Abstract
Purpose
This retrospective cohort study aimed to determine whether age influences treatment discontinuation among insured patients undergoing in vitro fertilization (IVF). We hypothesized that the youngest patients would be the least likely to discontinue treatment.
Methods
All women age 18–42 who underwent their first fresh, non-donor IVF cycle from 2002 to 2013 were followed until a live birth was achieved, until they discontinued treatment at our center (not presenting for treatment for a one-year period), or until they completed six fresh or frozen embryo transfer cycles, whichever occurred first.
Results
Of 11,361 women included, 4336 (38.2 %) discontinued treatment at our center before achieving a live birth or undergoing six IVF cycles. Discontinuation differed by age for cycles 2–4 (all P ≤ 0.004), with the proportion among women age 40–42 averaging 6–7 % higher than the other groups; discontinuation per cycle was similar among women <30 compared to women age 30–<35 and 35–<40. This continued in cycles 5 and 6, and in the sixth, 35.2, 32.0, 32.3, and 40.2 % of women among the four age groups discontinued treatment, respectively (P = 0.17). In cycles 2–5, women in the oldest two age groups with secondary infertility consistently discontinued treatment more frequently than those with primary infertility.
Conclusions
We found that women in the oldest age group were more likely to discontinue IVF treatment than younger women. Surprisingly, we found that the youngest women discontinued treatment in a similar fashion to women age 30–<40.
Keywords: Infertility, In vitro fertilization, Treatment discontinuation
Introduction
Most couples undergoing in vitro fertilization (IVF) report that the treatment regimen is demanding and creates high levels of anxiety due to financial burdens, large time commitments, and emotional distress. Thus, it is not surprising that treatment discontinuation is relatively common, with as many as 25–37 % of couples stopping treatment after their first unsuccessful attempt [1, 2]. With such high proportions of treatment discontinuation, researchers worldwide have focused on the reasons behind these patient decisions. The most commonly cited reason for treatment discontinuation for non-insured patients is financial strain [3–5]. For patients with insurance coverage, the most common reasons for treatment discontinuation are perception of poor prognosis [2, 6] and psychological burden [2, 6–8].
Older female age has been consistently associated with discontinuation of IVF treatment, which is thought to be due to poorer prognosis [1, 2, 9, 10]. If older patients discontinue treatment because they believe their prognosis is poor, there could be a linear relationship between age and treatment discontinuation, with the youngest patients being least likely to discontinue treatment. However, among patients without insurance coverage for IVF, younger patients may not have the same financial resources as older patients, and younger patients may thus be more likely to discontinue treatment for financial reasons. We hypothesized that in insured populations, there would be a direct relationship between age and treatment discontinuation, with the youngest patients being the least likely to discontinue treatment. The purpose of this study was to determine whether age influences treatment discontinuation among insured women undergoing IVF.
Materials and methods
Study population and participants
This retrospective cohort study included all insured women who were age 18–42 at the start of their first fresh, non-donor IVF cycle at a large, academic-affiliated infertility treatment center from January 1, 2000 to December 31, 2013 in Massachusetts. Although Massachusetts mandates insurance coverage of IVF, this mandate only extends to in-state policies. Not all patients are covered by in-state policies, and those without individual insurance coverage for IVF were excluded from this study. Subsequent frozen-embryo transfer cycles and canceled cycles were included as distinct cycles, as has been done previously [11, 12]. We followed women until delivery of a live born infant was achieved, until they discontinued treatment at our center, defined as not receiving care for a period of at least 1 year, or until they had completed six fresh and frozen autologous embryo transfer cycles, whichever occurred first. Women who did not experience a live birth were eligible to return to care for a subsequent cycle. Women who did not return to care included those who discontinued IVF treatment altogether, those who transferred their care to another infertility treatment center, and those who chose to proceed with oocyte donation or a gestational carrier.
