Abstract
Products that are manufactured for use in a clinical trial, with the intent of gaining US Food and Drug Administration (FDA) approval for clinical use, must be produced under an FDA approved investigational new drug (IND) application. We describe work done toward generating reliable methodology and materials for preserving ovarian cortical tissue through a vitrification kit and reviving this tissue through a warming and recovery kit. We have described the critical steps, procedures, and environments for manufacturing products with the intent of submitting an IND. The main objective was to establish an easy-to-use kit that would ensure standardized procedures for quality tissue preservation and recovery across the 117 Oncofertility Consortium sites around the globe. These kits were developed by breaking down the components and steps of a research protocol and recombining them in a way that considers component stability and use in a clinical setting. The kits were manufactured utilizing current good manufacturing practice (cGMP) requirements and environment, along with current good laboratory practices (cGLP) techniques. Components of the kit were tested for sterility and endotoxicity, and morphological endpoint release criteria were established. We worked with the intended down-stream users of these kits for development of the kit instructions. Our intention is to test these initial kits, developed and manufactured here, for submission of an IND and to begin clinical testing for preserving the ovarian tissue that may be used for future restoration of fertility and/or hormone function in women who have gonadal dysgenesis from gonadotoxic treatment regimens or disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0846-6) contains supplementary material, which is available to authorized users.
Keywords: Good manufacturing practice, Vitrification, Ovary, Oncofertility
Purpose
Current Good Manufacturing Practice (cGMP) regulations are enforced by the US Food and Drug Administration in order to ensure the quality of manufactured drug products. These guidelines are listed in the 21 Code of Federal Regulations (CFR) part 211 [1]. cGMPs include a set of guidelines for facility standards, proper documentation, and implementation of procedures. Additionally, equipment within facilities used for drug manufacturing must be monitored and calibrated under cGMP guidelines and personnel must be properly trained and proficient in the facility procedures in order to be compliant. Current good laboratory practices (cGLPs) are additional guidelines that ensure that the data generated by testing the manufactured products has been collected in a scientifically sound manner and is reliable to support the product application. Taken together, these guidelines and training requirements are constructed to protect the consistency and sterility of the materials throughout the manufacturing process and ensure a quality, standardized product.
We sought to create Vitrification and Warming & Recovery kits for use by Oncofertility® Consortium members and partners for preserving and reviving ovarian cortical tissue. This tissue would be isolated under institutional review board (IRB)-approved procedures for preserving tissue in patients at risk for gonadal dysgenesis due to chemotherapy, radiation, and/or disease. We hope to gain approval of these kits as an Investigational New Drug (IND) through the FDA, and therefore, defining the production and manufacturing of quality medias for this approval are achieved by adhering to the cGMP guidelines [2]. Standard operating procedures (SOPs) for formulating Vitrification and Warming and Recovery medias that have been made for research purposes must be converted to fulfill the manufacturing and release guidelines put forth by the FDA with the goal of submitting the IND and performing clinical trials for establishing this procedure in clinical practice. Clinical use of this vitrified and recovered tissue could include autotransplant of the tissue back into the patient or isolation of specific cell types within the vitrified tissue for cytotherapy for restoring fertility and hormone production as previously reported [3]. An easy-to-follow kit would ensure standardized procedures for quality tissue preservation and recovery across 117 sites around the globe [oncofertility.northwestern.edu].
Choosing the vitrification protocol
Other laboratories have created ovarian tissue vitrification formulations with common cryoprotectants, mainly ethylene glycol and dimethyl sulfoxide, developed from embryo and oocyte cryopreservation protocols and generally involves plunging the tissue directly into liquid nitrogen. Table 1 displays examples of these vitrification solutions in which bovine, non-human primate, or human ovarian tissues were used to test the vitrification protocol. An additional method was developed within the Oncofertility Consortium to investigate a closed system of vitrification that may reduce cross-contamination between samples and from the environment, with intended clinical use. Synthetic polymers such as polyvinylpyrrolidone (PVP), a polyvinyl alcohol and vinyl acetate copolymer (Super cool X-1000), and a polyglycerol (Super cool Z-1000) were used to bind nucleators, and prevent ice formation and de-vitrification in an ethylene glycol-based cryoprotectant solution [4, 5]. These synthetic polymers were chosen over others because they have been shown to decrease crystallization, and improve functionality of tissues warmed and recovered after vitrification when compared to solutions without these polymers, and did not increase tissue toxicity [6, 7].
Table 1.
Vitrification solutions for ovarian tissue preservation
| Vitrification solution components | Analyses (ovary) | Citations | ||||
|---|---|---|---|---|---|---|
| EG | DMSO | G | S | Polymer | ||
| X | X | In vitro culture, histology, apoptosis, and secreted E2 and P4 in human | [15] | |||
| X | X | X | Autotransplant, serum E2, growth of primordial follicles in bovine; morphology in human | [16] | ||
| Xenograft and recovery of primordial or secondary follicle-containing bovine tissue into mice | [17] | |||||
| X | X | PrOH, PVP | Histology and ultrastructure in human | [11, 12] | ||
| X | X | PVP | Histology, follicle count, and ultrastructure in cynomolgus macaques | [18] | ||
| Autotransplant, histology and ultrastructure, vascularization of graft, serum E2 and P4, ova stimulation, and embryo development through ICSI in cynomolgus macaques | [19] | |||||
| Autotransplant, serum E2 and P4, ova stimulation, and embryo development through ICSI, 2 successful deliveries in human | [20] | |||||
| X | X | X | PVP K-10 | Apoptosis, proliferation, and fibrosis of human xenografted in mice | [21] | |
| Autotransplant, histology, follicle count, proliferation, apoptosis, graft vascularization and fibrosis, AMH immunohistochemistry in Papio anubis | [22] | |||||
| Autotransplant, histology, follicle count, proliferation, apoptosis, graft vascularization and fibrosis, AMH immunohistochemistry in cynomolgus macaques | [23] | |||||
| X | X | PVP K-12, X-1000, Z-1000 |
Histology, proliferation, follicle counts, in vitro growth and hormone secretion in rhesus macaque | [7] | ||
| In vitro growth and hormone secretion of follicles isolated from vitrified rhesus macaque tissue | [8] | |||||
Examples of vitrification solutions that have been used for human, bovine, and non-human primate ovarian tissue preservation. A brief description of their analysis is listed.
