Abstract
Aim
Cetuximab is an anti‐epidermal growth factor receptor antibody used for the treatment of metastatic colorectal cancer and head and neck cancer. Hypersensitivity reactions (HSRs) are associated with cetuximab use. The aim of the study was to evaluate the utility of anti‐cetuximab immunoglobulin E (IgE) detection in order to identify patients at risk of HSR to cetuximab.
Methods
We included patients ready to receive a first cetuximab infusion in a prospective cohort carried out at nine French centres. Pretreatment anti‐cetuximab IgE levels were measured. We compared the proportion of severe HSRs in the low anti‐cetuximab IgE levels (≤29 IgE arbitrary units) subgroup with that in a historical cohort of 213 patients extracted from a previous study.
Results
Of the 301 assessable patients (mean age: 60.9 ± 9.3 years, head‐and‐neck cancer: 77%), 66 patients (22%) had high anti‐cetuximab IgE levels, and 247 patients received cetuximab (including 38 with high anti‐cetuximab levels). Severe HSRs occurred in eight patients (five grade 3 and three grade 4). The proportion of severe HSRs was lower in the low anti‐cetuximab IgE levels subgroup vs. the historical cohort (3/209 [1.4%] vs. 11/213 [5.2%], odds ratio, 0.27, 95% confidence interval, 0.07–0.97), and higher in high vs. low anti‐cetuximab IgE levels subgroup (5/38 [13.2%] vs. 3/209 [1.4%]; odds ratio, 10.4, 95% confidence interval, 2.4–45.6). Patients with severe HSRs had higher anti‐cetuximab IgE levels than patients without reaction (median, 45 vs. 2 IgE arbitrary units, P = 0.006).
Conclusions
Detection of pretreatment anti‐cetuximab IgE is feasible and helpful to identify patients at risk of severe cetuximab‐induced HSRs.
Keywords: anaphylaxis, anti‐immunoglobulin E antibodies, cetuximab, hypersensitivity
What is Already Known about this Subject
Hypersensitivity reactions (HSRs) are not rare and are potentially life‐threatening adverse events associated with cetuximab.
HSR appears to be an IgE‐mediated anaphylactic mechanism because of a cross‐reactivity with galactose‐α‐1,3 galactose present on cetuximab.
High pretreatment levels of anti‐cetuximab IgE have been observed in patients who experienced HSRs.
What this Study Adds
HSRs reported in the study were immediate‐onset episodes, implying the presence of preexisting anti‐cetuximab IgEs.
A strong association is prospectively verified between the presence of circulating anti‐cetuximab IgE and the risk of HSR.
Anti‐cetuximab IgE detection could be helpful to physicians in order to identify patients at higher risk of severe HSR.
Tables of Links
| TARGETS | |
|---|---|
| Enzymes 2 | RAS family |
| EGFR | RAF family |
These Tables lists key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Cetuximab is a chimeric immunoglobulin (Ig)G1 monoclonal antibody directed against epidermal growth factor receptor. Currently, it is used in daily practice for metastatic colorectal cancer and for locally advanced or metastatic head‐and‐neck cancer 3, 4, 5, 6, 7. Hypersensitivity reactions (HSRs) are adverse events often associated with cetuximab use 8. The frequency of severe HSRs to cetuximab was found to be <5% 3, 4, 7, 9, although it appears to vary according to the geographical region. A higher incidence of 14–30% has been observed in Arkansas, North Carolina and Tennessee in the USA 10, 11, 12, 13, and even fatal reactions have been reported 14, 15, 16, 17.
Several approaches have been attempted to identify patients at a higher risk of HSRs to cetuximab. Smokers, men and patients with a history of allergy were suggested to be at a higher risk of HSR 12, 18, while no clear relationship was found with tumour location 12, 13, 14, 18, 19. Importantly, the link between these factors and the risk of HSR to cetuximab is not strong enough to help clinicians identify patients at a high risk of severe HSR 19.
High pretreatment levels of anti‐cetuximab IgE have been observed in patients who experienced HSR at the first cetuximab administration 10. The basis for the HSR appears to be an IgE‐mediated anaphylactic mechanism because of a cross‐reactivity with galactose‐α‐1,3 galactose, an oligosaccharide epitope that is present on cetuximab 20.
