Abstract
Purpose
It was studied whether morphokinetics of blastocoele re-expansion and hatching in vitrified-warmed blastocysts is predictive of implantation, clinical pregnancy, and live birth.
Methods
In 144 patients aiming for single warmed blastocyst transfer, blastocysts were cultured in a new time-lapse system (Miri® TL) immediately after warming. Video sequences with an image interval of 5 min were annotated and the corresponding morphokinetic variables were correlated with pregnancy outcome. In detail, tRE (start of re-expansion), tCRE (completion of re-expansion), tAH (hatching from the manipulated zona pellucida), and presence of collapses were recorded.
Results
In the pregnant group, tRE and tCRE were significantly lower (0.69 ± 0.45 h and 2.16 ± 0.94 h) as compared to the non-pregnant group (1.23 ± 1.08 h and 2.70 ± 1.20 h). Both variables and the duration of re-expansion (tCRE-tRE) allowed for distinction between “non-pregnant,” “loss of pregnancy,” and live birth/ongoing pregnancy. Presence and number of collapses showed no correlation with outcome.
Conclusions
Time-lapse imaging of vitrified-warmed blastocysts offers additional selection criteria allowing for prediction of implantation potential. As a consequence, cumulative pregnancy rate could be increased and time-to-pregnancy reduced.
Keywords: Blastocyst vitrification, Blastocyst re-expansion, Morphokinetics, Time-lapse imaging, Hatching
Introduction
The proportion of births following transfer of cryopreserved blastocysts has increased dramatically due to a remarkable improvement in the efficiency of freezing techniques [1]. In particular, vitrification turned out to be a reliable alternative to the slow freezing method [2, 3]. In parallel, a rethinking process led to policies like “freeze-all blastocysts” [4, 5] or “single embryo/blastocyst transfer” [6, 7] which in turn resulted in an increasing proportion of vitrified blastocysts available.
In order to achieve optimal survival rates and, as a consequence, to increase cumulative (singleton) pregnancy rates, there is a tendency to vitrify only blastocysts of a certain quality [8]. In detail, both cell lineages inner cell mass (ICM) and trophectoderm (TE) have to be of moderate to optimal quality based on cell number and cohesion of these cells. Thus, blastocyst scoring is generally done before vitrification and morphological information on warmed blastocysts is rare. This is a shortcoming since the dehydration process during cryopreservation cells and cell lineages may undergo considerable morphological changes. Accordingly, blastocyst morphology before and after vitrification may differ substantially regardless of whether an open or a closed carrier is used [9].
Since cryopreserved blastocysts are shrunk after thawing/warming, it is more difficult to assess and score such blastocysts as compared to fresh ones [10]. As a consequence, for proper morphological analysis, embryologists tend to wait until re-expansion occurs. While some authors recommend checking for 24-h survival [3, 11], others state that most of the cryopreserved blastocyst are already re-expanded after 2–4 h [10]. Indeed, our group [8] demonstrated that re-expansion within 2 h after warming allows for prediction of implantation, pregnancy, and live birth.
In terms of embryo scoring, morphokinetics has taken over the leading role from pure morphological evaluation and it was only a matter of time before time-lapse data dealing with vitrified/warmed blastocysts were published [12]. This report roughly classified vitrified/warmed blastocysts by whether they had shrunk or not at 5 to 6 h after warming and then retrospectively screened the video sequences for evidence of temporary re-expansion [12].
Since in this preliminary study [12] the time-lapse incubator was not primarily used to measure the exact timing of particular events of the warming process, the present study was set up to generate more robust data on early morphokinetic variables of warmed blastocysts. By doing so, we aimed for a decision aid of clinical relevance, e.g., determining whether to transfer a warmed blastocyst or to warm an additional one on time.
Materials and methods
In order to gain maximum insight into the implantation behavior of warmed blastocysts, only single blastocyst transfers were prospectively included in this 6-month study. Since annotation of dynamic variables was performed retrospectively and had no influence on the decision of transferring or warming additional blastocysts, IRB approval had not been sought. Not to forget that culturing embryos in a time-lapse incubator (Miri TL®, Esco Medical, Berlin, Germany) is a routine culture technique in our lab.
