Abstract
Autophagy is a catabolic pathway that promotes the degradation and recycling of cellular components. Proteins, lipids, and even whole organelles are engulfed in autophagosomes and delivered to the lysosome for elimination. In response to stress, autophagy mediates the degradation of cell components, which are recycled to generate the nutrients and building blocks required to sustain cellular homeostasis. Moreover, it has an important role in cellular quality control, particularly in neurons, in which the total burden of altered proteins and damaged organelles cannot be reduced by redistribution to daughter cells through cell division. Autophagy occurs in all cells and tissues, and it is regulated by the Atg genes. The importance of this pathway has been recently recognized by the Nobel Prize in Physiology and Medicine award to Professor Yoshinori Ohsumi who was the discoverer of the first Atg genes in yeast in the 1990s. Research has only begun to examine the role of autophagy in the visual system. Both the retina and the eye are exposed to a variety of environmental insults and stressors, including genetic mutations and age-associated alterations that impair their function. Here, we review studies that have sought to explain autophagy's importance for retinal ganglion cells, and their implications for diseases like glaucoma and optic neuropathies.
Autophagy
In cells, the main catabolic pathways are the ubiquitin-proteasome pathway and the autophagy-lysosomal system, through which degradation of cell components occurs inside lysosomes.1,2 Autophagy means self-eating and is named to distinguish from heterophagy, where the material to be degraded comes form outside the cell. There are three main types of autophagy, which are classified according to how material to be degraded reaches the lysosomes. During macroautophagy, called from now on autophagy, cytoplasmic material is delivered to the lysosome via an intermediate organelle, the autophagosome, which will fuse with lysosomes. Other forms of autophagy include chaperone-mediated autophagy that occurs only in mammalian cells and allows for the degradation of specific proteins harboring the amino acid sequence KFERQ. Finally, in microautophagy material destined for degradation reaches the lysosomal lumen through invagination of the lysosomal or endosomal membrane.2
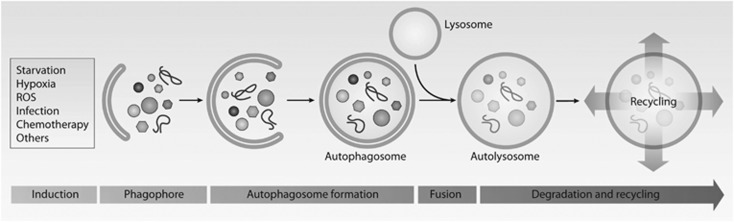
Autophagy is triggered by numerous stimuli, from starvation and hypoxia to infection and chemotherapy (Figure 1). This induction phase triggers the formation of the phagophore or isolation membrane that finally closes to form the autophagosome. Autophagosome then fuses with lysosomes, in which acidic hydrolases degrade the engulfed material. Last, the resulting of metabolites will be released from the lysosomal compartment to the cytoplasm and used to manintain cellular functions. Autophagy is a highly dynamic process, whereby autophagosomes form and fuse with the lysosome in <10 min. Thus, blockade of the maturation or lysosomal fusion of the autophagosome, or impairment of lysosomal function or biogenesis, leads to the accumulation of autophagosomes, ultimately disrupting or diminishing autophagic flux.2 Autophagy occurs in all cell types and tissues, and a basal level of autophagy is required to ensure that cells (particularly, post-mitotic cells such as neurons) remain free of damaged cellular components.3, 4 However, autophagy can also be induced in response to stressors associated with a variety of conditions and situations (eg, nutrient starvation, metabolic stress); in this scenario, autophagy mediates the recycling of intracellular components to generate ATP and new ‘building blocks', thus sustaining cell survival.2
Figure 1.
Signals that induce autophagy promote the formation of a pre-autophagosomal phagophore, which surrounds parts of the cytoplasm, including whole organelles. The membrane nucleates to close and forms an autophagosome, which then fuses with the lysosome to form the autolysosomes where degradation takes place. The final products, including amino acids, lipids, and nucleotides, can be then translocated back to the cytosol and recycled for new anabolic reactions to sustain cell homeostasis.