E2 estradiol, P4 progesterone, DMSO dimethyl sulfoxide, EG ethylene glycol, G glycerol, PrOH 1,2-propanediol (a.k.a. propylene glycol), PVP polyvinylpyrrolidone, S sucrose, X-1000 and Z-1000 21st Century Medicine Super cool polymers
Methods
GMP manufacturing of kit reagents
Environmental requirements
These procedures were performed at the Northwestern Memorial Hospital (NMH) Mathews Center for Cellular Therapy (MCCT) under the direction of Ann LeFever. The MCCT is a fully operational current Good Tissue Practice (cGTP) and cGMP compliant clinical facility dedicated to manufacturing support of human cellular and tissue-based products intended for human use and is an FDA-registered establishment for such manufacturing. The facility has a Quality Assurance/Quality Control (QA/QC) Laboratory, receiving area, control room, library, and classified areas (International Organization for Standardization (ISO) Class 5, ISO Class 7, and ISO Class 8). The ISO Class 8 areas consist of separate material staging, material airlocks for passage of materials in and out of the facility, and gowning and de-gowning areas. An ISO Class 7 clean corridor connects the work suites with the gowning, de-gowning, and material staging areas. The classified areas of the facility are clean rooms, and they are supplied with high efficiency particulate air (HEPA) filtered air. The Building Automation System (BAS) with an integral Environmental Monitoring System (EMS) continuously monitors the MCCT including critical parameters such as temperature, humidity, and differential pressure. The system also monitors critical parameters of dedicated laboratory equipment, including incubators and refrigerators. The BAS is equipped with an emergency callout system for off-site and off-hours personnel notification.
Personnel
Three key personnel were given the authority for approving the SOPs developed for manufacturing these media kits, as well as overseeing the production of the kit under cGMP. This approval was given in writing by Teresa K Woodruff (Research Director), Ann V LeFever (Laboratory Director), and Monica M Laronda (Quality Assurance Officer).
Appropriate hygiene is essential for the personnel involved in the manufacturing of sterile media. Appropriate personal protective equipment (PPE) is required for the use of the MCCT GMP facility, which included gloves, face mask, bouffant cap, hood, jump suit, booties, and long surgical gloves for the ISO 7 work suites. These items are put on carefully in an order outlined by the MCCT Gowning Procedure SOP, in which each person using the suit had demonstrated proficiency. Only the minimum number of personnel should be present during the production of materials. For our media production, 1 person pipetted or weighed the ingredients while the second person noted the progress of large quantities and reviewed all work and calculations to minimize measuring errors. Each person involved initialed the SOPs at the completion of a task.
Kit components
The media created for Oncofertility Consortium Vitrification and Oncofertility Consortium Warming and Recovery kits were based on a previously established protocol for rhesus macaque ovarian tissue with modifications for ease of use as a kit and conversion to what would be needed for a clinical lab receiving human tissue [7, 8]. The list of solutions for each kit and the components within each solution are for research use only. The Equilibration Solution 1 (ES1) contained glycerol (Sigma G9021), Sage OFC Holding Media (Origio ART-8040), and Quinn’s AdvantageTM Serum Protein Substitute (SPS, ART-3011). The CryoMedium (CM) contained Sage OFC Holding Media and SPS. The Vitrification Solution (VS) contained glycerol, ethylene glycol (Sigma 03750), Sage OFC Holding Media, and SPS, and the Vitrification Solution with Polymers (VS+PXZ) contained Poly(N-vinylpyrrolidone), MW 2500 (PVP-25, Polysciences 16693), Super cool X-1000 (21st Century Medicine), and Super cool Z-1000 (21st Century Medicine) in addition to the VS base components. The Warming Solution (WS1) contained sucrose (Sigma ARK2195B), SAGE OFC Holding Media, and SPS. The CM for the Warming and Recovery kit is the same formulation as the Vitrification kit. The L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (AA2P, Sigma A8960) would be added to each medium by the end user and was kept as a lyophilized powder for a prolonged media shelf-life. All reagents were vetted through the cGMP Supply Management protocols, Certificates of Analysis reviewed to confirm specifications met kit requirements and additional sterility testing was conducted to confirm sterility and endotoxin levels in the individual kit components.
The containers for these media were PETG Certified Clean Containers in the appropriate volumes of 30, 60, 125, 250, or 500 ml (Nalgene, 2019) and sealed with the appropriate shrink band (Nalgene 312160) sealed with a 1500 watt Dual Temperature Heat Gun (Drill Master), and examples of manufactured bottles are shown in Suppl. Fig. S2a. The AA2P in each kit was packaged in amber serum tubing bottles (Wheaton 223693) and ultra-pure straight plug stoppers (Wheaton 224100-400) and capped securely with aluminum seals (Wheaton 224182-01). The original kits also included individually wrapped, high security 2-ml tissue straws (IMV Technologies 018960). Single-use, disposable, sterile supplies were used throughout.