A total of 151 patients met the inclusion criteria (day 5 vitrification, single transfer of vitrified-warmed blastocyst) within the study period. It should be noted that seven patients had to be excluded from further analysis since their embryo transfer had to be canceled due to the fact that no blastocyst(s) survived the warming procedure. None of the patients were included twice in the study. The overall blastocyst survival rate after warming was 87.8% (144/164).
The mean age of the 144 female patients was 32.6 ± 5.1 years which was younger than that of their male partners (39.6 ± 8.2). Approximately half of the patients suffered from secondary infertility, and medical indication was as expected in average IVF clients (less than 15% PCOS and approx. 10% endometriosis). In the case of male subfertility, it should be noted that all sperms were ejaculated since no cases of TESE were seen. In their fresh cycles, every fifth patient (20.8%) was stimulated according to an agonist protocol, whereas almost 80% had an antagonist protocol prescribed. Depending whether the patients achieved pregnancy with the fresh blastocyst transfer, the duration of blastocyst cryostorage varied from 2 months (minimum interval recommended in our clinic) to 92 months (mean ± SD 12.6 ± 14.6 months).
Vitrification
All blastocysts considered for vitrification were scored according to the previously published guidelines of Alpha and ESHRE [13], focusing on expansion, inner cell mass, and trophectoderm. As a general rule, day 5 embryos were only considered for vitrification if both cell lineages inner cell mass (ICM) and trophectoderm (TE) were of good to moderate quality based on cell number and cohesion [8]. In addition, only a minimal cytoplasmic loss due to fragmentation and exclusion of blastomeres was accepted. In all cases, vitrification was done at room temperature using a commercially available kit (GM501 VitriStore Freeze; Gynemed, Lensahn, Germany). In short, all blastocysts had to be pre-incubated in a medium containing phosphate-buffered saline (PBS). This step was followed by two sequential incubations in vitrification media showing increasing molarities of the cryoprotectants ethylene glycol and dimethyl sulphoxide (DMSO). In the first vitrification medium, timing was strictly adjusted to the grade of expansion, so that early blastocysts were incubated for 1 min, full blastocysts for 2 min, and expanded or hatching blastocysts for 3 min. Exposure time in highly concentrated vitrification medium 2 was kept to a minimum of 30 s. Within this last crucial period, blastocysts shrank and had to be placed on the tip of a Kitazato Cryotop (Gynemed) in very small volumes of vitrification medium 2 (approximately 0.1–0.2 μl), followed by direct plunging into liquid nitrogen and storage in a container (“open system”). It should be noted that all blastocysts were individually vitrified.
Warming
Special care was taken to ensure very high warming rates, which required rapid plunging into the first warming solution which was at 37 ° and contained 0.5 M sucrose. The other three warming solutions of the warming kit (VitriStore Thaw, Gynemed) were kept at room temperature and differed from each other by a descending concentration of sucrose (0.25, 0.125, and 0 M). After the last incubation step (0 M sucrose), the warmed blastocysts were transferred into a conventional 4-well dish containing pre-warmed culture medium (BlastAssist, Origio, Måløv, Denmark). It is important to note that the whole warming process took only 8 min for all grades of blastocysts.
In order to reduce a potential negative impact of cryopreservation on the zona pellucida constitution [9], all blastocysts were hatched artificially using a diode laser (Octax, MTG, Bruckberg, Germany). The principle of complete hatching was chosen which involves a full-thickness defect of the zona pellucida [14]. Assisted hatching was never done in case blastocysts had already started to leave the zona before vitrification.
Time-lapse imaging
Immediately after assisted hatching, blastocysts were pipetted from the 4-well dish to a CultureCoin® which is a culture dish specially designed for the Miri® TL time-lapse incubator from Esco Medical. In vitro culture in the separate chambers of the Miri® TL incubator was done under reduced oxygen (5%) with 6.5% CO2. The volume of culture medium (BlastAssist) used was 30 μl (under mineral oil).