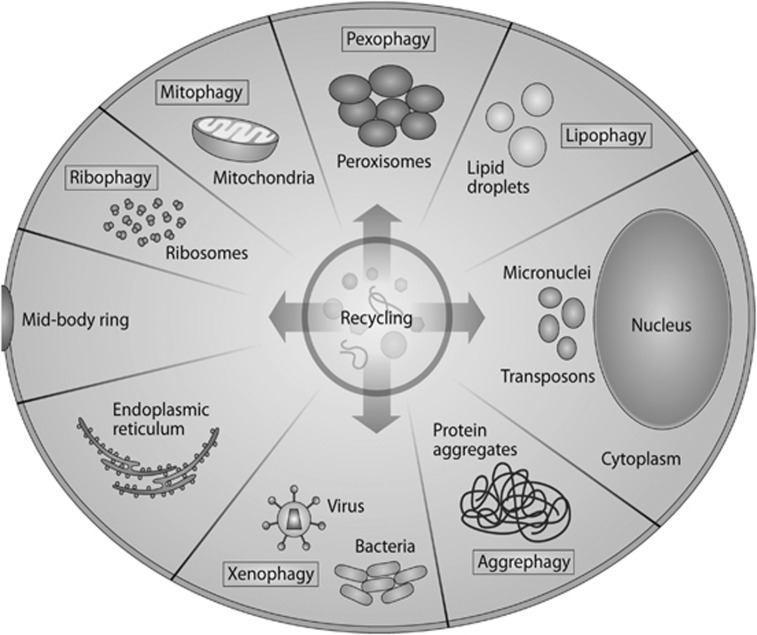
In the past, autophagy was considered a nonselective process by which randomly enclosed cargo was degraded in bulk. However, recent findings suggest that autophagy is in fact a highly selective process (Figure 2). Autophagosomes specifically target many organelles and cytoplasmic components, including mitochondria, ribosomes, peroxisomes, lipid droplets, misfolded proteins, and intracellular pathogens.5 During selective autophagy, the ubiquitination of substrates enables their specific degradation by mediating their binding to autophagy receptors such as p62 and OPTN, which in turn link the ubiquitinated cargo to the autophagosomal membrane mediated by the presence of a LIR domain (LC3-interacting domain).5 Mitophagy is the selective degradation of mitochondria that have been delivered to the lysosomes via the autophagy pathway.6 Generally, this process involves the elimination of damaged and depolarized mitochondria via the PINK1-Parkin pathway, although mitochondria can also be eliminated during development via a process known as programmed mitophagy, which has been observed for example during lens maturation.6, 7
Figure 2.
Selective autophagy. Subcellular structures are specifically targeted for lysosomal degradation by autophagy. Depending on the cargo, the processes are named differently: mitophagy for the specific elimination of mitochondria; ribophagy for ribosomes; and lipophagy for the degradation of lipid droplets. Pexophagy degrades peroxisomes and aggrephagy degrades intracellular protein aggregates and misfolded proteins such as those observed in many neurodegenerative conditions. Xenophagy denotes the degradation of intracellular pathogens such as viruses and intracellular bacteria. Other cellular components, such as the endoplasmic reticulum, micronuclei, glycogen, and transposons, can also be specifically targeted by autophagosomes for degradation.
Physiological functions of autophagy
Autophagy is one of the main intracellular quality control pathway, mediating the degradation of misfolded or aggregated proteins and damaged organelles. In line with this function, deletion of the autophagy regulators ATG5 and ATG7 in many cells types, including neuronal precursors and hepatocytes, results in the accumulation of damaged (ubiquitinated) proteins and organelles (eg, mitochondria), leading to increased levels of ROS and damage to intracellular components.3, 4, 8
Moreover, autophagy is induced in cells in response to many stressors including starvation, hypoxia, and infection, and finely tuned regulation of this process is essential to ensure the maintenance of cell and tissue homeostasis.9 In eukaryotes, the activation of autophagy during periods of starvation is an evolutionarily conserved response.9 In these conditions, protein and lipid degradation allows the cell to adapt its metabolism and fulfill its energy needs. Pharmacological and genetic downregulation of autophagy results in rapid cell death in starvation conditions.10 Autophagy also has a central role in maintaining energy levels in various tissues at birth, when the supply of maternal nutrients via the placenta ceases,11 and ATG-deficient cancer cells use autophagy to maintain levels of TCA cycle metabolites.12 Recent evidence has also demonstrated an essential metabolic role of autophagy in adult tissues. Conditional deletion of ATG7 in adult mice results in severe alterations in glucose homeostasis, and the depletion of liver glycogen stores in fasting animals.13 Moreover, these mice die of neurodegeneration at around 3 months of age highlighting again the essential homeostatic function of autophagy for the nervous system.
