Abstract
Purpose
The purpose of this study is to assess outcomes after magnetic-activated cell sorting (MACS) technology on obstetric and perinatal outcomes compared with those achieved after swim up from randomized controlled trial.
Methods
This is a two-arm, unicentric, prospective, randomized, and triple-blinded trial and has a total of 237 infertile couples, between October 2010 and January 2013. A total of 65 and 66 newborns from MACS and control group, respectively, were described.
Results
MACS had no clinically relevant adverse effects on obstetric and perinatal outcomes. No differences were found for obstetric problems including premature rupture of membranes 6.1% (CI95% 0–12.8) vs. 5.9% (CI95% 0–12.4), 1st trimester bleeding 28.6% (CI95% 15.9–41.2) vs. 23.5% (CI95% 11.9–35.1), invasive procedures as amniocentesis 2.0% (CI95% 0–5.9) vs. 3.9% (CI95% 0–9.2), diabetes 14.3% (CI95% 4.5–24.1) vs. 9.8% (CI95% 1.6–17.9), anemia 6.1% (CI95% 0–12.8) vs. 5.9%(CI95% 0–12.4), 2nd and 3rd trimesters 10.2% (CI95% 1.7–18.7) vs. 5.9% (CI95% 0–12.4), urinary tract infection 8.2% (CI95% 0.5–15.9) vs. 3.9% (CI95% 0–9.2), pregnancy-induced hypertension 6.1% (CI95% 0–12.8) vs. 15.7% (CI95% 5.7–25.7), birth weight (g) 2684.10 (CI95% 2499.48–2868.72) vs. 2676.12 (CI95% 2499.02–2852.21), neonatal height (cm) 48.3 (CI95% 47.1–49.4) vs. 46.5 (CI95% 44.6–48.4), and gestational cholestasis 0%(CI95% 0–0) vs. 3.9% (CI95% 0–9.2), respectively, in MACS group compared with control group.
Conclusions
Our data suggest that MACS technology does not increase or decrease Powered by Editorial Manager® and ProduXion Manager® from Aries Systems Corporation adverse obstetric and perinatal outcomes in children conceived when this technology was performed, being the largest randomized control trial with live birth reported results with MACS.
Keywords: Ovum donation program, MACS technology, Sperm selection, Perinatal outcome, Obstetrics
Introduction
Currently, routine semen analysis is carried out to evaluate male factor infertility and is based on parameters such as sperm concentration, motility, and morphology [1]. Nevertheless, normal semen results do not guarantee fecundity, so it is important to acknowledge the limitations of semen analysis results in predicting the health and functional capacity of the male reproductive organs and cells [2]. Furthermore, semen analysis does not provide information regarding molecular sperm characteristics. For this reason, it has been suggested that these characteristics should be included in the semen analysis of individuals seeking fertility evaluation [3].
Among the molecular aspects of sperm linked to male infertility, the process of apoptosis has recently received special attention [4, 5]. One of the early markers of apoptosis is the loss of membrane integrity, which leads to externalization of phospholipid phosphatidylserine (PS) (a molecule with a high affinity for annexin V (AV)) [6, 7]. Therefore, AV (used as an apoptotic sperm marker) conjugated with magnetic microspheres, which are exposed to a magnetic field in an affinity column, can separate apoptotic from non-apoptotic sperm. This procedure is called magnetic-activated cell sorting (MACS) and was used in 1995 by Pesce and De Felici to isolate and purify the primordial germ cells (PGCs) of mouse embryos (Mini MACS Magnetic Separation System) [8].
This hypothesis has generated great interest, and there are numerous groups currently working in the evaluation of MACS as a method to reduce apoptotic sperm and improve sperm and embryo quality [9–11].