Sterility and endotoxin testing
The MCCT and the Clinical Microbiology Laboratories of Northwestern Memorial Hospital (both are CAP/CLIA certified) provide quality assurance and quality control support of products manufactured within the MCCT.
Suitability of kit reagents for tissue vitrification and revival of tissue
To establish the suitability of the various cGMP manufactured buffers for tissue vitrification and revival of tissue function, a series of processes were conducted utilizing bovine ovarian tissue. These non-clinical evaluations were not done within the GMP facility.
Preparation of test tissue
Two shipments of 4–5 bovine ovaries each were obtained from young cows at the Aurora Packing Company (Aurora, IL) and transported to the lab in BoviPro Oocyte Holding Medium (1182/1210). The single best ovary, based on size and appearance (lack of cysts and with smooth even surface epithelium), was chosen from each of the two shipments. Upon arrival, the ovaries are rinsed in fresh medium and the excess fat was removed. Ovaries were first bisected along the hilus and cut into quarters. Ovarian tissue was processed into 500-μm-thick sections of ovarian cortex using a Thomas Stadie-Riggs Tissue Slicer (Suppl. Video 1, Thomas Scientific, 6727C10). The first slice was considered cortex tissue and was used for our experiments. Tissue was sliced into 5 × 5 mm square pieces and pieces that did not contain visible corpus luteum were used. This experiment was performed two separate times. For each experiment, five cortical pieces were not put through the vitrification protocol but were fixed in 10% neutral buffered formalin (Azer Scientific, NBF-4-G) to represent fresh tissue. Twenty cortical pieces were vitrified per experiment.
Vitrification procedure
The vitrification procedure was performed as written in the kit instructions (Suppl. Fig. S1). The vial of AA2P was dissolved in 2 ml of CM to make a 100 μM AA2P solution. The AA2P solution was added to ES1, CM, VS, and VS+PXZ at 1:1000 to create a concentration of 100 nM AA2P per solution. Seventy milliliter each of Equilibration Solution 2 and 3 (ES2, ES3) were made in sterile Nalgene bottles by combining 17.5 ml VS with 52.5 ml CM for ES2 and 35 ml VS with 35 ml CM for ES3. In a biosafety cabinet, five 60-mm petri dishes were arranged on a slide warmer set to 37 °C and 10 ml each of ES1, ES2, ES3, VS, and VS+PXZ were mixed thoroughly, aliquoted into each dish and allowed to warm for 15 min. A vitrification validation test was performed to ensure that the VS+PXZ would solidify without forming crystals when held in liquid nitrogen vapors for 10 min in a 2-ml high security straw. The remainder of the straws needed were sealed on one side, labeled and pre-loaded with 2 ml of VS+PXZ. Up to 10 pieces of ovarian tissue were placed in 1 petri dish starting with ES1. Tissue was incubated in ES1 for 7 min. The contents of the dish were agitated by swirling the dish two times every 30 s. Tissue was then transferred to the dish with ES2 solution, ES3, and VS solution and incubated in the same way as with ES1. The tissue was then transferred to VS+PXZ for 30 s, swirled twice, and immediately loaded into the straws containing 2 ml of VS+PXZ each. One or 2 pieces of tissue were loaded into the straw at a time and either pushed down with a sterile pipet tip or left alone until the pieces settled before loading the rest of the tissue, up to 10 pieces (Suppl. Fig. S2b). The straw was then sealed and placed into liquid nitrogen vapor for 10 or more minutes before plunged into the liquid nitrogen (Suppl. Fig. S2c). The straws were stored in liquid nitrogen inside pre-chilled canisters until ready to warm and recover. The location and quantity of tissue in storage was noted. Of note, the preferred method for incubating the tissue in each solution is by constant shaking at 37 °C, as stated in the original article describing this vitrification method [7]. However, our lab and the consulting fertility clinic lab were not equipped to do this within our sterile hood and, therefore, swirled the dish at regular intervals. Further analysis will be done to compare these two methods.
Warming and recovery procedure
The AA2P for this kit was prepared in the same way with 2 ml of CM to create a 100 μM solution. AA2P solution was added at 1:1000 in WS1 and CM. Seventy milliliter each of Warming Solutions 2, 3, and 4 (WS2, WS3, WS4) were made in sterile bottles by combining 52.5 ml WS1 with 17.5 ml CM for WS2, 35 ml of WS1 and 35 ml with 35 ml of CM for WS3, and 17.5 ml of WS1 with 52.5 ml of CM for WS4. In a biosafety cabinet, six 60-mm petri dishes were arranged on a slide warmer and 10 ml each of WS1, WS2, WS3, WS4 and two plates of CM. Straws were removed from storage into a Dewar flask with liquid nitrogen. Straws were removed one at a time with forceps and not touched at an area with the vitrification solution. The straw was held at room temperature for 1 min then plunged into and stirred in a 45 °C water bath for 30 s. The contents were poured into an empty 100-mm petri dish, then the tissue was immediately transferred to the warm WS1. The contents from each straw were put into a separate set of dishes. The tissue was incubated in WS1, WS2, WS3, and WS4 for 5 min each and swirling two times every 30 s. The tissue was then transferred to CM and incubated two times for 10 min each, swirling two times every 30 s. Media from the first CM plate was reserved for anaerobic, aerobic, fungal sterility, and endotoxin testing. The tissue was fixed in 10% neutral buffered formalin to represent vitrified, warmed, and recovered tissue.