It was ensured that every warmed blastocyst was placed in the time-lapse incubator in less than 10 min after beginning of the warming procedure. Since it was planned to annotate morphokinetic variables of the early warming process, it was of utmost importance to preset image frequency to 5 min (seven focal planes) in order not to miss the beginning of these early events. Images were taken by a built-in Zeiss objective (×20) with a numerical aperture of 0.35 specialized for 635 nm illumination (red light). All videos (average length of 4.46 ± 1.10 h) were analyzed with the Miri® TL Viewer software. Time in the Miri TL® depended on the time scheduled for transfer. However, no correlation between length of the video and morphokinetic data or treatment outcome was observed.
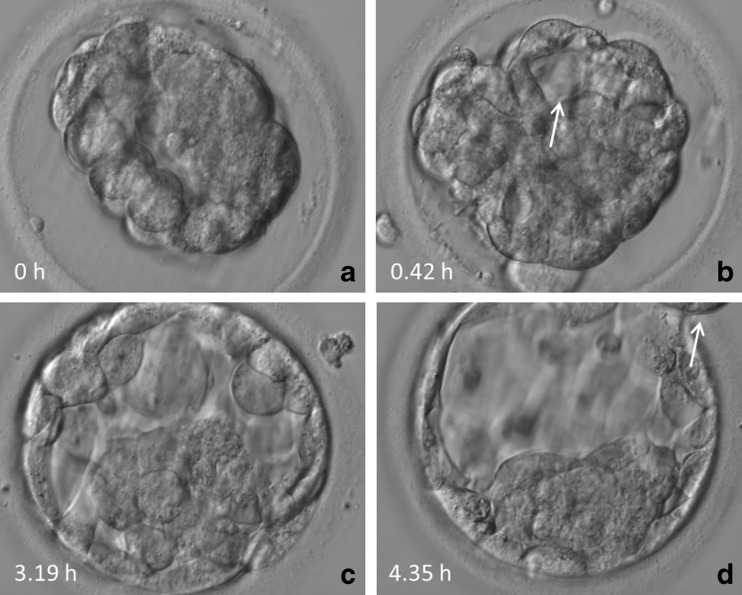
In particular, we focused on three morphokinetic parameters (Fig. 1a–d):
-
i.
Time of the start of re-expansion (tRE; first frame in which the blastocoele forms or increases in size)
-
ii.
Time of completion of re-expansion (tCRE; first frame the blastocyst occupies the whole perivitelline space)
-
iii.
Time of artificial hatching (tAH; first frame in which the trophectoderm blebbs out of the manipulated zona pellucida)
Fig. 1.
Temporal changes of a vitrified and warmed blastocyst. a Original blastocyst immediately after transfer to CultureCoin®. b Start of re-expansion (tRE) of blastocoel as indicated by an arrow. c Completion of re-expansion (tCRE). d Start of hatching (tAH) as indicated by an arrow
Based on these annotation parameters, the duration of the re-expansion process is calculated tCRE-tRE. It should be noted that not all blastocysts went through all three stages during the recorded video sequence. Apart from the abovementioned morphokinetic parameters, blastocyst development after warming was also checked for the presence of collapses.
Statistics
According to the results of the Kolmogoroff-Smirnov test for normal distribution either a chi-square test, a Mann-Whitney U test (after Bonferroni-Holms correction), a Fisher’s Exact test, or a Kruskal-Wallis test was performed. A Mann-Whitney U test and an Independent Samples Median test were used to compare distributions and medians, respectively. A logistic regression has been done where appropriate. Level of significance was defined as P < 0.05.
The primary goal of this study was to determine whether the means of tRE, tRCE, and tHA are different between the groups “clinical pregnancy” and “no pregnancy.” Sample size calculation based on parametric tests revealed the necessity of at least 53 patients in each group (alpha = 5%, power = 80%, medium effect size). The fact that we had even more patients included ensures a high power even for non-parametric testing.