In this context, and in order to avoid oocyte quality bias, our group performed a prospective, randomized, controlled trial (RCT) within our ovum donation (OD) program to determine the clinical relevance of using MACS to remove presumptive apoptotic AV+ sperm cells from samples from unselected males without previous signs of sperm apoptosis on fertilization rates, early embryo development and morphological features, and implantation, pregnancy, and live birth rates following intracytoplasmic sperm injection (ICSI) performed as part of our OD program. Our results revealed similar results for the parameters studied when MACS was applied and in the control group (in which only swim up was performed) [12]. Once the efficiency of sperm selection by MACS has been evaluated, it should be further validated by confirming the safety of the technique through analyzing obstetric and perinatal outcomes of the babies conceived following transfer of embryos done from ICSI with MACS in order to compare these outcomes with those observed in pregnancies and live births achieved in ICSI conducted with swim up only.
Materials and methods
Study design and study population
This is an obstetric and perinatal data analysis of the infants born after transferring embryos obtained using either sperm selected by MACS (study group) or sperm selected by swim up (control group) in a RCT.
The study was approved by the Institutional Review Board of the Instituto Valenciano de Infertilidad, Valencia, Spain (0810-C-051-MM). Before signing informed consent forms, patients received detailed information about the scientific basis of MACS, including previous results obtained and potential benefits and limitations of the technique.
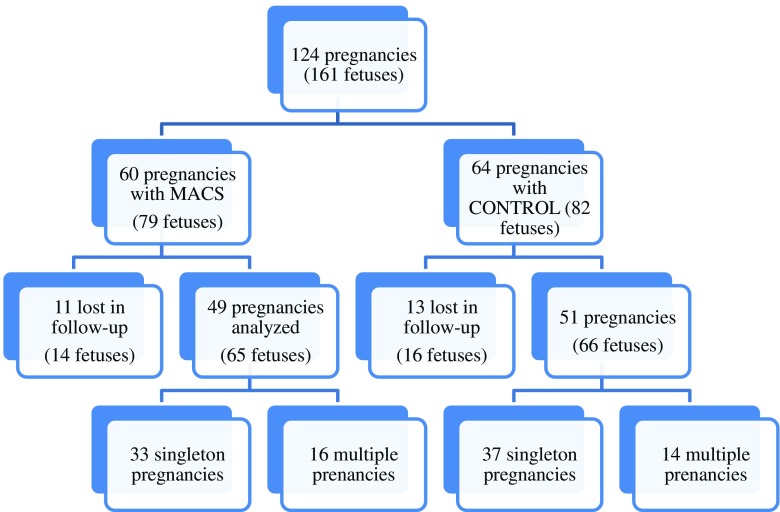
All births of which we had notification during the period between October 2010 and December 2012 were included in the study. Unfortunately, we did not receive data for all the women that underwent treatment in our center; our total notification rates were 81.7 and 79.7 % for MACS and swim up cycles, respectively. Consequently, 161 infants (N = 124 deliveries) were included in the analysis: 79 in the MACS group and 82 in the control group. It was impossible to gather information on the perinatal outcome of 23 pregnancies, and 30 infants were lost in the follow-up (16 children from the control group and 14 from the study group). Consequently, the final sample analyzed consisted of 66 newborn infants (N = 51 deliveries) in the control group and 65 (N = 49 deliveries) in the study group (Fig. 1).
Fig. 1.
Comparison of pregnancies following MACS vs. controls
The IVF cycle and embryo transfer were performed at the University Institute IVI Valencia (Spain). However, the pregnancies were monitored and babies were delivered in the women’s places of residence according to local protocols: Spain (12.1%), Italy (35.9%), Germany (15.5%), France (10.8%), UK (12.1%), and other European countries (16.4%).
IVF procedures
In our OD program, IVF cycles were performed according to standard procedures and protocols for ovarian stimulation for donors, while endometrial preparation for recipients was performed as previously described [13].