In a previous retrospective study, conducted on patients treated between October 2005 and March 2009 at our centre, we described the standardisation of an enzyme‐linked immunosorbent assay test to detect serum anti‐cetuximab IgE 21. In that historical cohort, the overall rate of severe HSRs was 5.2% (11/213 patients). Statistical analysis indicated a cut‐off value of 29 anti‐cetuximab IgE arbitrary units (EAU) above which patients may be considered at a higher risk of severe HSR to cetuximab 21.
The present study was conducted prospectively to evaluate the utility of pretreatment measurement of serum anti‐cetuximab IgE levels in patients naive to cetuximab to identify those who may be at a high risk of severe HSR.
Methods
Study design
This was a multicentre, prospective, diagnostic trial carried out between January 2010 and February 2013, at nine centres across France. Patients receiving a first infusion of cetuximab were included and classified according to their pretreatment levels of serum anti‐cetuximab IgE.
The primary objective was to compare the proportion of severe HSR at the first infusion of cetuximab in patients with low levels of anti‐cetuximab IgE, to that observed in the historical cohort of 213 patients treated with cetuximab without prior testing for their anti‐cetuximab IgE levels 21. Secondary objectives were to compare the proportion of severe HSR to cetuximab between patients with low or high levels of anti‐cetuximab IgE, and to examine the association of previously reported risk factors for HSR to cetuximab in the present prospective cohort.
All patients provided a written informed consent before undergoing any study‐specific procedures. The study was conducted in accordance with the principles of the Declaration of Helsinki. The study was approved by the local Ethics Committee (Comité de Protection des Personnes Nord‐Ouest III, Caen, France). This study is registered with ClinicalTrials.gov (NCT01436617) and with EudraCT (2009‐016968‐37).
Anti‐cetuximab IgE assay
The detection of anti‐cetuximab IgE was centralised at the Laboratory of Immunology and Immunopathology, Caen University Hospital, Caen, France, and was performed using an enzyme‐linked immunosorbent assay as previously described 21. The results were expressed as EAU using a standard calibration established with a control serum containing known concentration of anti‐cetuximab IgE antibodies. The lower limit of quantification (LLOQ) was between 3 and 5 EAU according to the batch of calibration serum. Consequently, when results were lower than the LLOQ, they were given as LLOQ/2. According to the receiver operating characteristic analysis performed in the historical cohort, a cut‐off value of 29 EAU had been previously selected in order to predict severe HSR with a sensitivity of 87.5% and a specificity of 82.1% 21. The calculated positive predictive value and negative predictive value (NPV) of the test were 33.3% and 98.5%, respectively. Anti‐cetuximab IgE levels ≤29 were defined as low, and levels >29 EAU as high.
Patients and treatment
Inclusion criteria for patients were age ≥18 years, histologically confirmed colon or head‐and‐neck cancer, naive to cetuximab, and adequate liver, renal and bone marrow functions to receive cetuximab and associated treatments. Cetuximab infusion, premedication and concomitant treatments were applied according to the recommended protocols at each participating centre. The serum anti‐cetuximab IgE levels were determined in all patients before cetuximab infusion, and patients were excluded if these data were not available. Concerning the historical cohort, the premedication usually administrated before the cetuximab infusion was made up of a combination of corticosteroids and antihistamine.
The treating physician was informed of each patient's anti‐cetuximab IgE levels before the first administration of cetuximab. Patients with low IgE levels were administered cetuximab according to the centre's standard procedures. For patients with high IgE levels, the decision of cetuximab treatment was reevaluated in a multidisciplinary oncology experts meeting. In case of patients with high IgE levels, the two possible options were to provide cetuximab treatment as planned, or suggest another equally effective or less effective treatment. In all cases, the options and their consequences were clearly explained to the patient, who was then allowed to choose between the treatments. If the patient consented to receive cetuximab treatment, the first and second (if applicable) infusions were monitored closely by a physician, with resuscitation equipment readily available in case of an HSR.
Clinical data
For each patient, data were collected on the following parameters: weight, age at cetuximab administration, site of primary tumour, date of diagnosis, previous tumour treatments, concomitant and previous medications, and location of metastases in case of metastatic disease. In addition, history of allergy (against drug, food or insects), asthma, eczema, allergic rhinitis, and shock or angioedema were recorded.