Autophagy and cell death during RGC development
Programmed cell death, together with proliferation and differentiation, is an essential process during the development of the nervous system. During neurogenesis, neurons and glia are generated in large numbers, and subsequently, they die in a process that depends on trophic signaling that refines the cytoarchitecture and connectivity of the nervous system. Coupled to this process, the elimination of dead cells by professional phagocytes or neighboring cells is essential for proper neuronal development. We have demonstrated that autophagy has an essential role in cell corpse removal during retinal development.14 Inhibition of autophagy results in the accumulation of apoptotic cells in a well-circumscribed area of the retinal neuroepithelium, a region of active differentiation in which proliferating neuroblasts exit their final cell cycle to differentiate into RGCs.15 We have further demonstrated that autophagy has a metabolic role in this process as its inhibition reduces the ATP levels essential for the phosphatidylserine exposure in the surface of apoptotic cells.15 Supplying retinal explants with permeable Krebs cycle intermediates, such as methypyruvate restores ATP, phosphatidylserine exposure, and the elimination of apoptotic cells. Interestingly, this process is associated to early neural cell death of RGCs as autophagy inhibition at later stages has no effect on the removal of apoptotic cells.14, 16
Autophagy has a role in the differentiation of multiple cell types, including erythrocytes, lymphocytes, and adipocytes.17 Previous studies by our group have demonstrated the involvement of autophagy in vitro model of neuronal differentiation from olfactory bulb neural stem cells. We have shown that stem cells isolated from the olfactory bulb of autophagy-deficient mice (Ambra1 and ATG5 knockouts) develop fewer differentiated neurons in culture, and display significant alterations in neuritogenesis and axonogenesis.18 We are currently investigating the retinal phenotype of those mice and have preliminary evidence of reduced numbers of RGCs at the early phases of retinal neurogenesis.
Role of autophagy in RGCs
Retinal ganglion cells are the only projecting neurons of the retina. Their axons form the optic nerve and transmit visual information to the brain.19 The role of autophagy in RGCs has attracted much research attention with a view to identifying potential neuroprotective strategies for glaucoma. Both retinal hypoxia and axonal damage of the optic nerve have been shown to induce autophagy.20, 21, 22 The cytoprotective role of autophagy in RGCs was first demonstrated in vivo in mice subjected to optic nerve transection and subsequently treated with rapamycin.23
Since the discovery of ATG genes, many autophagy-deficient animals have been generated and this has helped to understand the role of autophagy in different tissues. In general, full autophagy deficiency results in embryonic or early postnatal death,24 and thus the retinal phenotype cannot be assessed in the ‘classical' models of complete autophagy insufficiency. However, models with partial autophagy deficiency such as the Atg4B-deficient mice survive into adulthood and thus are a great model where they study the role of autophagy in adult tissues. ATG4B-deficient mice are born with no major histopathological or biochemical alterations, although they display reduced (but not abolished) autophagy in many tissues25 including the retina.23 Under normal physiological conditions, the retinas from ATG4B−/− mice do not show any alteration, but ATG4B−/− mice are more susceptible to stress as optic nerve axotomy in these animals results in reduced RGC survival as compared with wild-type mice.23, 26 These data suggest that even a slight reduction in retinal autophagy levels can alter the capability of retinal ganglion cells to respond to axonal stress. No mouse with an RGC-specific deficiency in autophagy has been generated to date. However, transduction of RGCs using viral vectors with RGC-specific serotypes such as AAV2 has been used to study the effects of autophagy downregulation in these cells.23 Downregulation of ATG5 in RGCs makes these cells more vulnerable to optic nerve axotomy; fewer surviving RGCs are found post axotomy in ATG5flox/flox mice injected with AAV2-GFP-Cre vs those injected with the AAV2-GFP control vector,26 indicating a cytoprotective role of autophagy in RGC survival in conditions of axonal damage.