An embryologist with no clinical involvement randomly assigned (in a 1:1 proportion) the 263 patients to MACS treatment (experimental group) or conventional sperm preparation (control group) by means of computer-generated randomization using Statistical Package for the Social Sciences 17 (SPSS Inc., Chicago, IL). Semen specimens from the control group (n = 125) were prepared by swim up, as previously described [14]. Samples from the study group (n = 138) were prepared by swim up followed by MACS and incubation with AV-conjugated microbeads (MB) in order to remove AV+ sperm cells [12]. All the cycles were performed according to ICSI procedures [15]. All the embryo transfers were performed under ultrasound guidance. Embryos were transferred on day 3.
Data source and outcome measurements
Data on pregnancies, deliveries, and obstetric/perinatal outcome were obtained medical records, reports sent by the referral centers, and the survey completed by the patients, and were scrutinized by an obstetrician (V.S.), a gynecologist responsible for our infertility program (J.R.), and an embryologist (L.R.).
The main IVF parameters recorded have been described previously by Romany et al. 2014 [12] and constituted the first report of the finalized RCT. The main pregnancy outcome measures were gestational hypertension, preterm birth, and other pregnancy-related complications. The main delivery outcome measures were gestational age at delivery, route of delivery, and puerperal problems. The main neonatal outcome measures were gender, weight at birth, Apgar scores, admission to the neonatal intensive care unit, perinatal mortality, neonatal complications, and incidence of congenital malformations. Small for gestational age (SGA) was defined as birth weight below the 10th percentile. Low weight at birth (LWB) was defined as less than 2500 g and very low weight at birth (VLWB) was defined as less than 1500 g. Perinatal mortality included stillbirths after 28 weeks of gestation and neonatal deaths up to 7 days of life [16]. Major malformations were defined as anomalies or malformations that create significant medical problems for the patient or that require specific surgical or medical management. Minor malformations were described as features that vary from those that are most commonly seen in the normal population but that do not cause increased morbidity.
Statistical analysis
The categorical and continuous variables were expressed as proportions and means with 95% confidence intervals (95%CI), respectively. Categorical data were compared using the chi-square analysis and a Fisher’s exact test, where appropriate. Continuous data were compared with the Student’s t test. A P value of less than 0.05 was considered significant.
All statistical analyses were performed using the Statistical Package for Social Sciences, version 20 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of the study population
No statistically significant differences were found for baseline characteristics, except for smoking habit (P = 0.024), as shown in Table 1. Regarding the incidence of previous medical conditions, the proportion of women under medication prior to conception, smoking habit, and cause of infertility did not show significant differences. No differences were found between the fertility profile of couples for whom data was obtained and those for whom data was missing (data not shown).
Table 1.
Characteristics of the IVF cycles and baseline characteristics of the study population
| MACS group (N = 49) | Control group (N = 51) | P | |
|---|---|---|---|