In case of an HSR to cetuximab, the time interval between the beginning of the infusion and reaction, the clinical manifestation of the HSR, as well as the details of its management were recorded. Serum assays for histamine and tryptase were performed.
Case definition and grading system
HSRs to cetuximab were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 3.0, in the same manner as in our previous retrospective cohort study 21. The grades for all HSRs were described by an independent clinician blinded to the anti‐cetuximab IgE levels and to the grades given by the investigators. Severe HSRs were defined as grades 3, 4 or 5.
Statistical design and analyses
All HSR proportions were analysed in patients who received cetuximab. A cohort of interest was defined as patients with low pretreatment levels of anti‐cetuximab IgE (≤29 EAU). The study was designed to test whether the proportion of severe HSR (grade 3 or above) in the cohort of interest would be lower than that observed in the historical cohort (11/213 = 5.2%) in which the pretreatment anti‐cetuximab IgE levels had not been determined prior to treatment. In the studies that have assessed the indications for treatment with cetuximab, the minimal reported rate of severe HSR was 1.2% 3, 4, 7, 9. Assuming that the severe HSR proportion in the cohort of interest would be equal or lower to this proportion of 1.2% (i.e. lower by at least 4% compared with the historical cohort), 135 patients would be needed in the cohort of interest from Basse‐Normandie to detect this reduction as significant with a one‐sided Fisher exact test (α = 5%, β = 20%). Given that 75% of the patients from Basse‐Normandie had low pretreatment levels of anti‐cetuximab IgE, the required overall sample size was increased to 180 patients from Basse‐Normandie. Secondly, the protocol was amended to open new investigator centres in different geographical areas to assess if the rate of HSR depended on the geographical region 3, 4, 7, 9, 10, 11, 12, 13, 22, 23. Assuming that 80% of the study patients would be from Basse‐Normandie, the number of patients to enrol was therefore increased to 225 patients. Additionally, we had to take into account that cetuximab treatment was not continued for 20% of recruited patients. Thus at least 285 patients had to be enrolled.
The comparison of the rate of severe HSRs in the present prospective cohort with that in the historical cohort was done in a stepwise manner. In the first step, reflecting the primary objective, the proportion of severe HSRs in the cohort of interest specifically from the region of Basse‐Normandie was compared by a one‐sided Fisher exact test with the one observed in the historical cohort which had been entirely constituted from this region. Secondly, it was compared between the cohort of interest from Basse‐Normandie and that from outside this region by a two‐sided Fisher exact test. Lastly, if no geographical disparity is found in the second step, then the severe HSR rate would be compared between the entire cohort of interest from the present prospective cohort and the historical cohort by a one‐sided Fisher exact test. The odds ratio (OR) of the rate of severe HSR in a given cohort of interest to that in the historical cohort was determined with a two‐sided 95% confidence interval (CI). The sensitivity and the NPV of anti‐cetuximab IgE assay associated with the risk of severe HSR were estimated with exact binomial 95% CI. As it was possible for patients with high levels of anti‐cetuximab IgE, not to receive cetuximab, specificity and positive predictive value could not be calculated.
Factors that have been reported as possibly related to the occurrence of a severe HSR were investigated as described below. Mean age was compared by a Student t test, levels of anti‐cetuximab IgE by a Mann–Whitney U test, and qualitative factors (sex, history of allergy, premedication, and site of cancer) were compared by a Fisher exact test. P‐values and CI are two‐sided unless otherwise specified. Significance was set at P ≤ 0.05. The statistical software R version 3.0.1 (R foundation for statistical computing, Vienna, Austria) was used for all analyses.
Results
Patient population
This study included 303 patients (Figure 1). Of these patients, two patients were excluded because of either previous cetuximab treatment or lack of data on pretreatment anti‐cetuximab IgE levels. Of the 301 assessable patients, 66 (21.9%) had anti‐cetuximab IgE levels (>29 EAU) and 38 (57.6%) received cetuximab. There were 235 patients with low anti‐cetuximab IgE levels (≤29 EAU), of whom 209 (88.9%) received cetuximab. Concerning the main cohort of interest, 138 patients from Basse‐Normandie who had low levels of anti‐cetuximab IgE were treated with cetuximab.
Figure 1.