Embryo transfer cycles
Women underwent standard ovarian stimulation protocols, which have been described previously, for ovarian stimulation, monitoring, and oocyte retrieval [7]. Embryos were generally transferred 3 or 5 days after oocyte retrieval, and the number transferred adhered to national guidelines [13]. Cryopreservation of embryos that were considered viable using morphologic criteria was conducted at the cleavage stage on day 3 or at the blastocyst stage on days 5 or 6 after oocyte retrieval. Patients who underwent frozen embryo transfer received exogenous estradiol with or without a GnRH agonist prior to transfer, as described previously [11]. Luteal phase support was provided through 10 weeks of gestation.
Statistical analyses
Demographic characteristics at the start of the first cycle, along with cycle characteristics and outcomes, were collected from each patient’s electronic medical record for up to six fresh and frozen cycles. Descriptive data are presented as mean and standard deviation, median, and interquartile range or proportion. Treatment discontinuation per cycle was calculated as the number of women who did not return to care, as defined above, divided by the number of women who were eligible to return to care. Female patient age at the start of the first cycle was stratified into the following groups based on categories defined by the Society for Assisted Reproductive Technology (SART): <30, 30–<35, 35–<40, and 40–42 years. Differences in the proportion of treatment discontinuation between age groups were calculated using the chi square test. Differences in means were calculated using a t test, and differences in medians were calculated using the Mann–Whitney U test. A secondary analysis was performed stratifying women according to whether their infertility was primary or secondary in order to examine whether parity altered the patterns of treatment discontinuation by age. P values less than 0.05 were considered to be statistically significant, and all tests were two sided. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Stata 12 (StataCorp, College Station, TX).
Ethical approval
The institutional review board at Beth Israel Deaconess Medical Center approved this study.
Results
Characteristics at first cycle
A total of 11,361 women were included in the analysis. Of these women, 12.1 % were age <30, 34.0 % were age 30–<35, 37.7 % were age 35–<40, and 16.2 % were age 40–42 at the time of their first cycle. Primary infertility was present in 90.0, 81.7, 71.6, and 67.4 % of patients in each of the four age groups, respectively. Similarly, the presence of male factor infertility decreased with age, while levels of cycle day 3 follicle-stimulating hormone tended to increase with age (Table 1). In the first cycle, the age groups differed with respect to the use of intracytoplasmic sperm injection and assisted hatching (both P < 0.001), as well as the number of oocytes retrieved, embryos transferred, and embryos cryopreserved (all P < 0.001). As expected, younger women showed more favorable prognoses based on these parameters (Table 2).
Table 1.
Patient characteristics at the start of the first IVF cycle
| Characteristic | <30 years n = 1375 | 30–<35 years n = 3862 | 35–<40 years n = 4279 | 40–42 years n = 1845 |
|---|---|---|---|---|
| Age (years) | 27.9 ± 1.8 | 32.6 ± 1.4 | 37.4 ± 1.5 | 41.4 ± 0.8 |
| Body mass index (kg/m2) | 25.9 ± 6.0 | 25.2 ± 5.5 | 25.5 ± 5.4 | 25.7 ± 5.4 |
| Gravidity | ||||
| 0 | 964 (70.8) | 2320 (60.5) | 2041 (48.1) | 701 (38.2) |
| 1 | 235 (17.3) | 884 (23.0) | 1128 (26.6) | 475 (25.9) |
| ≥2 | 162 (11.9) | 633 (16.5) | 1071 (25.3) | 658 (35.9) |
| Parity | ||||
| 0 | 1203 (90.0) | 3094 (81.7) | 3014 (71.6) | 1228 (67.4) |
| 1 | 108 (8.1) | 579 (15.3) | 971 (23.1) | 439 (24.1) |
| ≥2 | 25 (1.9) | 116 (3.1) | 223 (5.3) | 156 (8.6) |
| Cycle day 3 FSH | 5.6 ± 2.6 | 6.5 ± 4.5 | 7.4 ± 4.5 | 8.0 ± 4.1 |
| Infertility diagnosisa | ||||
| Tubal factor | 194 (14.1) | 477 (12.4) | 632 (14.8) | 223 (12.1) |
| Ovulatory dysfunction | 241 (17.5) | 493 (12.8) | 297 (6.9) | 93 (5.0) |
| Diminished ovarian reserve | 4 (0.3) | 52 (1.3) | 124 (2.9) | 89 (4.8) |
| Endometriosis | 73 (5.3) | 247 (6.4) | 231 (5.4) | 67 (3.6) |
| Uterine factor | 23 (1.7) | 69 (1.8) | 106 (2.5) | 69 (3.7) |
| Male factor | 442 (32.1) | 984 (25.5) | 900 (21.0) | 304 (16.5) |
| Unexplained | 351 (25.5) | 1416 (36.7) | 1703 (39.8) | 732(9.7) |
Data are presented as mean ± standard deviation or n (%)
FSH follicle-stimulating hormone
aMultiple diagnoses may be reported
Table 2.