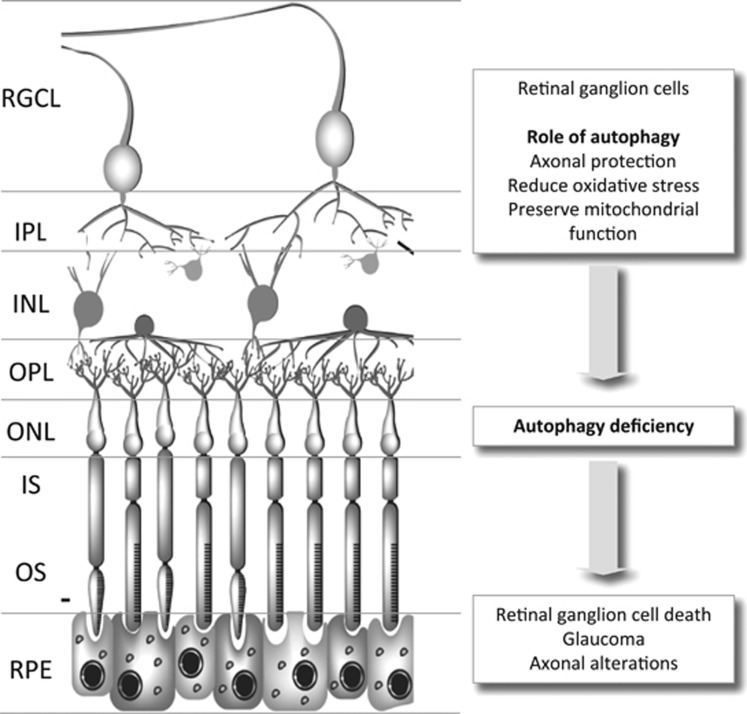
Why is then autophagy good for RGCs? As increased levels of oxidative stress have been observed in experimentally-induced glaucoma, increased autophagy may serve as a means of reducing oxidative stress.23, 27 In line with this view, cells from autophagy-deficient animals show increased levels of ROS, possibly owing to impaired autophagy-mediated elimination of oxidized cellular components.27, 28 In this regard, as mitochondria are the main source of intracellular ROS; eliminating damaged mitochondria by mitophagy could be seen as a potential cytoprotective role for autophagy in RGCs (Figure 3). In addition, excessive oxidative stress has also been shown to preferentially damage lysosomal membranes, inducing cell death as well as a decreased autophagic flux.29, 30 The duration of the insult may also strongly influence the response to stress. For example, although acute damage may induce a cytoprotective response, prolongation of this stimulus can give rise to a deleterious response.31
Figure 3.
Role of autophagy in retinal ganglion cells and disease condition related to autophagy alterations. INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; RGCL, retinal ganglion cell layer; RPE, retinal pigmented epithelium.
It remains to be determined whether manipulation of autophagy can be exploited as a potential means of inducing RGC neuroprotection, to date, rapamycin in the only pharmacological compound tested in animal models of glaucoma.32 Other better-tolerated rapalogs could also be candidate neuroprotectants. However, the inhibition of mTOR activity by rapalogs could result in much broader effects than autophagy upregulation alone, underscoring the need for new autophagy regulators that act independently of mTOR. Therapies aimed at restoring lysosomal function could also be indicated for conditions involving lysosomal damage caused by elevated calcium levels in RGCs (eg, traumatic lesions, ischemia).
Autophagy and aging in the retina
The decline in visual function that commonly accompanies aging is thought to be a consequence of retinal degeneration and the loss of retinal cells.33 Moreover, aging is linked to a general decline in the activity of proteolytic pathways,34, 35 including autophagy and the ubiquitin-proteasome system.36 In the retina, a recent report shows a decrease in the retinal mRNA expression of several autophagy regulators that correlated with decreased autophagic flux in 2-year-old mice.37 This effect was accompanied by lipofuscin accumulation, morphological alterations in RPE cells, and photoreceptor cell death.37 Surprisingly, chaperone-mediated autophagy was upregulated in the aged retinas, in marked contrast with the age-associated decrease in the activity of this pathway described in other organs.38, 39 Interestingly, a similar upregulation of CMA in mice with Atg5 deletion in neuronal precursors, supporting a generalized increase in CMA specifically when macroautophagy is impaired, suggests that maintenance of cellular homeostasis depends on cross-talk between different proteolytic pathways.37 Elucidating the underlying mechanism in retinal aging would facilitate the design of new therapeutic strategies to prevent visual dysfunction associated both with the normal aging process and with age-related retinal pathologies.
In conclusion, all these data point out to an essential role of autophagy for RGC health from development to aging. Future investigations will be aimed to determine the specific functions of autophagy for RCGs and axonal homeostasis.