Histological analysis of tissue and follicle counting
All tissue processing and hematoxylin and eosin (H&E) staining was performed by the Northwestern University Center for Reproductive Sciences Histology Core. Fixed tissue was processed using an automated tissue processor (Leica) and embedded in paraffin. Serial sections were cut 5 μm thick and stained with H&E using a Leica Autostainer XL (Leica Microsystems). TUNEL analysis was performed using the DeadEnd™ Fluorometric TUNEL System (Promega, G3250) and following kit instructions. Controls for detection of TUNEL-positive cells were created by digesting fresh bovine tissue sections with DNase I (Promega, M6101; Suppl. Fig. S3). Negative controls were created by performing all steps in the kit instructions and excluding the Images were obtained using a Nikon E600 fluorescent microscope and Metamorph software and analyzed using ImageJ software (NIH). Sections from 4–5 pieces per group were analyzed in duplicate from different slides. Over 10,000 individual cells were counted as positive or negative for GFP (TUNEL) expression for each group (fresh or vitrified/recovered) and the data is represented as a percent of positive cells detected within each piece of tissue.
Follicles were counted in five pieces per group and were analyzed in duplicate from sections 100 μm away as to not count the same follicles twice. Follicles were identified as containing centralized oocytes with surrounding granulosa cells and as primordial or primary if only a single layer of granulosa cells was present. Images of each section were coded to the individual counting the follicles and measuring the area of each section. The images were analyzed using Photoshop (Adobe, CC2015). The data is represented as the average number of total follicles per surface area.
Premises and equipment
The MCCT is constructed with appropriate lighting, ventilation, and sufficient space in accordance with cGMP CFR title 21 guidelines [2]. The facility is also constructed with clean surfaces for easy testing and decontamination. The work suite used for manufacturing this media had undergone a complete sanitization and analysis of sterility prior to manufacturing to prevent the introduction, transmission, or spread of contaminants. This required the use of preapproved cleaning agents, such as 70% isopropyl alcohol and documentation of cleaning and sanitization on the MCCT log sheet. Additionally, all equipment used was calibrated according to MCCT standards prior to manufacturing and included laminar flow hoods, scales, and pipets. Data was analyzed using a two-tailed Student’s t test.
Storage instructions and stability
Written procedures were established to describe the process for testing and accepting materials, including chemical components, for media manufacturing and are described in more detail below. The components were kept sealed and treated as containing potential contaminants until it was determined that they met our endotoxin and sterility standards. The Human Ovarian Tissue Vitrification Kit and Human Ovarian Tissue Warming & Recovery Kit were stored at 4 °C and are stable until the date listed. Expiry date was dictated by the expiry date of the components and, in this case, was determined by the shelf life of the OFC Holding Media which was listed as expiring 1 year following production. AA2P was not added until the day of vitrification. The solutions should be used within 24 h following addition of AA2P.
Results
Development of media kits to be used in clinical labs
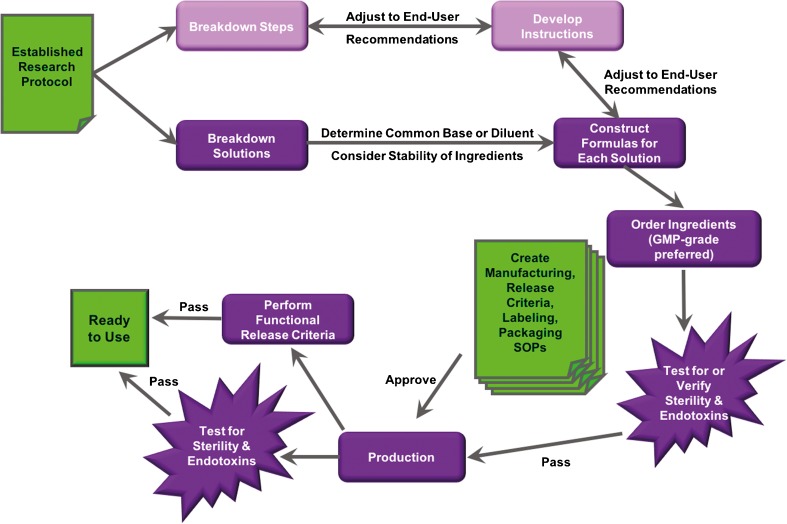
A flow chart for this process is illustrated in Fig. 1. Vitrification of ovarian tissue has already been tested as a potential way to preserve and restore quality ovarian tissue in rhesus monkeys and humans [7–12]. The goal for developing the Human Ovarian Tissue Vitrification Kit and Human Ovarian Tissue Warming & Recovery Kit were to maintain consistent media formulations and sets of instructions for handling the human ovarian tissue for establishing ovarian tissue vitrification as a way to preserve and restore quality ovarian tissue in future clinical trials. These kits must consider the down-stream users, and strong communication with the clinical labs that would use the kits for the first human trials was established and maintained throughout this process.
Fig. 1.
Flow chart of kit development. Schematic for developing solutions from an established research protocol into a clinical grade kit
The instructions considered the equipment within the lab of the down-stream user. The medias within each kit were designed to require only simple dilutions, and therefore, the components that were consistent within each medium was created as the diluent, here called CM. Additionally, the volumes chosen also considered the amount of ovarian tissue received from each participant in the trial as the kits are designed to be used for 1 participant only. The AA2P required in each medium is quickly degraded, and therefore, we decided to include this in its most stable form, lyophilized powder in a light-block container, and instruct the down-stream user to add AA2P to each medium within 24 h of use. We began production once we had determined the quantity of materials required for each kit and scaled to create 10 kits for use by the clinical lab for human tissue, with an additional 4 kits for testing the media under our SOP for release criteria and ease of use.
Production
Establishing SOPs for each point of production
SOPs were written and approved by the Research Director, Laboratory Director and Quality Assurance Officer. These were written in a standard format that included the location of production (MCCT), protocol number, version number, implementation and revision date, and author. They consisted of the “principal” or reason for creating the SOP, the “scope” or to whom this document applies, a list of reagents and equipment, if applicable, the “operational procedure,” “references,” and “revisions.” A Job Aid was also created to list the SOPs required for manufacturing complete kits. The personnel responsible for manufacturing the media reviewed each SOP prior to performing the tasks. A Document Control system was established to oversee the utilization and review of procedures.