Clinical characteristics of the first cycle
| Characteristic | <30 years n = 1375 | 30–<35 years n = 3862 | 35–<40 years n = 4279 | 40–42 years n = 1845 | P value |
|---|---|---|---|---|---|
| Fresh cycles | 1375 (100.0) | 3862 (100.0) | 4279 (100.0) | 1845 (100.0) | – |
| Manipulation | |||||
| ICSIa | 503 (36.6) | 1181 (30.6) | 1086 (25.4) | 369 (20.0) | <0.001 |
| Assisted hatching | 5 (0.4) | 32 (0.8) | 118 (2.8) | 190 (10.3) | <0.001 |
| Oocytes retrieveda | 13.3 ± 8.0 | 11.9 ± 6.9 | 10.2 ± 6.7 | 8.6 ± 5.9 | <0.001 |
| Embryos cryopreserveda | <0.001 | ||||
| 0 | 607 (48.2) | 1800 (51.1) | 2348 (64.1) | 1182 (81.7) | |
| 1–3 | 271 (21.5) | 735 (20.9) | 674 (18.4) | 136 (9.4) | |
| ≥4 | 381 (30.3) | 985 (28.0) | 641 (17.5) | 128 (8.9) | |
| Embryos transferredb | 1.8 ± 0.7 | 1.8 ± 0.7 | 2.0 ± 1.0 | 2.7 ± 1.5 | <0.001 |
Data are presented as mean ± standard deviation or n (%)
ICSI intracytoplasmic sperm injection
aCalculated only among fresh cycles
bCalculated among all cycles
Treatment discontinuation by age
For all age groups, treatment discontinuation was least common after the first unsuccessful cycle and ranged from 14.7 % of women <30 years of age to 20.9 % of women age 40–42 (Table 3). The likelihood of treatment discontinuation increased with each cycle for all women. The proportion of women who attempted a cycle and then discontinued treatment after that cycle differed significantly with age for unsuccessful cycles 2 through 4 (all P ≤ 0.002). The statistical difference was driven by women age 40–42, who had the highest proportion of treatment discontinuation per cycle; interestingly, the proportions were similar across the three younger (age <40) age groups (all P ≥ 0.18). After the second unsuccessful cycle, 27.8 % of women in the oldest group did not return for a third cycle, whereas only 17.2–20.5 % of women in each of the other three groups did not return for a third cycle (P < 0.001). This pattern continued in subsequent cycles, including the sixth cycle, whereby the proportion who discontinued treatment was 35.2 % for women age <30, 32.0 % for women age 30–<35, 32.2 % for women age 35–<40, and 40.2 % for women age 40–42 (P = 0.17).
Table 3.