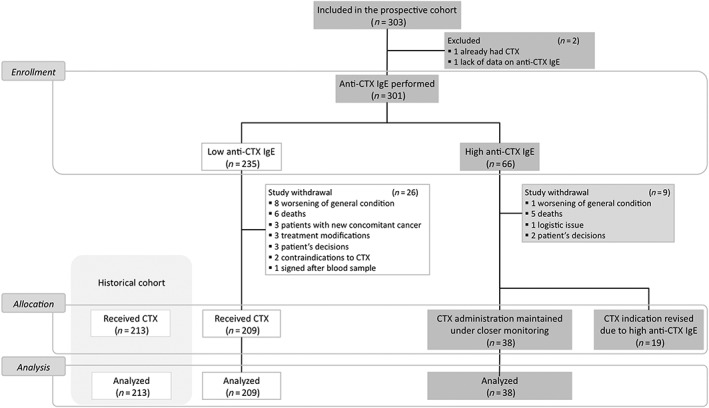
Study flowchart. CTX, cetuximab; IgE, immunoglobulin E
The baseline characteristics of the present study population were similar to those of the historical cohort 21, except for the site of tumour (Table 1). The proportion of patients treated with cetuximab for head‐and‐neck cancer was relatively higher in the present prospective cohort than in the historical cohort probably because of the more recent addition of this type of tumour to cetuximab indications.
Table 1.
Baseline patient characteristics
| Historical cohort(n = 213) | Present prospective cohort | ||
|---|---|---|---|
| Patients receiving cetuximab (n = 247) | Overall population (n = 301) | ||
| Age (years), mean ± SD | 62.1 ± 10 | 61.1 ± 9.1 | 60.9 ± 9.3 |
| Male | 159 (76.6%) | 197 (79.8%) | 244 (81.1%) |
| History of allergy | 36 (16.9%) | 30 (12.1%) | 41 (13.6%) |
| Type of cancer | |||
| Head‐and‐neck cancer | 100 (46.9 %) | 189 (76.5 %) | 232 (77.1 %) |
| Colon cancer | 107 (50.2%) | 58 (23.5 %) | 69 (22.9 %) |
Data are presented as n (%) unless otherwise specified. SD, standard deviation
Hypersensitivity reactions to cetuximab
Among the 247 patients who received cetuximab for the first time, 12 (4.9%) experienced an HSR, of whom eight patients (3.2%) had a severe reaction (five grade 3 and three grade 4 reactions). There were no deaths due to an HSRs to cetuximab. All HSRs occurred within 1 h after the beginning the first cetuximab administration. The 12 cases of HSR to cetuximab and their management are described in Table 2.
Table 2.
Description of the cases of hypersensitivity reactions to cetuximab
| Cases | Location | Premedication dexchlorpheniramine + corticosteroids | Grade | Time (min) | IgE value (EAU) | Anti‐cetuximab IgE assay | Histamine (normal <6 nmol l −1 ) | Tryptase (normal <12.5 μg l −1 ) | Treatment a Medications |
|---|---|---|---|---|---|---|---|---|---|
| A | Colon | Yes | 1 | 35 | 9 | Low | ND | ND | – |
| B | Colon | Yes | 1 | 15 | 1.5 | Low | 3.1 | 5.2 | – |
| C | Head‐and‐neck | Only dexchlorpheniramine | 2 | 40 | 15 | Low | 9.3 | 27.8 | – |
| D | Head‐and‐neck | Yes | 2 | 15 | 39 | High | ND | ND | CORT |
| E | Colon | Yes | 3 | 40 | 1.5 | Low | 3.8 | 5.1 | CORT, TER, IPR |
| F | Head‐and‐neck | Yes | 3 | 15 | 35 | High | 83.3 | 13.7 | – |
| G | Head‐and‐neck | Yes | 3 | 20 | 55 | High | 1.3 | 2 | CORT, DEX, TER, IPR |
| H | Colon | Yes | 3 | 25 | 2.5 | Low | 12 | ND | CORT, DEX |
| I | Head‐and‐neck | Yes | 3 | 15 | 490 | High | ND | ND | DEX, CORT, TER |
| J | Head‐and‐neck | Yes | 4 | 15 | 134 | High | 661 | 31.6 | CORT, EPI |
| K | Head‐and‐neck | Yes | 4 | <1 | 480 | High | 100 | 64 | CORT, EPI, TER |
| L | Head‐and‐neck | Yes | 4 | 30 | 4 | Low | 20.8 | 8.6 | EPI |
Treatment administered in addition to cetuximab infusion stop. In patient A, cetuximab infusion was stopped temporarily and resumed. IgE, immunoglobulin E; EAU, IgE arbitrary units; ND, not determined;
CORT, corticosteroids; DEX, dexchlorpheniramine; TER, terbutaline; IPR, ipratropium; EPI, epinephrine; SD, standard deviation
Anti‐cetuximab IgE levels and risk of hypersensitivity reaction to cetuximab
Primary objective
The proportion of severe HSRs to cetuximab in the cohort of interest (low levels subgroup) from the Basse‐Normandie region was nonsignificantly lower than that in the historical cohort conducted in the same region (2/138 = 1.4% vs. 11/213 = 5.2%, one‐sided P = 0.060; OR, 0.27, 95% CI, 0.06–1.24; two‐sided P = 0.087), indicating a decrease of 3.8% in the observed rate of severe HSRs.