Results
Morphokinetics
Morphokinetic analysis showed that nine blastocysts (6.3%) did not start to re-expand in the given time of the video sequence (4.6 ± 1.2 h). A 33.3% ongoing pregnancy rate in this group indicates that re-expansion had started later (tRE >5 to 6 h). In the group of blastocysts that did re-expand, the average tRE was 1.0 ± 0.97 h after the blastocysts were transferred into the CultureCoin®. tRE ranged from 0.17 to 6.18 h, meaning that sometimes re-expansion started as early as 10 min (2 frames) after loading the dish.
Completion of re-expansion (tCRE) was not observed in 35 blastocysts (24.3%) within a given time (4.55 ± 1.16 h). The average tCRE was 2.44 ± 1.12 h with the earliest completion after 0.51 h (approx. 31 min). Related to the latter parameter, duration of the re-expansion process, calculated as tCRE-tRE, was 1.74 ± 0.86.
Hatching from an artificial gap could not be observed in 70 blastocysts (48.6%). As expected, tAH was close to tCRE with a mean of 2.92 h (±0.95 h). Thus, on average, it took less than an hour (0.91 ± 0.69 h; range from 0.16 to 3.07 h) to leave the zona pellucida once full re-expansion had been reached. The earliest artificial hatching was annotated at 40 min. Collapses during the warming process were noted in 23 cases (16.0%), but this had no effect on further implantation behavior. In addition, the presence and number of collapses was not caused by assisted hatching. Morphokinetics of collapses was not related to treatment outcome (P = 0.39).
Pregnancy outcome
Overall, biochemical pregnancy rate (also reflecting the implantation rate) was 62/144 (43.1%). The corresponding live birth rate, or ongoing pregnancy rate, respectively, was found to be 34.7% since 6 missed abortions each with and without heart activity occurred. The (monozygotic) multiple pregnancy rate was as a high as 3.2%. All in all, 52 healthy children have been delivered.
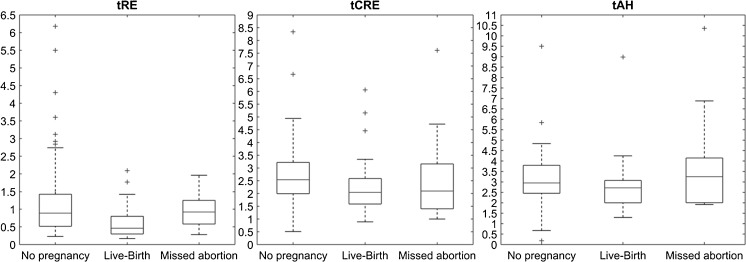
Figure 2 and Table 1 show that both tRE (P < 0.001) and tCRE (P = 0.018) were able to distinguish between “non-pregnant” and “hCG positive.” In addition, the same annotation variables allowed to differ between “non-pregnant,” “loss of pregnancy,” and live birth (P = 0.003 for tRE and P = 0.04 for tCRE). Moreover, medians of these three groups varied in tRE and tCRE (P = 0.003 and P = 0.04, respectively). Logistic regression revealed that tRE was the most effective prediction parameter.
Fig. 2.
Comparison of tRE, tCRE, and tAH and its correlation with pregnancy outcome
Table 1.
Morphokinetic annotation of dynamic events during the warming process
| Variable | tRE | tCRE | tAH |
|---|---|---|---|
| No pregnancy | 1.30 ± 1.19a,c | 2.64 ± 1.14b,d | 3.07 ± 1.30 |
| Positive β-hCG | 0.69 ± 0.45a,c | 2.30 ± 1.29b,d | 3.05 ± 1.77 |
| Live birth | 0.60 ± 0.42c | 2.16 ± 1.06d | 2.76 ± 1.32 |
| Pregnancy losses | 1.00 ± 0.48 | 2.75 ± 1.80 | 4.06 ± 2.66 |
Values are mean ± standard deviation (in hours)
tRE start of re-expansion, tCRE completion of re-expansion, tAH start of artificial hatching
a P > 0.001 (Mann-Whitney U test)
b P > 0.05 (Mann-Whitney U test)
c P > 0.01 (Kruskal-Wallis test)
d P > 0.05 (Kruskal-Wallis test)
Duration of re-expansion in those blastocysts that resulted in pregnancy was significantly shorter as compared to re-expansion in the non-pregnant group (P < 0.05).