Treatment discontinuation by age group and cycle number
| <30 years | 30–<35 years | 35–<40 years | 40–42 years | |||||
|---|---|---|---|---|---|---|---|---|
| Cycle | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) |
| 1 | 1375 | NA | 3862 | NA | 4279 | NA | 1845 | NA |
| 2 | 779 | 134/913 (14.7) | 2209 | 383/2592 (14.8) | 2721 | 494/3215 (15.5) | 1279 | 337/1616 (20.9) |
| 3 | 457 | 95/552 (17.2) | 1313 | 290/1603 (18.1) | 1688 | 436/2124 (20.5) | 810 | 312/1122 (27.8) |
| 4 | 239 | 70/309 (22.7) | 719 | 234/953 (24.6) | 1005 | 341/1346 (25.3) | 463 | 235/698 (33.7) |
| 5 | 122 | 55/177 (31.1) | 402 | 147/549 (26.8) | 588 | 245/833 (29.4) | 246 | 152/398 (38.2) |
| 6 | 59 | 32/91 (35.2) | 210 | 99/309 (32.0) | 330 | 157/487 (32.2) | 131 | 88/219 (40.2) |
aDenominator is the number of women eligible to return for that cycle (the number of women in the previous cycle minus the number of women with a live birth)
Among women who continued or discontinued treatment after their first failed cycle, there was no difference in the mean number of oocytes retrieved (10.0 ± 7.0 vs. 10.0 ± 6.3, respectively; P = 0.90) or in the median number of embryos frozen (0.0 [0.0–1.0] vs. 0.0 [0.0–1.0], respectively; P = 0.15). However, in all subsequent cycles, women who discontinued treatment had significantly fewer mean oocytes retrieved (all P < 0.001) and significantly fewer median embryos frozen (all P < 0.001).
Figure 1 shows the proportion of patients discontinuing treatment after each unsuccessful cycle, as well as the proportion of patients who become pregnant after each cycle attempt. As expected, although women age <30 were the most likely to experience a live birth after each cycle, the proportion discontinuing treatment was similar to the proportions among women age 30–<40 (Table 4).
Fig. 1.
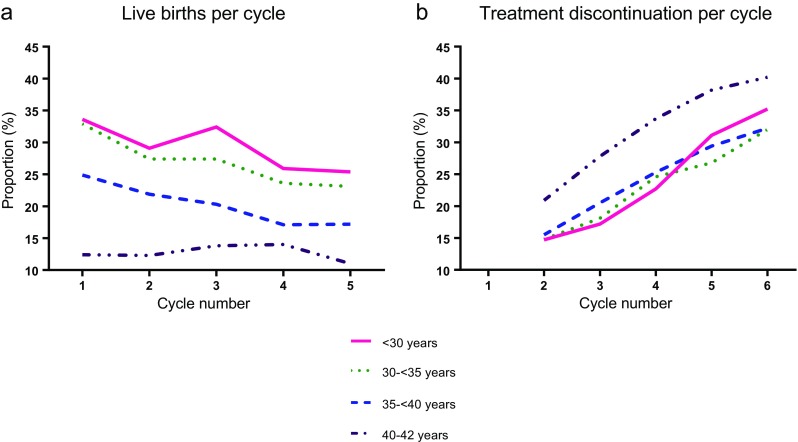
a Live birth and b treatment discontinuation by age at the time of the first cycle and cycle number
Table 4.
Treatment discontinuation by age group and cycle number, among women with primary infertility
| <30 years | 30–<35 years | 35–<40 years | 40–42 years | |||||
|---|---|---|---|---|---|---|---|---|
| Cycle | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) | Cycle cohort | Did not return for treatmenta N/Total N (%) |
| 1 | 1203 | NA | 3094 | NA | 3014 | NA | 1228 | NA |
| 2 | 685 | 114/799 (14.3) | 1786 | 281/2067 (13.6) | 1930 | 320/2250 (14.2) | 879 | 215/1094 (19.7) |
| 3 | 395 | 79/474 (16.7) | 1061 | 222/1283 (17.3) | 1196 | 285/1481 (19.2) | 581 | 200/781 (25.6) |
| 4 | 201 | 61/262 (23.3) | 589 | 181/770 (23.5) | 733 | 226/959 (23.6) | 326 | 161/487 (33.1) |
| 5 | 100 | 46/146 (31.5) | 323 | 124/447 (27.7) | 608 | 165/443 (27.1) | 175 | 105/280 (37.5) |
| 6 | 48 | 26/74 (35.1) | 170 | 81/251 (32.3) | 245 | 123/368 (33.4) | 96 | 60/156 (38.5) |
aDenominator is the number of women eligible to return for that cycle (the number of women in the previous cycle minus the number of women with a live birth)
Figure 2 shows the secondary analysis stratifying women by primary and secondary infertility. In general, the proportion of women discontinuing treatment was similar for those with primary compared to secondary infertility. For women in the oldest two age groups, those with secondary infertility consistently discontinued treatment more frequently than those with primary infertility, with the exception of cycle 6 where sample sizes were small.