Secondary objectives
There was no geographical disparity in the proportion of severe HSR between the cohort of interest from Basse‐Normandie and that from outside this region (2/138 = 1.4% vs. 1/71 = 1.4%, P > 0.999). Moreover, the characteristics of the patients in these two populations were not different. These results allowed to compare the HSR rate in the entire population with the historical cohort conducted only in the Basse‐Normandie region. The overall severe HSR rate in the cohort of interest from the entire study population was lower as compared with the historical cohort (3/209 = 1.4% vs. 11/213 = 5.2%, one‐sided P = 0.029; OR, 0.27, 95% CI, 0.07–0.97; two‐sided P = 0.053, Figure 2).
Figure 2.
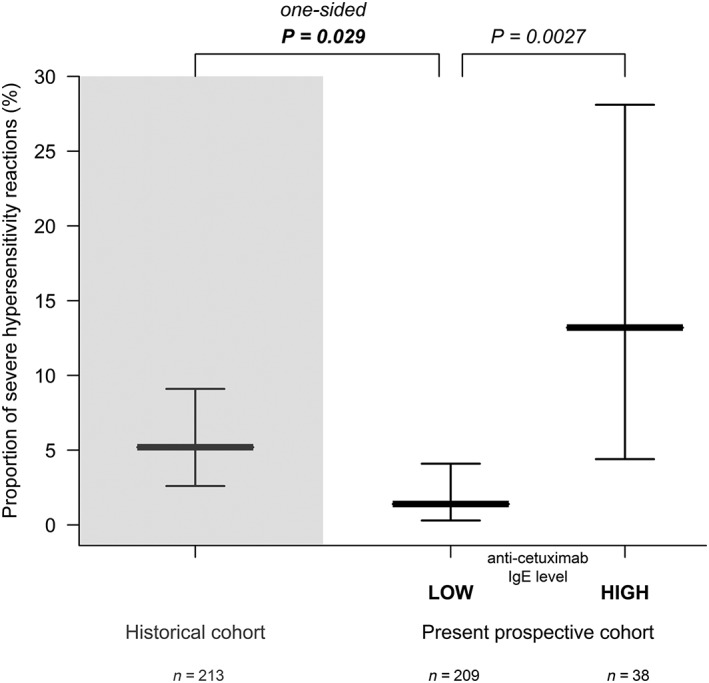
Proportion of severe hypersensitivity reactions to cetuximab. Proportion of severe hypersensitivity reactions (bold line) and two‐sided 95% confidence interval (thin line) in the historical retrospective cohort and the present prospective cohort, according to the levels of anti‐cetuximab immunoglobulin (Ig)E (low levels, ≤29, or high levels, >29 IgE arbitrary units)
The proportion of severe HSRs in patients with high levels of anti‐cetuximab IgE was significantly higher than that in those with low levels (5/38 = 13.2% vs. 3/209 = 1.4%, P = 0.0027; OR, 10.4, 95% CI, 2.4–45.6).
The sensitivity and the NPV of anti‐cetuximab IgE assay associated with the risk of severe HSRs were 5/8 = 63% (95% CI, 24–91%) and 206/209 = 98.6% (95% CI, 95.9–99.7%), respectively. In the historical cohort, the sensitivity and the NPV were 87.5% and 98.5% respectively.