| Maternal agea | 40.1 (38.8–41.3) | 40.7 (39.5–42.0) | |
| Age of donors | 25.2 (24.1–26.3) | 25.5 (24.0–27.0) | NS |
| Patient BMI (kg/m2) | 22.1 (20.1–24.1) | 22.7 (20.6–24.7) | NS |
| Donor BMI (kg/m2) | 22.9 (22.0–23.4) | 21.9 (21.0–22.9) | NS |
| Previous miscarriages (≥1) | 26.7 (14.3–39.1) | 37.9 (24.6–51.2) | NS |
| Parous women (≥1) | 33.3 (20.1–46.5) | 26.6 (14.5–38.7) | NS |
| Previous preterm deliveries (≥1) | 3.4 (1.7–8.5) | 7.7 (0.4–15.0) | NS |
| Previous medical disorders | 16.3 (5.9–26.6) | 9.8 (1.6–17.9) | NS |
| Previous surgical procedures | 44.9 (30.9–58.8) | 23.5 (11.9–35.1) | NS |
| Smoking habit | 3.8 (0–9.1) | 0 (0–0) | 0.024 |
| Maternal age | 40.1 (38.8–41.3) | 40.7 (39.5–42.0) | NS |
| Cause of infertilityb | |||
| Advanced maternal age | 67.3 (54.2–80.5) | 51.0 (37.3–64.7) | NS |
| Low response | 20.4 (9.1–31.7) | 29.4 (16.9–41.9) | NS |
| Endometriosis | 6.1 (0–12.8) | 2.0 (0–5.8) | NS |
| Idiopathic disease | 2.0 (0–5.9) | 11.8 (2.9–20.6) | NS |
| Other | 4.1 (0–9.6) | 5.9 (0–12.4) | NS |
| Controlled ovarian stimulation | |||
| Days of stimulation (donors) | 10.4 (9.8–11.1) | 11.3 (9.4–13.1) | NS |
| Total gonadotropin dose (donors) (IU) | 1982 (1833–2131) | 2106 (1939–2273) | NS |
| E2 on day of hCG (donors) (pg/ml) | 2619 (2191–3046) | 2567 (2044–3091) | NS |
| Days of endometrial preparation (recipients) | 15.76 (14.7–16.8) | 15.6 (14.5–16.6) | NS |
| Number of oocytes MII oocytes | 10.74 (9.88–11.59) | 10.86 (9.93–11.79) | NS |
Values are expressed as means (95% confidence interval) or N (%)
E2 estradiol level, MII metaphase II, NA not available, NS not significant, BMI body mass index
aMaternal age >38 years
bOne patient may be categorized in more than one cause of infertility
A randomization effectiveness test was performed for the characteristics of the women participating in the trial and of the IVF cycles; no statistical differences were found between the groups in length of ovarian stimulation, doses of gonadotropins administered, estradiol levels for donors/oocyte recipients, endometrial preparation, number of MII oocytes, and number of embryos transferred (Table 1). Male characteristics and sperm parameters (concentration, motility, and morphology) have been described in a previous manuscript [12].
Pregnancy, delivery, and neonatal outcomes
The proportion of singleton births was 67.3% (33 of 49 pregnancies) and 72.5% (37 of 51 pregnancies) in the MACS group and control group, respectively; a difference that was not statistically significant (Fig. 1). Table 2 reflects data corresponding to pregnancy, delivery, and neonatal outcomes, and shows that MACS technology had no effect on any outcome measures when compared with current procedures. A similar frequency of the 2nd and 3rd trimester bleeding was reported. Route of delivery and neonatal gender, as well as other pregnancy-related complications, including anemia, gestational cholestasis, diabetes, pregnancy-induced hypertension, preterm premature rupture of membranes, preterm birth, and very early preterm birth rates, were comparable in the two groups. No differences were found between groups in gestational age at delivery, weight at birth, LWB, VLWB, and SGA (Table 2). Other measurements, such as neonatal height and Apgar scores, were also comparable. The rate of birth defects, even when classified as major and minor malformations, was also similar in the two groups (Table 2). Admissions to the neonatal intensive care unit (NICU) and length of stay in the NICU were also comparable between groups. There was only one perinatal mortality in the control group.
Table 2.
