase inhibitor therapy beyond RECIST disease progression in patients with advanced EGFR-mutated non-small-cell lung cancer (NSCLC): retrospective GFPC 04-13 study. Target Oncol. 2016;11:167–174. doi: 10.1007/s11523-015-0387-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 2013;79:33–39. doi: 10.1016/j.lungcan.2012.09.016. [DOI] [PubMed] [Google Scholar]