Fig. 2.
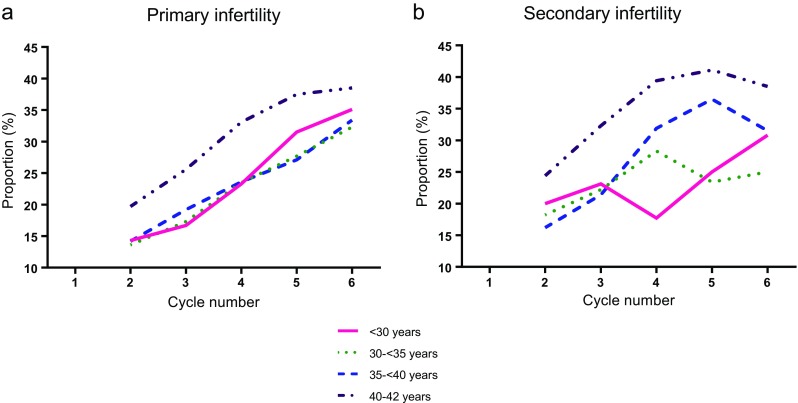
Treatment discontinuation by age at the time of the first cycle and cycle number among women with a primary and b secondary infertility
Discussion
Consistent with the literature [1, 2, 9, 10], we found that older women were more likely than younger women to discontinue IVF treatment prior to having a live birth. This makes intuitive sense, as it is not logical to continue to withstand emotionally, physically, and financially stressful treatment if the prognosis is poor. Unexpectedly, a proportion of women age <30, who have the best treatment prognosis (Table 2, Fig. 1), discontinued treatment prior to a live birth in an equivalent fashion to women age 30–<35 and 35–<40. Women age 35–<40 and 40–42 with secondary infertility consistently discontinued treatment more often than women with primary infertility with the exception of the final cycle.
While patients without insurance coverage discontinue treatment sooner than patients with coverage due to financial pressures [3–5], our study was restricted to insured patients in a state with mandated insurance coverage of six IVF cycles. In general, younger patients may not have the financial resources to continue treatment. However, if prognosis and financial resources are the main determinants of treatment discontinuation, one would anticipate that in states or countries with insurance mandates, the likelihood of discontinuation would steadily increase with age. However, we saw no difference in the proportion of women discontinuing treatment among those age <40. In our population, which had up to six IVF cycles covered by insurance, women age <30 continued to discontinue treatment after each cycle in a similar fashion to women in their 30s.
The likelihood of live birth among women age <30 who undergo IVF is excellent. Previous work from this center has shown that after six cycles, 58 % of patients age <25 and 69 % of patients age 25–<30 will achieve a live birth [14]. Why then do women with such positive prognoses for pregnancy discontinue treatment at the same rate as women who have lower chances for success? There are five potential explanations for this behavior in younger women: (1) the burden of treatment is higher for younger women, as they may have experienced less loss in their lives and have thus not developed adequate coping skills or resilience, and they may lack social support from friends who also are experiencing infertility; (2) younger women are more likely to take time off from treatment, as they perceive that they have more time to pursue conception; (3) younger women are more likely to obtain treatment elsewhere; (4) younger women are more likely to conceive spontaneously and thus discontinue treatment; and (5) the reproductive healthcare team may not be effectively communicating to these patients their excellent odds of conceiving a healthy pregnancy. It is probable that some patients experience a combination of all explanations. While conceiving spontaneously and seeking care elsewhere may not be seen as true treatment discontinuation, these women do leave treatment at our clinic, which is of interest to our center. Whether women seek care elsewhere or eventually return for treatment at our center is the subject of ongoing work. The reason for the observed findings is unknown, and a follow-up study is underway to answer this question. However, there is mixed evidence with regard to the provider-patient communication explanation. In the French study cited previously [2], treatment discontinuation was significantly associated with the number of frozen embryos; women with the most frozen embryos were the most likely to discontinue treatment. Given that a high number of frozen embryos is a good prognostic indication of cycle success, one would expect these patients to be the least likely to discontinue treatment, which suggests that their odds of achieving pregnancy may not be effectively communicated to them. In the present study, treatment discontinuation was seen most often in women who may be considered to have poorer prognoses. One possible reason for this discrepancy is that state-mandated insurance covers donor egg cycles among the six IVF cycles that women age <40 are eligible for; thus, in order to maximize their chance of a successful pregnancy, the women with poorer prognoses may have elected to move onto donor egg, which in our study was considered to be treatment discontinuation, regardless of age.
