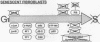Abstract
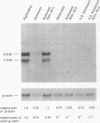
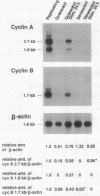
Senescent human diploid fibroblasts (HDF) contain no detectable cdc2 mRNA or p34cdc2 protein. Similarly, young quiescent HDF have only low levels of cdc2 mRNA and protein. After serum stimulation, quiescent HDF accumulate increasing amounts of cdc2 mRNA and protein and go through DNA synthesis and mitosis. In contrast, serum-stimulated senescent HDF fail to accumulate detectable amounts of cdc2 mRNA and protein and fail to enter S phase. Mitosis is likewise deficient in senescent cells even when they have been induced to synthesize DNA by simian virus 40 large tumor antigen. Since p34cdc2 or its homologues appear to be required for DNA synthesis and mitosis in eukaryotes, a lack of these molecules in serum-stimulated senescent HDF could be an important reason for their inability to enter S phase or mitosis. Nuclear microinjection of cdc2 DNA into senescent HDF causes rounding up of the cells but no induction of DNA synthesis. Since cyclins A and B are important cofactors of the protein kinase activity of p34cdc2 or its homologues, we analyzed expression of these genes in serum-stimulated senescent HDF and determined that they contain little or no cycA or cycB mRNA. These deficiencies may be relevant to the lack of DNA synthesis and mitosis in senescent HDF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang Z. F., Chen K. Y. Regulation of ornithine decarboxylase and other cell cycle-dependent genes during senescence of IMR-90 human diploid fibroblasts. J Biol Chem. 1988 Aug 15;263(23):11431–11435. [PubMed] [Google Scholar]
- Draetta G., Beach D., Moran E. Synthesis of p34, the mammalian homolog of the yeast cdc2+/CDC28 protein kinase, is stimulated during adenovirus-induced proliferation of primary baby rat kidney cells. Oncogene. 1988 Jun;2(6):553–557. [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Cristofalo V. J. Analysis of the G1 arrest position of senescent WI38 cells by quinacrine dihydrochloride nuclear fluorescence. Evidence for a late G1 arrest. Exp Cell Res. 1986 Nov;167(1):87–94. doi: 10.1016/0014-4827(86)90206-5. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Cristofalo V. J. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol. 1985 Oct;125(1):122–126. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Tanese N., Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. doi: 10.1016/0022-2836(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lin B. T., Gruenwald S., Morla A. O., Lee W. H., Wang J. Y. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. EMBO J. 1991 Apr;10(4):857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Smith J. R. Entry into S phase is inhibited in human fibroblasts by rat liver poly(A)+RNA. Exp Cell Res. 1985 Oct;160(2):544–549. doi: 10.1016/0014-4827(85)90201-0. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Knepper J. E., Butel J. S., Smith J. R., Pereira-Smith O. M. Mitogenic effects of the proto-oncogene and oncogene forms of c-H-ras DNA in human diploid fibroblasts. Mol Cell Biol. 1986 Aug;6(8):2990–2993. doi: 10.1128/mcb.6.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. The cell cycle: spinning farther afield. Science. 1991 Jun 14;252(5012):1490–1492. doi: 10.1126/science.1828620. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Russell P., Reed S. I. Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Mol Cell Biol. 1990 Jul;10(7):3847–3851. doi: 10.1128/mcb.10.7.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M., Gordon L., Beeson M. Carcinogen-transformed human cells are inhibited from entry into S phase by fusion to senescent cells but cells transformed by DNA tumor viruses overcome the inhibition. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5287–5291. doi: 10.1073/pnas.79.17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y., Yasuda H., Kamijo M., Nakaya K., Nakamura Y., Ohba Y., Nishimura S. In vitro phosphorylation of the tumor suppressor gene RB protein by mitosis-specific histone H1 kinase. Biochem Biophys Res Commun. 1989 Oct 16;164(1):580–586. doi: 10.1016/0006-291x(89)91759-2. [DOI] [PubMed] [Google Scholar]
- Th'ng J. P., Wright P. S., Hamaguchi J., Lee M. G., Norbury C. J., Nurse P., Bradbury E. M. The FT210 cell line is a mouse G2 phase mutant with a temperature-sensitive CDC2 gene product. Cell. 1990 Oct 19;63(2):313–324. doi: 10.1016/0092-8674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- Thomas N. S. Regulation of the product of a possible human cell cycle control gene CDC2Hs in B-cells by alpha-interferon and phorbol ester. J Biol Chem. 1989 Aug 15;264(23):13697–13700. [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma gene and cell growth control. Trends Biochem Sci. 1990 May;15(5):199–202. doi: 10.1016/0968-0004(90)90162-5. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Dell'Orco R., Stein G., Stein J. Histone gene expression remains coupled to DNA synthesis during in vitro cellular senescence. Exp Cell Res. 1987 Oct;172(2):397–403. doi: 10.1016/0014-4827(87)90397-1. [DOI] [PubMed] [Google Scholar]