Nineteen patients did not finally receive the drug due to high anti‐cetuximab IgE level: anti‐cetuximab IgE levels of these patients were not significantly different than levels of patients with a high level who were treated (median, 55 EAU; interquartile range 39.5–122.5 vs. 57.5 EAU; interquartile range 35–101.8, respectively, P = 0.49).
Patients with severe HSR had significantly higher levels of anti‐cetuximab IgE than patients without severe HSR (median, 45 EAU; interquartile range, 3.5–220.5 vs. 2 EAU; interquartile range, 1–12, P = 0.0059).
Factors related to severe hypersensitivity reactions
A comparison of various characteristics between patients who did not have a severe HSR and those who did (Table 3) revealed that high levels of anti‐cetuximab IgE was the only factor significantly associated with the occurrence of severe HSR to cetuximab. No significant association was noted between severe HSR and sex, age, history of allergies or tumour location (Table 3). Further, none of these factors was found to be related to HSR of any severity grade (data not shown).
Table 3.
Univariate analysis of factors related to the occurrence of a severe hypersensitivity reaction to cetuximab
| No severe HSR | Severe HSR Grade 3 or 4 | P | |
|---|---|---|---|
| n = 239 | n = 8 | ||
| Age (years), mean ± SD | 61.1 ± 8.9 | 62 ± 12.9 | 0.85 |
| Male | 191 (79.9 %) | 6 (75 %) | 0.67 |
| History of allergy | 28 (11.7 %) | 2 (25 %) | 0.25 |
| Type of cancer | |||
| Head‐and‐neck cancer | 183 (76.6 %) | 6 (75%) | 1 |
| Premedication | |||
| Dexchlorpheniramine | 216 (91.5%) | 8 (100 %) | 1 |
| Corticosteroids | 232 (98.3 %) | 8 (100 %) | 1 |
| Anti‐cetuximab IgE assay | |||
| High | 33 (13.8 %) | 5 (62.5%) | 0.0027 |
HSR, hypersensitivity reaction; SD, standard deviation; IgE, immunoglobulin E.
Data are presented as n (%) unless otherwise specified
Discussion
This is the first large‐scale prospective study to confirm the strong association between the presence of circulating anti‐cetuximab IgE before the first infusion of cetuximab, and the occurrence of hypersensitivity reactions to cetuximab. It demonstrates that assaying for preexisting anti‐cetuximab IgEs could help assess the risk of HSR and tailor the therapeutic strategy accordingly. These considerations are clinically meaningful because prevention of severe allergic reactions to cetuximab is a major challenge, and also a financial burden, especially in regions with high incidence 16, 24.
The study was powered to detect a difference of at least 4% decrease in the proportion of severe HSRs between patients with low pretreatment anti‐cetuximab IgE levels from the present prospective cohort and the historical cohort that had not been prospectively screened for presensitisation to cetuximab 21. However, the calculated difference was a decrease of 3.8%, after verifying lack of geographical disparity. Although our statistical assumptions concerning the main objective were not fully reached (expected at least a decrease of 4%, observed a decrease of 3.8%), the decrease in the severe HSR rate remains of clinical interest.
This study confirms that severe HSR to cetuximab is not a rare adverse event. In the historical cohort, the proportion was of 5.2%, an intermediate between low rates from the pivotal studies of cetuximab (1–3.5%) 3, 4, 7, 9, and the high rates described in some areas such as North Carolina or Tennessee (up to 22%) 11, 12. In the present prospective study, we noted an overall low rate of severe HSR of 3.2%, accounting for 1.4 or 13.2% in subgroups of cetuximab‐treated patients with low or high levels of anti‐cetuximab IgE, respectively. This observed overall proportion is indeed biased because some patients with high levels of anti‐cetuximab IgE declined cetuximab treatment (19/66), notably patients with an alternative treatment option. Considering the severe HSR rate of 13.2% observed in the group of patients with high levels of anti‐cetuximab IgE, we can speculate that between two and three (19 × 0.132) severe HSRs were avoided in the 19 untreated patients with high pretreatment levels of anti‐cetuximab IgE and that seven or eight severe HSRs (57 × 0.132) could have been avoided if all patients with high pretreatment levels of anti‐cetuximab IgE had received an alternative treatment.