A strength of this study is its restriction to patients with insurance coverage, which allowed us to largely exclude the influence of financial resources as a cause of treatment discontinuation among patients undergoing IVF, though financial burden may still affect treatment discontinuation in this setting, as many women do not have full coverage, and younger women may be more vulnerable to high out-of-pocket costs. Another strength is our ability to include over 11,000 women in the analysis, making our study, to our knowledge, the largest published to date on this topic. Using this large sample size, we were able to provide more granular information than prior studies by stratifying women age <35 into two groups and calculating the proportion of women who discontinued treatment after each cycle. However, all patients were from a single treatment center in a state with an insurance mandate for IVF treatment, which is a limitation of the work, and these results may not be generalizable to patients receiving care in states without insurance mandates. Despite our large sample size, the power to detect a statistically significant difference between the proportion of women age <30 who discontinued treatment after the first cycle (14.7 %) and the proportion of women age 30–<35 (14.8 %) and 35–<40 (15.5 %) was only 5 and 9 %, respectively. However, these differences are not clinically relevant, and thus lack of power is not a concern. Additionally, we do not know what happened to the women who discontinued care, and they may have conceived spontaneously or pursued treatment at another center. Finally, we were unable to control for factors that may be associated with treatment discontinuation, such as psychological burden and perception of poor prognosis [2, 6–8].
Counseling patients after a failed cycle is challenging for health care professionals. One is caught between emotionally supporting the patient who is mourning the cycle failure while simultaneously encouraging her to try again with the same procedure that is causing her distress. Additionally, a provider often must accomplish both objectives in a single visit. This might be an opportune time for a mental health professional to counsel all patients, but especially the younger patient. This can accomplish several goals, including providing emotional support, reviewing which coping skills worked for the patient to build resilience during previous life challenges, and teaching the patient new and effective strategies to counteract stress.
These findings suggest that the ideal post-IVF failure visit has three goals. The first is to provide appropriate patient support, which includes empathizing with the patient, listening to her describe her disappointment and frustration, and answering her questions as to why the cycle failed. The second is to describe the protocol for the next cycle and to be absolutely clear about her chance for success. This includes a presentation of the number and quality of frozen embryos and/or blastocysts and what they represent in terms of her prognosis. The third is to ask the patient what resources she would need to undergo another cycle. This includes her own resources such as family and friends, as well as resources the infertility clinic can provide, including nurse counseling, a visit with a mental health professional, and/or attendance at a support group or mind/body program.
The results of this study indicate that young patients with a likely good prognosis may discontinue treatment for different reasons than older patients, as it is hypothesized that older patients discontinue treatment due to a poor prognosis. These results highlight the need for patient counseling to be age-specific, specifically with regards to informing younger patients that despite their failed cycle(s), they often do have good prognoses. Although many clinics may understand that counseling is needed to support older age groups, it appears that it is just as important for the entire team to understand that younger patients may need additional support and alternative dedicated counseling.
Acknowledgments
This work was conducted with support from the Domar Foundation and from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05) and financial contributions from Harvard University and its affiliated academic health care centers.