Discussion
Since all freezing techniques involve careful removal of water from cells, there is an intracellular risk of ice crystal formation. The situation is somewhat more severe at the blastocyst stage since the amount of watery fluid in the blastocoelic cavity is considerable. As a result of the presence/size of the blastocoele, vitrified blastocysts experience several morphological changes during cryopreservation finally resulting in a well-aimed collapse. As a consequence, it is more difficult to accurately score the quality of blastocyst after warming as compared to a fresh one [8, 10]. Time-lapse imaging here offers a beneficial tool for exactly tracing early dynamic events of the warming process.
Indeed, present data provide first evidence that early onset of re-expansion (assessed as the first sign of an increase of the blastocoelic cavity) is of positive predictive power in terms of implantation, pregnancy, and live birth. This very first morphokinetic variable obviously reflects resumption of cellular metabolism and/or osmotic capacity. In accordance with blastocoele formation during fresh in vitro culture, it is very likely that with tRE trophectoderm cells are viable and capable of metabolic activity. The event of re-expansion is comparable with the situation found during regular (fresh) blastocoele development, when trophectoderm cells actively pump in sodium ions [15] which is followed by passive influx of water due to the existing osmotic imbalance [16]. In other words, tRE seems to accurately reflect viability of the warmed blastocyst. It turned out that in the majority of cases which resulted in ongoing pregnancies or live births, tRE was annotated so early that an image interval of 10–15 min would not allow for proper scoring. Since the Miri® TL incubator enables a frame frequency of 5 min, this optimal setting has been chosen.
As the completion of the re-expansion process was also found to be a significant predictor of outcome, it is evident that the duration of the re-expansion process (tCRE-tRE) is also time-dependent. The earlier this process is completed, the better the prognosis of the warmed blastocyst. To date, embryologists either waited overnight [3, 11] to confirm blastocyst survival and mitotic activity or a fixed time was chosen, e.g., 2 h [8] beyond which re-expansion was considered to be delayed. The latter scenario was associated with significantly reduced rates of implantation and clinical pregnancy [8]. However, it should be kept in mind that even if it is assumed that all viable blastocysts will re-expand after several more hours, any delay in this process could be a manifestation of altered osmotic and/or metabolic conditions, which does not preclude occurrence of pregnancy.
One dynamic event of warmed blastocysts that often goes with re-expansion is hatching through the laser-drilled gap. In our hands, tAH more or less directly followed tCRE in less than an hour since the zona pellucida did not hinder proceeding of re-expansion until (artificial) hatching. The process of assisted hatching is preventively chosen in order to guard against hatching problems due to freezing-related zona hardening [3].
One morphokinetic phenomenon especially known for blastocysts is the incident of one or more collapses [17, 18]. Per definition, collapses are “a separation of all or part of the TE cells from the zona pellucida” during blastocyst growth [17]. Marcos et al. [17] stressed that blastocysts that exhibit collapse(s) are as likely to hatch as those that do not, but since their implantation potential seems to be limited they recommended not to replace them if alternatives are available. Recently, Bodri and co-workers [18] specified that collapse(s) are not independent predictors of live birth rate since they are confounded by stronger predictors such as female age. In the present setting, collapses happened in 16% of the warmed blastocysts which parallels data from literature [17]. However, it should not be forgotten that both papers dealing with time-lapse imaging of collapsing blastocysts [17, 18] worked on fresh blastocysts and it is in no way clear whether our warmed blastocysts showed collapses during in vitro culture of the fresh cycles or if the underlying cause of this phenomenon is the same. There is indeed evidence that vitrification may increase the frequency of contractions during the (spontaneous) pre-hatching stage [19]. The latter observation could not be confirmed in the present study but it should be clarified that we artificially hatched the zona pellucida (which did not affect formation or morphokinetics of collapses).