Establishing sterile and endotoxin-free components
Each component and material ordered for use in the MCCT was shipped directly to the site and opened under the established SOP for reviewing and recording shipments received. Components that met high sterility and high purity standards were preferred. The Certificate of Analysis (C of A) and Certificate of Sterility (C of S) documents for each component used to make the media were examined. If these documents did not state that the product met sterility standards (GMP, USP, Pharma) and endotoxin levels were not confirmed to be less than 1 EU/ml, we proceeded with the sterilization protocol within the GMP facility. If sterility standards were met, then the product could be used as is for the media kit.
Components that did not meet this standard were sterilized with a 0.2-μm polyethersulfone filter system with attached sterile reservoir. The components purchased for AA2P storage were sterilized with ethylene oxide treatment in the NMH facility, which undergoes regular calibration and testing. AA2P was tested for sterility by dissolving the lyophilized powder in a sterile diluent at the same concentration that will be used in the kit. Stock solutions of media components were created when necessary. In this case, a 20% PVP-25 solution was made with sterile water and filter sterilized before taking samples for endotoxin and sterility testing. Sterility testing of these solutions included 14-day anaerobic, 14-day aerobic, and 28-day fungal testing as performed by the MCCT SOPs. Endotoxin testing was performed with Endosafe according to the MCCT SOPs. Passive air testing was also performed using open settle plates in close proximity within the biological safety cabinets. Aseptic technique was used throughout all processes.
Manufacturing batches
Production took place in a Level II vertical laminar flow biosafety cabinet in an ISO Class 7 work suite by two trained personnel who had reviewed the approved SOPs for manufacturing each medium. Media were made in large quantities then aliquoted into 14 containers (28 containers for the CM since it is contained in both kits). Appropriate mixing of the media was done with a serological pipet in between each aliquot. The bottles aliquoted from one batch preparation were considered of the same lot, and therefore, sterility and endotoxin testing results from media collected from the first, middle, and last bottles were considered the results for that lot. After labeling each bottle appropriately (see below), each bottle was sealed with shrink bands and stored at 4 °C.
Labeling
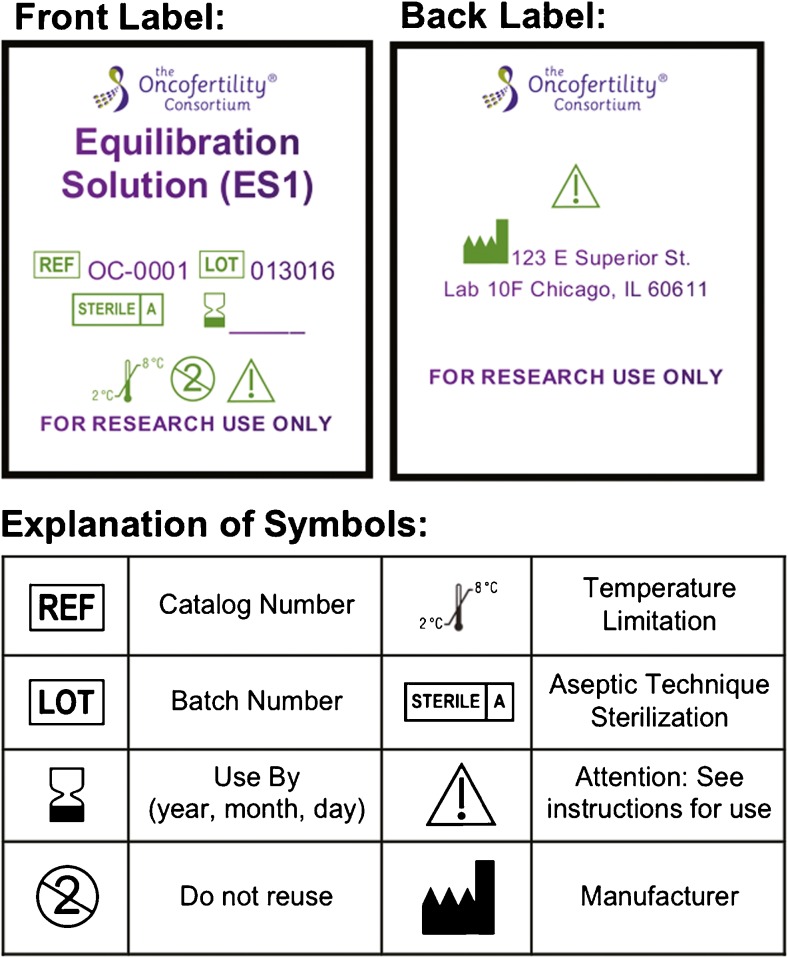
Each bottle was labeled with the specific media label prepared for that lot. An example of a media label with the required information is included in Fig. 2. According to the Code of Federal Regulations, the label on the “drug or drug product” must contain the name and address of the manufacturer, packer, and distributor [13]. Additionally, the label must clearly display the unique reference and lot number and expiration date. The labels created for each product also contained the Oncofertility Consortium logo, product name, and symbols for recommended storage temperature. Additionally, each label identified the contents as a single-use product for “research use only,” and that instructions should be read prior to using the product.
Fig. 2.
Product labeling. Example of product labeling with required and recommended details in short hand or symbol form
The product information insert that is part of the packaging listed the contents for each kit and a legend with descriptions for the symbols used on the product labels (Fig. 2). The package insert also stated, “These products are intended for the ultra-rapid vitrification of human ovarian tissue. All media preparations and procedures must be carried out in a biosafety cabinet.” A list of precautions and warnings are also declared on the packaging insert.