References
- Boya P, Esteban-Martinez L, Serrano-Puebla A, Gomez-Sintes R, Villarejo-Zori B. Autophagy in the eye: development, degeneration, and aging. Prog Retin Eye Res 2016; 55: 206–245. [DOI] [PubMed] [Google Scholar]
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 2013; 15(7): 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441(7095): 885–889. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441(7095): 880–884. [DOI] [PubMed] [Google Scholar]
- Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol 2016; 26(1): 6–16. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 2013; 20(1): 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney PA. Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 2015; 1853(10 Pt B): 2775–2783. [DOI] [PubMed] [Google Scholar]
- Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev 2011; 25(8): 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 2015; 16(8): 461–472. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005; 25(3): 1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T et al. The role of autophagy during the early neonatal starvation period. Nature 2004; 432(7020): 1032–1036. [DOI] [PubMed] [Google Scholar]
- Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 2011; 25(5): 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 2014; 4(8): 914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Mellen MA, de la Rosa EJ. How autophagy is related to programmed cell death during the development of the nervous system. Biochem Soc Trans 2008; 36(Pt 5): 813–817. [DOI] [PubMed] [Google Scholar]
- Mellén MA, de la Rosa EJ, Boya P. The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ 2008; 15(8): 1279–1290. [DOI] [PubMed] [Google Scholar]
- Mellén MA, de la Rosa EJ, Boya P. Autophagy is not universally required for phosphatidyl-serine exposure and apoptotic cell engulfment during neural development. Autophagy 2009; 5(7): 964–972. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol 2010; 12(9): 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez P, Arroba AI, Cecconi F, de la Rosa EJ, Boya P, De Pablo F. Atg5 and Ambra1 differentially modulate neurogenesis in neural stem cells. Autophagy 2012; 8(2): 187–199. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res 2003; 22(4): 483–543. [DOI] [PubMed] [Google Scholar]
- Wu BX, Darden AG, Laser M, Li Y, Crosson CE, Hazard ES 3rd et al. The rat Apg3p/Aut1p homolog is upregulated by ischemic preconditioning in the retina. Mol Vis 2006; 12: 1292–1302. [PubMed] [Google Scholar]
- Kim SH, Munemasa Y, Kwong JM, Ahn JH, Mareninov S, Gordon LK et al. Activation of autophagy in retinal ganglion cells. J Neurosci Res 2008; 86(13): 2943–2951. [DOI] [PubMed] [Google Scholar]
- Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci USA 2010; 107(13): 6064–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Muela N, Germain F, Marino G, Fitze PS, Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ 2012; 19(1): 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Komatsu M. Pathophysiological role of autophagy: lesson from autophagy-deficient mouse models. Exp Anim 2011; 60(4): 329–345. [DOI] [PubMed] [Google Scholar]
- Mariño G, Fernández AF, Cabrera S, Lundberg YW, Cabanillas R, Rodríguez F et al. Autophagy is essential for mouse sense of balance. J Clin Invest 2010; 120(7): 2331–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Muela N, Boya P. Axonal damage, autophagy and neuronal survival. Autophagy 2012; 8(2): 286–288. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 2014; 10(10): 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa Y, Kitaoka Y. Autophagy in axonal degeneration in glaucomatous optic neuropathy. Prog Retin Eye Res 2015; 47: 1–18. [DOI] [PubMed] [Google Scholar]
- Serrano-Puebla A, Boya P. Lysosomal membrane permeabilization in cell death: new evidence and implications for health and disease. Ann N Y Acad Sci 2015; 1371: 30–44. [DOI] [PubMed] [Google Scholar]
- Gomez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev 2016; 32: 150–168. [DOI] [PubMed] [Google Scholar]
- Koch JC, Lingor P. The role of autophagy in axonal degeneration of the optic nerve. Exp Eye Res 2016; 144: 81–89. [DOI] [PubMed] [Google Scholar]
- Russo R, Berliocchi L, Adornetto A, Amantea D, Nucci C, Tassorelli C et al. In search of new targets for retinal neuroprotection: is there a role for autophagy? Curr Opin Pharmacol 2013; 13(1): 72–77. [DOI] [PubMed] [Google Scholar]
- Militante J, Lombardini JB. Age-related retinal degeneration in animal models of aging: possible involvement of taurine deficiency and oxidative stress. Neurochem Res 2004; 29(1): 151–160. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011; 146(5): 682–695. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest 2015; 125(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez N, Athonvarangkul D, Singh R. Autophagy and aging. Adv Exp Med Biol 2015; 847: 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Muela N, Koga H, Garcia-Ledo L, de la Villa P, de la Rosa EJ, Cuervo AM et al. Balance between autophagic pathways preserves retinal homeostasis. Aging Cell 2013; 12(3): 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem 2000; 275(40): 31505–31513. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 2008; 14(9): 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]