Although this is first time-lapse evidence that dynamics of the warming process allow for a relatively precise prediction of further fate of the blastocysts, it is not the first work on time-lapse analysis of vitrified and warmed day 5 embryos. Maezawa et al. [20] used the time-lapse technique to distinguish viable embryos from shrunken ones. If a snapshot 5–6 h after warming revealed that the blastocyst actually was collapsed, these authors retrospectively screened the video for signs of (temporary) re-expansion. The Japanese group could further prove that collapsed blastocysts have a lower oxygen consumption rate than expanded ones; however, this metabolic imbalance was not reflected in implantation potential.
Based on time-lapse imaging, others [20] found a tendentially higher rate of positive serum hCG if the warmed blastocyst showed “pronounced” re-expansion after 30 min of warming. This trend reached the level of statistical significance if the warmed blastocysts had a “100% trophectoderm diameter re-expansion” (which would be comparable to tCRE) after 90 min. Galán Rivas et al. [21] used the time-lapse technique to quantify certain morphological structures of warmed blastocysts such as the initial and final thickness of the zona pellucida, the initial and final area of the ICM, and the presence of collapses, with the latter showing no correlation with implantation.
To summarize, annotation of three new morphokinetic events (tRE, tCRE, tAH) showed that it is the early onset and the duration of the re-expansion process that is meaningful in terms of further post-warming development and implantation behavior. Theoretically, with this knowledge, one does not need to wait for a period of time until blastocyst survival is controlled for. Much rather, decision whether to transfer the warmed blastocyst/s or warm additional embryos can be made in less than an hour, which definitely is of clinical relevance for a busy embryological staff. As a consequence, cumulative pregnancy rate could be increased and time-to-pregnancy would be reduced.
Acknowledgments
The authors would like to thank G. Schappacher-Tilp for statistical expertise.
Footnotes
Capsule Time-lapse imaging of vitrified-warmed blastocysts, particularly the start of re-expansion, offers additional selection criteria allowing for prediction of implantation potential which may result in reduced time-to-pregnancy.
References
- 1.Liebermann J, Tucker MJ. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application. Fertil Steril. 2005;86:20–6. doi: 10.1016/j.fertnstert.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Vanderzwalmen P, Bertin G, Debauche C, Standaert V, van Roosendaal E, Vandervorst M, et al. Births after vitrification at morula and blastula stages: effect of artificial reduction of the blastocoelic cavity before vitrification. Hum Reprod. 2002;17:744–51. doi: 10.1093/humrep/17.3.744. [DOI] [PubMed] [Google Scholar]
- 3.Vanderzwalmen P, Bertin G, Debauche C, Standaert V, Bollen N, van Roosendaal E, et al. Vitrification of human blastocysts with the Hemi-straw carrier: application of assisted hatching after thawing. Hum Reprod. 2003;18:1504–11. doi: 10.1093/humrep/deg298. [DOI] [PubMed] [Google Scholar]
- 4.Ortega-Hrepich C, Stoop D, Guzmán L, Van Landuyt L, Tournaye H, Smitz J, et al. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome. Fertil Steril. 2013;100:1002–7. doi: 10.1016/j.fertnstert.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Freeze-all at the blastocyst or bipronuclear stage: a randomized clinical trial. Fertil Steril. 2015;104:1138–44. doi: 10.1016/j.fertnstert.2015.07.1141. [DOI] [PubMed] [Google Scholar]