Analysis of various patient characteristics did not show a correlation of any with the risk of HSR, except for high levels of preexisting anti‐cetuximab IgEs. The results of this study do not support the indications from previous studies, such as that of association of HSR with allergies, head‐and‐neck cancer or male sex 12, 13, 14, 18. Indeed, several reports have described a higher incidence of HSRs to cetuximab in patients treated for head‐and‐neck cancer or lung cancer 12, 13, 14, but these patients are usually more likely to have symptoms such as dyspnoea or bronchospasm, which are confounding symptoms for HSRs.
In our study, patients who had severe HSRs had in fact received appropriate premedication, but this did not seem to prevent HSRs to cetuximab unlike in previous studies 11, 25. Given the mechanism of HSRs involving preexisting IgE, it is not surprising that premedication with corticosteroids and antihistamine was not sufficient to avoid anaphylactic reaction in patients with high anti‐cetuximab IgE concentration.
The results presented here support the notion that cetuximab HSR is an IgE‐mediated anaphylactic reaction in a presensitised individual 26. All HSRs reported here were immediate‐onset, implying the presence of preexisting IgEs 27.
The NPV of testing for anti‐cetuximab IgE would permit a better selection of patients for cetuximab treatment, so that patients with high IgE levels will not be exposed to the risk of a potentially fatal HSR. Therefore, rather than denying this treatment, it would be advisable to test for presensitisation to cetuximab and then provide the treatment taking all the necessary precautionary measures, especially during the first infusion. It is often the element of surprise that can cost the life of patients experiencing severe reactions 14, 15, 19. Thus, screening for patients at a high risk of severe reactions would allow a better management of these cases.
In conclusion, anti‐cetuximab IgE detection appears to be an effective tool to help clinicians predict allergic accidents to cetuximab, and should be considered as a part of a comprehensive approach to personalised cancer treatment. In addition to testing for RAS and RAF mutations to predict the effectiveness of treatment with cetuximab in colon cancer, assaying anti‐cetuximab IgE levels will predict a patient's tolerance to treatment 28, 29 and avoid fatal events.
Competing Interests
K.B.L. had a consulting or advisory role for Sanofi and Merck Serono. F.D.F. received honoraria from Roche, Sanofi, Amgen and Merck Serono. M.P.G. had a consulting or advisory role for Roche, Sanofi, Amgen and GlaxoSmithKline. R.S. had a consulting or advisory role for Roche. All the remaining authors have declared no conflicts of interests.
This work was supported by funds from the “Société Nationale Française de GastroEntérologie” and by the “Ligue contre le Cancer” .
The Northwest Data Center (CTD‐CNO) is acknowledged for managing the data. It is supported by grants from the French National League Against Cancer (LNC) and the French National Cancer Institute (INCa).
We would like to thank Dr Anuradha Alahari for help in language and content editing of this manuscript and Dr Marian Degardin for his participation in project initiation.
Dupont, B. , Mariotte, D. , Dugué, A. E. , Clarisse, B. , Grellard, J.‐M. , Babin, E. , Chauffert, B. , Dakpé, S. , Moldovan, C. , Bouhier‐Leporrier, K. , Reimund, J.‐M. , Di Fiore, F. , Zanetta, S. , Mailliez, A. , Do, P. , Peytier, A. , Galais, M.‐P. , Florescu, C. , Schott, R. , Le Mauff, B. , and Gervais, R. (2017) Utility of serum anti‐cetuximab immunoglobulin E levels to identify patients at a high risk of severe hypersensitivity reaction to cetuximab. Br J Clin Pharmacol, 83: 623–631. doi: 10.1111/bcp.13140.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous‐cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–578. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–345. [DOI] [PubMed] [Google Scholar]
- 5. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2311–2319. [DOI] [PubMed] [Google Scholar]
- 7. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 8. Patel DD, Goldberg RM. Cetuximab‐associated infusion reactions: pathology and management. Oncology (Williston Park) 2006; 20: 1373–1382 ; discussion 82, 92–4, 97. [PubMed] [Google Scholar]
- 9. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non‐small‐cell lung cancer (FLEX): an open‐label randomised phase III trial. Lancet 2009; 373: 1525–1531. [DOI] [PubMed] [Google Scholar]