Obstetric and perinatal outcomes
| MACS group (N = 49) | Control group (N = 51) | P | |
|---|---|---|---|
| Pregnancy outcome | |||
| 1st trimester bleeding | 28.6 (15.9–41.2) | 23.5 (11.9–35.1) | NS |
| Invasive proceduresa | 2.0 (0–5.9) | 3.9 (0–9.2) | NS |
| Anemia (Hb <11 g/dl) | 6.1 (0–12.8) | 5.9 (0–12.4) | NS |
| Gestational cholestasis | 0 (0–0) | 3.9 (0–9.2) | NS |
| Diabetes | 14.3 (4.5–24.1) | 9.8 (1.6–17.9) | NS |
| 2nd and 3rd trimester bleeding | 10.2 (1.7–18.7) | 5.9 (0–12.4) | NS |
| PROM <37 weeks | 6.1 (0–12.8) | 5.9 (0–9.2) | NS |
| Pregnancy-induced hypertension | 6.1 (0–12.8) | 15.7 (5.7–25.7) | NS |
| Urinary tract infection | 8.2 (0.5–15.9) | 3.9 (0–9.2) | NS |
| Delivery outcome | |||
| Weeks at delivery | 36.6 (35.1–38.1) | 36.4 (35.1–38.8) | NS |
| Preterm births (<37 weeks) | 28.6 (15.9–41.2) | 31.3 (18.6–44.0) | NS |
| Very preterm births (<34 weeks) | 12.2 (3.0–21.4) | 10.2 (1.9–18.5) | NS |
| Cesarean section | 71.4 (58.7–84.0) | 66.7 (53.8–79.6) | NS |
| Neonatal outcome | |||
| (N = 65) | (N = 66) | ||
| Female neonates | 40.0 (26.3–53.7) | 54.5 (40.8–68.2) | NS |
| Birth weight (g) | 2684.1 (2499.5–2868.7) | 2676.1 (2499.0–2852.2) | NS |
| Birth weight female neonate (g) | 2627 (2345–2908) | 2564 (2325–2803) | NS |
| Birth weight male neonate (g) | 2722 (2465–2978) | 2841 (2572–3109) | NS |
| LBW (<2500 g) | 40.4 (26.6–54.1) | 38.0 (24.7–51.3) | NS |
| VLBW (<1500 g) | 6.1 (0–9.5) | 4.5 (0–10.2) | NS |
| Neonatal height (cm) | 48.3 (47.1–49.4) | 46.5 (44.6–48.4) | NS |
| Apgar score at 1 min | 8.8 (8.3–9.3) | 8.9 (8.6–9.2) | NS |
| Apgar score at 5 min | 9.7 (9.5–9.9) | 9.5 (9.2–9.7) | NS |
| Apgar score at 10 min | 9.8 (9.4–10.0) | 9.8 (9.5–10.0) | NS |
| Birth defects | 4.1 (0–9.6) | 2 (0–5.8) | NS |
| Major malformations | 0 (0–0) | 0 (0–0) | NS |
| Minor malformations | 4.1 (0–9.6) | 2 (0–5.8) | NS |
| Admission to NICU | 13.8 (4.1–23.5) | 12.1 (3.1–21.0) | NS |
| Days at the NICU | 15 (1–30) | 16 (10–23) | NS |
Values expressed as N (%) or mean (95% confidence interval)
LBW low birth weight, VLBW very low birth weight, SGA small for gestational age, NS not significant, Hb hemoglobin, PROM premature rupture of membranes
aChorionic villus sampling or amniocentesis: no abnormal results found
Discussion
The present study evaluates the obstetric and perinatal outcomes achieved after injecting oocytes with sperm selected by MACS technology by which potentially apoptotic sperm cells are removed from samples of unselected males.
Our group has performed a previous RCT to determine the clinical impact of using MACS to remove AV+ on fertilization rates, early embryo development and morphological features, and implantation, pregnancy, and live birth rates following intracytoplasmic sperm injection (ICSI) as part of our OD program. The results revealed similar results for all the parameters studied in the experimental group (MACS) and among controls (in which only swim up was performed) [12].
There are very few studies reporting newborns achieved after applying MACS technology, and none have followed up the said live births [17–19]. To the best of our knowledge, this is the first report that evaluates the safety of sperm selection by MACS by analyzing the obstetric and perinatal outcomes of babies conceived following the transfer of oocytes fertilized via ICSI with sperm selected by this method. Our results demonstrate the lack of apparent beneficial or deleterious effects of MACS on obstetric and perinatal outcomes in children conceived when this technology has been performed, showing comparable rates of pregnancy and live births following ICSI of sperm undergoing swim up only.