Caution
The user should read and understand all directions for use, precautions, and warnings before using Vitrification Kit.
Caution
Handle all human source material using universal precautions. Wear safety goggles and face mask.
Single use
Kit should be used for one patient. Discard any excess product that remains after procedure is complete.
Each set of instructions describes the protocols as listed here in the Methods section. Additionally, a C of A for each product that contains our release criteria (Table 2) with validation of sterility and results specific to each product is included with each kit.
Table 2.
Release criteria for vitrification kit
| Release criteria | Reviewer | |||
|---|---|---|---|---|
| Specification | Test method | Result | Initial/date | |
| Clarity/color | Clear, pink to peach | Visual | ||
| Fill volume | 35 ml | Visual | ||
| Endotoxin | <1.0 EU/ml | SOP # QM10.7, Endosafe | ||
| Sterility | Passes test | SOP # CTL1.2, HCT/P, Quality Assurance: Product Quality Testing | ||
| Morphological Endpoints (within kit) |
Fresh bovine cortex slice: fix 1/2, vitrify 1/2 and section to compare by hematoxylin and eosin stain | Oncofertility Consortium Vitrification Kit instructions 03/04/16 version 1 | ||
| Albumin | Donors negative for HCV, HIV-1, HIV-2 Abs and Hbs Ag | From Origio | Vendor C of A | |
| pH | 7.0–7.5 | SOP # NPC11.1 | ||
Each product within the Human Ovarian Tissue Vitrification and Warming & Recovery Kits underwent a pre-determined set of release criteria. Each task was completed from one aliquot reserved for testing. Each result was recorded, initialed, and dated by the Quality Assurance Officer or other designated personnel
Production and distribution of records
SOPs were developed for each step to ensure appropriate planning, execution, and documentation of each task toward manufacturing and testing of the kits and follow cGLP guidelines. During the manufacturing process, hard copies of the SOPs, wrapped in plastic lining for adequate sterilization, were marked with permanent ink as each task was completed and initialed by the two trained investigators at critical points of documentation, such as addition of large volumes to a media. These records were then scanned to create digital copies and stored on a secure server. The originals were kept in a binder with the Quality Assurance Officer. The endotoxin and sterility testing results were also scanned and uploaded as digital copies on the secure server.
Quality assurance and quality control
A set of release criteria was established prior to the production of the media (Table 2), and release criteria testing was conducted on aliquots of each manufactured product within the kit and for each batch of that product. These criteria represent the intended outcome of the manufactured product and outline the intended use of the kit as a whole. A separate release criteria form is produced for each product within the kits and is identified by the product name, catalog number, lot number, and expiry date. The release criteria form, which includes Table 2, is reviewed, initialed, and dated with the results for each lot of that product. A copy of this document, signed and dated by the Quality Assurance Officer, is included with each kit distribution. The product recipient should inspect the products within the kit and match the visual clarity and color as well as fill volume listed in the report to identify major unforeseen contamination, leakage, or pH change (in this case as indicated by the phenol red media color).
A test for identifying morphological endpoints of tissues vitrified with this kit has also been established for the release criteria of the Oncofertility Consortium Human Ovarian Tissue Vitrification and Warming and Recovery Kits. A more stringent functional test can be applied to the release criteria of future kits as needed. We chose to test bovine ovary slices upon completion of each manufactured batch, to ensure that the kit is reliable for down-stream use on human ovarian tissue. The VS+PXZ solution is put through an initial test to ensure that the solution vitrifies without forming crystals and is part of the initial test for every kit and is listed in the kit instructions. The bovine ovarian cortex tissue is sliced from the whole bovine ovaries and prepared in five by 5-mm pieces, mimicking what is prepared in the clinic for human tissue. Half of the bovine ovary pieces were saved to represent the initial condition of the tissue received, referred to here as “fresh.” The other half of the tissue pieces are put through the process as described in the kit instructions and vitrified. Upon completion of the protocol, the fresh tissue pieces were fixed. At least 24 h later, the vitrified tissue is put through the warming and recovery kit instructions and fixed in the same way. Additional sterility and endotoxin testing was performed on the CM used in the first plate of the warming and recovery protocol after the tissue had been washed in this media. This was to ensure that the whole process was performed with a sterile technique and that the kit components remained sterile after they were removed from the GMP environment.