- 10. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab‐induced anaphylaxis and IgE specific for galactose‐alpha‐1,3‐galactose. N Engl J Med 2008; 358: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keating K, Walko C, Stephenson B, O'Neil BH, Weiss J. Incidence of cetuximab‐related infusion reactions in oncology patients treated at the University of North Carolina Cancer Hospital. J Oncol Pharm Pract 2013; 20: 409–416. [DOI] [PubMed] [Google Scholar]
- 12. O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab‐related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 2007; 25: 3644–3648. [DOI] [PubMed] [Google Scholar]
- 13. Hansen NL, Chandiramani DV, Morse MA, Wei D, Hedrick NE, Hansen RA. Incidence and predictors of cetuximab hypersensitivity reactions in a North Carolina academic medical center. J Oncol Pharm Pract 2011; 17: 125–130. [DOI] [PubMed] [Google Scholar]
- 14. Grandvuillemin A, Disson‐Dautriche A, Miremont‐Salame G, Fourrier‐Reglat A, Sgro C. Cetuximab infusion reactions: French pharmacovigilance database analysis. J Oncol Pharm Pract 2013; 19: 130–137. [DOI] [PubMed] [Google Scholar]
- 15. Pointreau Y, Commins SP, Calais G, Watier H, Platts‐Mills TA. Fatal infusion reactions to cetuximab: role of immunoglobulin e‐mediated anaphylaxis. J Clin Oncol 2012; 30: 334 ; author reply 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tronconi MC, Sclafani F, Rimassa L, Carnaghi C, Personeni N, Santoro A. Fatal infusion reaction to cetuximab: the need for predictive risk factors and safer patient selection. J Clin Oncol 2011; 29: e680–e681. [DOI] [PubMed] [Google Scholar]
- 17. Dupont B, Mariotte D, Moldovan C, Grellard JM, Vergnaud MC, Laroche D, et al. Case report about fatal or near‐fatal hypersensitivity reactions to cetuximab: anticetuximab IgE as a valuable screening test. Clin Med Insights Oncol 2014; 8: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hopps S, Medina P, Pant S, Webb R, Moorman M, Borders E. Cetuximab hypersensitivity infusion reactions: Incidence and risk factors. J Oncol Pharm Pract 2013; 19: 222–227. [DOI] [PubMed] [Google Scholar]
- 19. Dupont B, Mariotte D, Clarisse B, Galais MP, Bouhier‐Leporrier K, Grellard JM, et al. Risk factors associated with hypersensitivity reactions to cetuximab: anti‐cetuximab IgE detection as a valuable screening test. Future Oncol 2014; 10. [DOI] [PubMed] [Google Scholar]
- 20. Saleh H, Embry S, Nauli A, Atyia S, Krishnaswamy G. Anaphylactic reactions to oligosaccharides in red meat: a syndrome in evolution. Clin Mol Allergy 2012; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mariotte D, Dupont B, Gervais R, Galais MP, Laroche D, Tranchant A, et al. Anti‐cetuximab IgE ELISA for identification of patients at a high risk of cetuximab‐induced anaphylaxis. MAbs 2011; 3: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herbst RS, Arquette M, Shin DM, Dicke K, Vokes EE, Azarnia N, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol 2005; 23: 5578–5587. [DOI] [PubMed] [Google Scholar]
- 23. Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined‐modality paradigm. J Clin Oncol 2006; 24: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 24. Foley KA, Wang PF, Barber BL, Long SR, Bagalman JE, Wagner V, et al. Clinical and economic impact of infusion reactions in patients with colorectal cancer treated with cetuximab. Ann Oncol 2010; 21: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siena S, Glynne‐Jones R, Adenis A, Thaler J, Preusser P, Aguilar EA, et al. Reduced incidence of infusion‐related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer 2010; 116: 1827–1837. [DOI] [PubMed] [Google Scholar]
- 26. Galili U. Anti‐Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013; 140: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi K, Watanabe T, Satoh T, Ishiguro M, Izawa M, Inoshiri S, et al. Severe infusion reactions to cetuximab occur within 1 h in patients with metastatic colorectal cancer: results of a nationwide, multicenter, prospective registry study of 2126 patients in Japan. Jpn J Clin Oncol 2014; 44: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy‐refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010; 11: 753–762. [DOI] [PubMed] [Google Scholar]
- 29. Karapetis CS, Khambata‐Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K‐ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–1765. [DOI] [PubMed] [Google Scholar]