- 6.Stoop D, Van Landuyt L, Van den Abbeel E, Camus M, Verheyen G, Devroey P. Should a single blastocyst transfer policy be a clinical decision or should it depend on the embryological evaluation on day 3? Reprod Biol Endocrinol. 2011;9:60. doi: 10.1186/1477-7827-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zander-Fox DL, Tremellen K, Lane M. Single blastocyst embryo transfer maintains comparable pregnancy rates to double cleavage-stage embryo transfer but results in healthier pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2011;51:406–10. doi: 10.1111/j.1479-828X.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 8.Ebner T, Vanderzwalmen P, Shebl O, Urdl W, Moser M, Zech NH, et al. Morphology of vitrified/warmed day-5 embryos predicts rates of implantation, pregnancy and live birth. Reprod Biomed Online. 2009;19:72–8. doi: 10.1016/S1472-6483(10)60049-1. [DOI] [PubMed] [Google Scholar]
- 9.Ebner T, Vanderzwalmen P, Wirleitner B. Atlas of vitrified blastocysts in human assisted reproduction. 1. Cambridge: Cambridge University Press; 2015. [Google Scholar]
- 10.Shu Y, Watt J, Gebhardt J, Dasig J, Appling J, Behr B. The value of fast blastocyst re-expansion in the election of a viable thawed blastocyst for transfer. Fertil Steril. 2008;91:401–6. doi: 10.1016/j.fertnstert.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 11.Van den Abbeel E, Camus M, Verheyen G, Van Waesberghe L, Devroey P, Van Steirteghem A. Slow controlled-rate freezing of sequentially cultured human blastocysts: an evaluation of two freezing strategies. Hum Reprod. 2005;20:2929–45. doi: 10.1093/humrep/dei134. [DOI] [PubMed] [Google Scholar]
- 12.Maezawa T, Yamanaka M, Hashimoto S, Amo A, Ohgaki A, Nakaoka Y, et al. Possible selection of viable human blastocysts after vitrification by monitoring morphological changes. J. Assist. Reprod. Genet. 2014;31:1099–104. doi: 10.1007/s10815-014-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ALPHA Scientists in Reproductive Medicine. ESHRE Special Interest Group Embryology Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 2011;22:632–46. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Ebner T, Moser M, Tews G. Possible applications of a non-contact 1.48 microm wavelength diode laser in assisted reproduction technologies. Hum Reprod Update. 2005;11:425–35. doi: 10.1093/humupd/dmi009. [DOI] [PubMed] [Google Scholar]
- 15.Biggers JD, Bell JE, Benos DJ. Mammalian blastocyst: transport functions in a developing epithelium. Am J Physiol. 1988;255:C419–32. doi: 10.1152/ajpcell.1988.255.4.C419. [DOI] [PubMed] [Google Scholar]
- 16.Veeck LL, Zaninovic N. An atlas of human blastocysts. 1. Boca Raton, London, New York, Washington: Parthenon Publishing; 2003. [Google Scholar]
- 17.Marcos J, Pérez-Albalá S, Mifsud A, Molla M, Landeras J, Meseguer M. Collapse of blastocysts is strongly related to lower implantation success: a time-lapse study. Hum Reprod. 2015;30:2501–8. doi: 10.1093/humrep/dev216. [DOI] [PubMed] [Google Scholar]
- 18.Bodri D, Sugimoto T, Yao Serna J, Kawachiya S, Kato R, Matsumoto T. Blastocyst collapse is not an independent predictor of reduced live birth: a time-lapse study. Fertil Steril. 2016;105:1476–83.e3. doi: 10.1016/j.fertnstert.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda Y, Kumagai J, Anzai M, Kabashima K, Togashi K, Miura Y, et al. Time-lapse monitoring reveals that vitrification increases the frequency of contraction during the pre-hatching stage in mouse embryos. J Reprod Dev. 2016;62:187–93. doi: 10.1262/jrd.2015-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzazadeh F, Cater E, Nice L, Campbell A. Data analysis study on the rate of blastocyst re-expansion after warming and its significance to outcome. Reprod Biomed Online. 2016;32(1):9. doi: 10.1016/S1472-6483(16)30154-7. [DOI] [Google Scholar]
- 21.Galán Rivas A, Coello A, Cobo A, Nohales M, Alegre L, Meseguer M. Morphology dynamics of warmed blastocysts are strong predictors of clinical outcome. Hum Reprod. 2016, in press.