Footnotes
Capsule
We found that women in the oldest age group were more likely to discontinue IVF treatment than younger women. Surprisingly, we found that the youngest women discontinued treatment in a similar fashion to women age 30–<40.
Contributor Information
Laura E. Dodge, Phone: 1 (617) 667-2254, Email: ledodge@bidmc.harvard.edu
Denny Sakkas, Phone: 1 (781) 434-6500, Email: dsakkas@bostonivf.com.
Michele R. Hacker, Phone: 1 (617) 667-2933, Email: mhacker@bidmc.harvard.edu
Rachael Feuerstein, Phone: 1 (781) 434-6500, Email: rfeuerst@hamilton.edu.
Alice D. Domar, Phone: 1 (781) 434-6500, Email: domar@domarcenter.com
References
- 1.Troude P, Ancelet S, Guibert J, Pouly JL, Bouyer J, de La RE. Joint modeling of success and treatment discontinuation in in vitro fertilization programs: a retrospective cohort study. BMC Pregnancy Childbirth. 2012;12:77. doi: 10.1186/1471-2393-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troude P, Guibert J, Bouyer J, de La RE. Medical factors associated with early IVF discontinuation. Reprod Biomed Online. 2014;28(3):321–9. doi: 10.1016/j.rbmo.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Khalili MA, Kahraman S, Ugur MG, Agha-Rahimi A, Tabibnejad N. Follow up of infertile patients after failed ART cycles: a preliminary report from Iran and Turkey. Eur J Obstet Gynecol Reprod Biol. 2012;161(1):38–41. [DOI] [PubMed]
- 4.Kulkarni G, Mohanty NC, Mohanty IR, Jadhav P, Boricha BG. Survey of reasons for discontinuation from in vitro fertilization treatment among couples attending infertility clinic. J Hum Reprod Sci. 2014;7(4):249–54. doi: 10.4103/0974-1208.147491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkhowa M, McConnell A, Thomas GE. Reasons for discontinuation of IVF treatment: a questionnaire study. Hum Reprod. 2006;21(2):358–63. doi: 10.1093/humrep/dei355. [DOI] [PubMed] [Google Scholar]
- 6.Lande Y, Seidman DS, Maman E, Baum M, Hourvitz A. Why do couples discontinue unlimited free IVF treatments? Gynecol Endocrinol. 2015;31(3):233–6. doi: 10.3109/09513590.2014.982082. [DOI] [PubMed] [Google Scholar]
- 7.Domar AD, Smith K, Conboy L, Iannone M, Alper M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil Steril 2010;94(4):1457–9. [DOI] [PubMed]
- 8.Verberg MF, Eijkemans MJ, Heijnen EM, Broekmans FJ, de KC, Fauser BC. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum Reprod. 2008;23(9):2050–5. doi: 10.1093/humrep/den219. [DOI] [PubMed] [Google Scholar]
- 9.McDowell S, Murray A. Barriers to continuing in vitro fertilisation—why do patients exit fertility treatment? Aust N Z J Obstet Gynaecol. 2011;51(1):84–90. doi: 10.1111/j.1479-828X.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 10.Soullier N, Bouyer J, Pouly JL, Guibert J, de La RE. Effect of the woman’s age on discontinuation of IVF treatment. Reprod Biomed Online. 2011;22(5):496–500. doi: 10.1016/j.rbmo.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–43. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 12.Malizia BA, Dodge LE, Penzias AS, Hacker MR. The cumulative probability of liveborn multiples after in vitro fertilization: a cohort study of more than 10,000 women. Fertil Steril. 2013;99(2):393–9. doi: 10.1016/j.fertnstert.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Practice Committee of Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertil Steril 2013;99(1):44–6. [DOI] [PubMed]
- 14.Humm KC, Dodge LE, Wu LH, Penzias AS, Malizia BA, Sakkas D, et al. In vitro fertilization in women under 35: counseling should differ by age. J Assist Reprod Genet. 2015;32(10):1449–57. doi: 10.1007/s10815-015-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