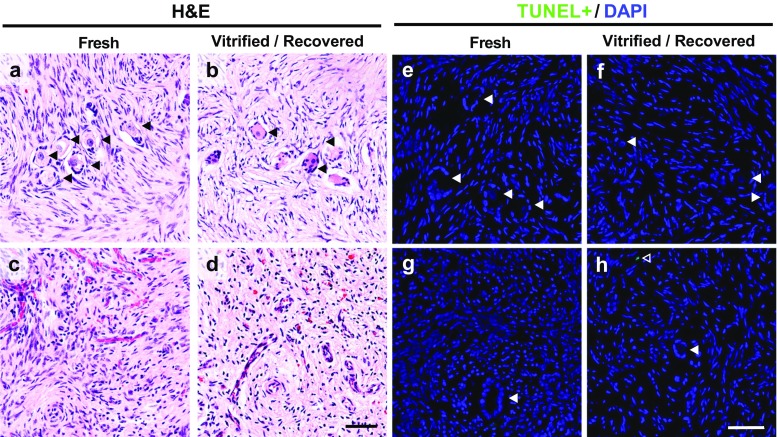
At least five pieces from each of the fresh and warmed/recovered tissue were analyzed through our Morphological Endpoint SOP. Sections from the ends and middle of each piece were stained with H&E and observed. Each of the two groups, the fresh and the vitrified/recovered sections, were observed in a blind fashion. Notes on the appearance of the tissue, describing presence of follicles, whether or not they appear healthy (healthy oocytes with healthy granulosa cells surrounding them), stroma cell shape and density (whether or not space is visible between cells), and intensity of blue versus pink staining were noted. A “Pass” result is given if these two groups of tissues appear similar in H&E. The characteristic of healthy stromal cells appeared elongated and stained mostly pink with a small granulated nuclear, or blue, stain (Fig. 3a–d). Healthy oocytes are ∼20–30 times larger than stromal cells, are round, and are mostly pink (Fig. 3a, c). Additionally, the number of follicles within sections from fresh tissue was compared with the number in warmed and recovered tissue sections. We identified approximately one follicle per 299.0 mm2 of surface area in fresh tissue sections (0.0033 follicles/mm2 ± 0.001) and approximately one follicle per 205.5 mm2 of surface area in warmed and recovered tissue sections (0.0049 follicles/mm2 ± 0.001). Overall, 97.5% of the follicles counted were primordial or primary and there was no significant difference among the two groups (mean ± standard error, Student’s t test, p = 0.459).
Fig. 3.
Ovarian tissue histology. Comparing bovine ovarian tissue cortex pieces that were fixed fresh or after vitrification. a–b Fresh tissue and vitrified and recovered tissue both contained follicles with a centralized oocyte and nuclear staining. c–d Fresh and vitrified and recovered tissue contained vessels with red blood cells within the stroma. e–h Representative images of fresh and vitrified and recovered tissue were stained for TUNEL (green) and DNA (DAPI, blue). Black or white arrowhead (◀), follicle; open arrowhead (◁), TUNEL-positive cell. Scale bar 50 μm
Additional testing on tissue health was performed on our bovine ovarian cortex slices to further confirm the morphological endpoint of the kit prepared in this batch. Sections of fresh and vitrified/recovered ovarian tissue was stained to identify cells undergoing apoptotic DNA degradation using a TdT-mediated dUTP Nick-End Labeling, or TUNEL, assay (Fig. 3e–h, Suppl. Fig. S3). The number of TUNEL-positive cells from four fresh and five vitrified/recovered tissue pieces were calculated and compared. We found that 1 cell out of 11,344 cells analyzed in four tissue samples (0.0081 ± 0.0058%) was positive for TUNEL in the fresh tissue samples and two cells out of 10,566 cells analyzed in five tissue samples (0.0178 ± 0.0246%) were positive for TUNEL in the vitrified/recovered samples and there was no significant difference among the two groups (mean ± standard error, Student’s t test, p = 0.668).
Conclusion
Translational research encompasses two phases of interpretation according to the National Institutes of Health (NIH), the dominant funding source for basic biological, and clinical research and training. The first phase requires translating basic research performed on the bench into clinical trials and studies in humans. The second phase requires translation from the clinical trials and studies to best practices in the clinic or community [14]. A clear understanding of cGLP and cGMP implementation must exist in order to bridge basic research toward clinical trials in humans. This manuscript describes the first steps toward translating bench research into a product that meets the criteria and provides data required for an IND submission to the FDA. These pathways of investigations are performed with the intended outcome of creating safe products for use on human ovarian tissues that may be transplanted back into the patient. Therefore, cGLP and cGMP guidelines were followed to ensure consistent, sterile product kits and good scientific methodologies of testing. These practices included controlled manufacturing environments, skilled personnel, establishment of SOPs, measures for sterility and functionality evaluation and appropriate documentation of procedures.
We describe the work done toward creating a reliable method for preserving ovarian cortical tissue through a vitrification kit and reviving this tissue through a warming and recovery kit, for eventual use in the clinic. These research protocols are established for rhesus macaque and are not currently intended for clinical use [7–9]. The next steps required developing the research protocols and media formulations into a simplified kit with the intention of future use within a fertility clinic as a standardized, replicable procedure to increase the reliability of predicted outcomes. Additional research on human ovarian tissue, including analysis of functional endpoints in vitro following use of the Vitrification and Warming and Recovery Kits, are ongoing to support our IND application. Furthermore, research into the ability of vitrified and recovered ovarian tissue to support hormone secretion and production of a fertilizable egg after transplantation into humans will also be required to demonstrate that full functionality can be preserved using these kits. While these steps describe development and manufacturing for specific kits, they can be translated to the development of other medias or kits that have the potential for translation and in which the researchers hope to submit an IND. Researchers at the apex of translational research should be encouraged to overcome the hurdle of understanding these specific FDA regulations and following the guidelines as these techniques are not much different from good scientific methodologies and general laboratory practices.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Vitrification Kit and Warming and Recovery Kit Instructions. Product insert kit information and instructions. (PDF 14.3 mb)
Oncofertility Consortium Vitrification Kit Use. Images of manufacturing media and vitrification of tissue. a Image of manufactured media bottles within GMP facility. b Image of bovine ovarian cortical tissue pieces within VS+PXZ—filled straw (black circles around pieces of tissue). c Image of sealed straws filled with tissue wedged within a liquid nitrogen Dewar in liquid nitrogen vapor (black arrow pointing to a straw containing bovine ovarian cortical pieces). The adjacent straws contain pieces of bovine placentomes (orange tissue). (PDF 39 kb)
Ovarian Tissue TUNEL Controls. Fresh bovine ovarian tissue that was digested with DNase I as a positive controls is shown with TUNEL-positive (green) cells alone or photo-merged with DAPI (blue) staining. (PDF 106 kb)
Standard Operating Procedure for Preparing Ovarian Tissue for Vitrification Using the Stadie-Riggs Tissue Slicer. Fresh bovine ovaries were prepared using the Stadie-Riggs tissue slicer to achieve 0.5 mm thick pieces of cortex. (MOV 292 mb)
Acknowledgments
This work is supported by the Watkins Chair of Obstetrics and Gynecology (TKW), the UH3TR001207 (NCATS, NICHD, NIEHS, OWHR, NIH Common Fund), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development U54HD076188 grant. The Oncofertility Consortium ® is funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and PL1CA133835, and the Center for Reproductive Health After Disease P50HD076188 grant from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI). MML acknowledges support from The Burroughs Wellcome Fund Career Award at the Scientific Interface and the John and Lillian Mathews Regenerative Medicine Endowment. This work was supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and a Cancer Center Support Grant (NCI CA060553). The authors thank Greg Fahy, from 21st Century Medicine, for gifting the Super Cool polymers used in this study; Clarisa Gracia and Jessica Brown, from the University of Pennsylvania, for giving us feedback on the operational procedures as performed in a fertility clinic; Cheryl Hanson, from the Mathews Center, for Good Manufacturing Practice training and feedback on procedures; and Steven Mullen, from Cook Regentec, for his role in developing the initial vitrification media recipes. We also thank Keisha Barreto from the Ovarian Histology Core at Northwestern University for her technical expertise.