After analyzing a small but valuable sample of over 71 babies, no adverse effects were observed in terms of obstetric and perinatal outcomes between pregnancies or babies conceived with sperm selected by MACS or by swim up. All the parameters analyzed were similar in both groups; no clinically relevant increase in obstetric or perinatal risks was detected when MACS technology was applied, suggesting that most of the concerns related to the use of high magnetic fields and the components of reagents used in the current protocol of AV beads and columns are theoretically overwhelmed and that it is not a damaging procedure [20].
There are some limitations to this study. For example, we only assessed the births of which we had been notified; unfortunately, data had to be partially obtained through medical questionnaires, as we were not able to collect the totality of the data for the pregnancies achieved as a result of our program. Furthermore, we only analyzed births at or beyond 24 weeks of gestation. Nevertheless, a previous randomized clinical trial by our group [12] showed similar early miscarriage rates in both groups, hinting that MACS technology is not related to early pregnancy loss.
It is also important to take into account that this study might have been underpowered to show a difference in perinatal outcome between groups. Given that most pregnancy complications occur at an incidence of 10% or lower, one would not expect a difference in these small study numbers. Moreover, sample-size calculations were not performed for an equivalence or non-superiority hypothesis, which would have required a huge sample, impossible to achieve in almost 99% of clinical settings. Subsequently, this study is also underpowered for a non-superiority or equivalence hypothesis.
Another limitation that should be underlined is the fact that pregnancies were monitored in the place of origin/residence; this is relevant, as different countries have different protocols of labor induction and interventions that might affect gestational age and birth weight.
We performed this trial to demonstrate the usefulness of MACS as a routine application preceding ICSI, which is why we used sperm samples from unselected males. However, we are unaware of the grade of apoptosis and percentage of DNA fragmentation or chromatin decondensation in our population pre- and post-MACS; subsequently, our patients did not necessarily have high DNA fragmentation. Therefore, it is possible that sperm with extraordinary levels of fragmented DNA account for the differences between control and the study group, and that their samples benefited particularly from the procedure. In short, the benefit of employing MACS before ICSI requires further investigation, particularly given that ICSI protocols tend to disguise the impact of sperm preparation methods.
Despite the aforementioned limitations, this study offers relevant evidence given that its conclusions are based on a RCT with two well-defined groups; 187 patients per arm were required for a one-sided significance level analysis, with the alpha error set at 0.05 and a test power of 80%. Despite the small sample size, this is the only trial to show the obstetric and perinatal outcome of babies conceived after the application of MACS technology in sperm samples of infertile patients within an OD program. Furthermore, the possible bias leads to suppose that ovarian hyperstimulation was eliminated by the OD, and we included the population of women from the two analyzed cohorts in the OD program and used female gametes with a good prognosis; therefore, all the women included in our series were infertile, so we can rule out the possibility that differences in infertility between the groups had any impact on the outcomes. Indeed, the fact that a single IVF center (IVI Valencia, Spain) was involved in this trial stands in its favor, as it means that the same protocols were followed with respect to IVF procedures and clinical management. In summary, despite the positive data we report regarding the birth of healthy children when MACS technology is applied, its benefits require further investigation in a larger sample.
In conclusion, the results of our study do not indicate a noticeable increase or decrease in obstetric or perinatal events when MACS technology is employed. However, it is necessary to investigate further in order to confirm that MACS has no benefit or negative impact on health parameters, and to do this, an important increase in sample size is mandatory, as is pediatric follow-up involving the evaluation of psychomotor development, educational progress, and prevalence of health problems to confirm the lack of long-term consequences.
The figure shows the number of pregnancies in the MACS group vs. controls. The number of singleton and multiple pregnancies is registered for each of the groups. Some of the originally recorded pregnancies were lost in the follow-up.
Compliance with ethical standards
The study was approved by the Institutional Review Board of the Instituto Valenciano de Infertilidad, Valencia, Spain (0810-C-051-MM).
Footnotes
Capsule IVF cycles where sperm has been prepared by magnetic activated cell sorting show no differences in obstetric and perinatal outocomes compared to standard sperm preparation.