Footnotes
Capsule The main objective of this study was to establish an easy-to-use kit for standardized ovarian tissue vitrification and recovery. We have described the critical steps, procedures, and environments for manufacturing media kits intended for an Investigational New Drug (IND) application for future clinical trials.
References
- 1.Food and Drug Administration. Current Good Manufacturing Practice in Manufacturing Processing, packing, or Holding of Drugs. [Internet]. 21; Apr 6, 2016. Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=1970e7dcc6a6fb934d0fb596aad264f9&mc=true&tpl=/ecfrbrowse/Title21/21cfr211_main_02.tpl
- 2.FDA US. Guidance for Industry CGMP for Phase 1 Investigational Drugs. Rockville; 2008.
- 3.Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–70. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wowk B, Leitl E, Rasch CM, Mesbah-Karimi N, Harris SB, Fahy GM. Vitrification enhancement by synthetic ice blocking agents. Cryobiology. 2000;40:228–36. doi: 10.1006/cryo.2000.2243. [DOI] [PubMed] [Google Scholar]
- 5.Badrzadeh H, Najmabadi S, Paymani R, Macaso T, Azadbadi Z, Ahmady A. Super cool X-1000 and Super cool Z-1000, two ice blockers, and their effect on vitrification/warming of mouse embryos. Eur J Obstet Gynecol. 2010;151:70–1. doi: 10.1016/j.ejogrb.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Fahy GM, Wowk B, Wu J, Phan J, Rasch C, Chang A, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48:157–78. doi: 10.1016/j.cryobiol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles. Cryobiology. 2012;65:1–11. doi: 10.1016/j.cryobiol.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28:1267–79. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–72. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–85. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 12.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod. 2011;26:594–603. doi: 10.1093/humrep/deq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. General Labeling Provisions. Code of Federal Regulations Title 21; Apr 1, 2015 pp. 1–17.
- 14.National Institutes of Health. RFA-RM-10-001: Institutional Clinical and Translational Science Award (U54) [Internet]. 2016 [cited 2016 May 11]. pp. 1–40. Available from: http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-10-001.html#SectionI
- 15.Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139:193–8. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod BioMed Online. 2009;18:568–77. doi: 10.1016/S1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 17.Bao RM, Yamasaka E, Moniruzzaman M, Hamawaki A, Yoshikawa M, Miyano T. Development of vitrified bovine secondary and primordial follicles in xenografts. Theriogenology. 2010;74:817–27. doi: 10.1016/j.theriogenology.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod BioMed Online. 2010;21:501–9. doi: 10.1016/j.rbmo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27:2420–9. doi: 10.1093/humrep/des178. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 21.Amorim CA, Dolmans M-M, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–2. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 22.Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28:2146–56. doi: 10.1093/humrep/det103. [DOI] [PubMed] [Google Scholar]
- 23.Dolmans M-M, Binda MM, Jacobs S, Dehoux JP, Squifflet JL, Ambroise J, et al. Impact of the cryopreservation technique and vascular bed on ovarian tissue transplantation in cynomolgus monkeys. J Assist Reprod Genet. 2015;32:1251–62. doi: 10.1007/s10815-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vitrification Kit and Warming and Recovery Kit Instructions. Product insert kit information and instructions. (PDF 14.3 mb)
Oncofertility Consortium Vitrification Kit Use. Images of manufacturing media and vitrification of tissue. a Image of manufactured media bottles within GMP facility. b Image of bovine ovarian cortical tissue pieces within VS+PXZ—filled straw (black circles around pieces of tissue). c Image of sealed straws filled with tissue wedged within a liquid nitrogen Dewar in liquid nitrogen vapor (black arrow pointing to a straw containing bovine ovarian cortical pieces). The adjacent straws contain pieces of bovine placentomes (orange tissue). (PDF 39 kb)
Ovarian Tissue TUNEL Controls. Fresh bovine ovarian tissue that was digested with DNase I as a positive controls is shown with TUNEL-positive (green) cells alone or photo-merged with DAPI (blue) staining. (PDF 106 kb)
Standard Operating Procedure for Preparing Ovarian Tissue for Vitrification Using the Stadie-Riggs Tissue Slicer. Fresh bovine ovaries were prepared using the Stadie-Riggs tissue slicer to achieve 0.5 mm thick pieces of cortex. (MOV 292 mb)