References
- 1.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male [corrected] Clinics (Sao Paulo) 2011;66(4):691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis SE. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134(1):31–40. doi: 10.1530/REP-07-0152. [DOI] [PubMed] [Google Scholar]
- 3.Delbes G, Herrero MB, Troeung ET, Chan PT. The use of complimentary assays to evaluate the enrichment of human sperm quality in asthenoteratozoospermic and teratozoospermic samples processed with Annexin-V magnetic activated cell sorting. Andrology. 2013;1(5):698–706. doi: 10.1111/j.2047-2927.2013.00106.x. [DOI] [PubMed] [Google Scholar]
- 4.Garrido N, Garcia-Herrero S, Meseguer M. Assessment of sperm using mRNA microarray technology. Fertil Steril. 2013;99(4):1008–22. doi: 10.1016/j.fertnstert.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Garrido N, Martinez-Conejero JA, Jauregui J, Horcajadas JA, Simon C, Remohi J, et al. Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril. 2009;91(4 Suppl):1307–10. doi: 10.1016/j.fertnstert.2008.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Glander HJ, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryostorage. Mol Hum Reprod. 1999;5(2):109–15. doi: 10.1093/molehr/5.2.109. [DOI] [PubMed] [Google Scholar]
- 7.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 8.Pesce M, De Felici M. Purification of mouse primordial germ cells by MiniMACS magnetic separation system. Dev Biol. 1995;170(2):722–5. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 9.Makker K, Agarwal A, Sharma RK. Magnetic activated cell sorting (MACS): utility in assisted reproduction. Indian J Exp Biol. 2008;46(7):491–7. [PubMed] [Google Scholar]
- 10.Dirican EK, Ozgun OD, Akarsu S, Akin KO, Ercan O, Ugurlu M, et al. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet. 2008;25(8):375–81. doi: 10.1007/s10815-008-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romany L, Meseguer M, Garcia S, Pellicer A, Garrido N. Magnetic activated sorting selection (MACS) of non-apoptotic sperm (NAS) improves pregnancy rates in homologous intrauterine insemination (IUI). Preliminary data. Fertil Steril. 2010;94(4):S14. doi: 10.1016/j.fertnstert.2010.07.055. [DOI] [Google Scholar]
- 12.Romany L, Garrido N, Motato Y, Aparicio B, Remohi J, Meseguer M. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertil Steril. 2014;102(6):1567–75.e1. doi: 10.1016/j.fertnstert.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25(9):2239–46. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 14.Meseguer M, Santiso R, Garrido N, Garcia-Herrero S, Remohi J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95(1):124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 16.Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohi J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014;102(4):1006–1015.e4. doi: 10.1016/j.fertnstert.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Polak de Fried E, Denaday F. Single and twin ongoing pregnancies in two cases of previous ART failure after ICSI performed with sperm sorted using annexin V microbeads. Fertil Steril. 2010;94(1):351.e15–351.e18. doi: 10.1016/j.fertnstert.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Rawe VY, Boudri HU, Alvarez Sedo C, Carro M, Papier S, Nodar F. Healthy baby born after reduction of sperm DNA fragmentation using cell sorting before ICSI. Reprod Biomed Online. 2010;20(3):320–3. doi: 10.1016/j.rbmo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Herrero MB, Delbes G, Chung JT, Son WY, Holzer H, Buckett W, et al. Case report: the use of annexin V coupled with magnetic activated cell sorting in cryopreserved spermatozoa from a male cancer survivor: healthy twin newborns after two previous ICSI failures. J Assist Reprod Genet. 2013;30(11):1415–9. doi: 10.1007/s10815-013-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said TM, Agarwal A, Zborowski M, Grunewald S, Glander HJ, Paasch U. Utility of magnetic cell separation as a molecular sperm preparation technique. J Androl. 2008;29(2):134–42. doi: 10.2164/jandrol.107.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]