Abstract
Aims
Pharmacovigilance databases are usually used to detect new potential signals that are relevant for drug safety. They are seldom used for explanatory purposes, e.g. to understand the mechanisms of adverse drug reactions (ADRs). The aim of the present study was to combine pharmacovigilance and pharmacodynamic data to investigate the association between dopamine D2, serotonin 5HT2A, and muscarinic M1 receptor occupancy and the risks of antipsychotic drug (AP)‐induced movement disorders.
Methods
First, we performed a case–noncase analysis using spontaneous reports from the World Health Organization (WHO) Global Individual Case Safety Report (ICSR) database, VigiBase®. We thus measured the risk of reporting movement disorders compared with all other ADRs [expressed as a reporting odds ratio (ROR)] for APs. Second, we performed a linear regression analysis to explore the association between the estimated risk of reporting for individual drugs and their receptor occupancy properties, for D2, 5HT2A and M1 receptors.
Results
Compared with second‐generation APs, first‐generation APs were found to be significantly more associated with the reporting of movement disorders in general but also with dystonia, Parkinsonism, akathisia and tardive dyskinesia, irrespective of gender. A significant inverse correlation was found between the ROR for movement disorders and the receptor occupancy of 5HT2A [P < 0.001; R2 = 0.51; slope = −0.014; 95% confidence interval (CI) (−0.029, 0.001)], M1 (P < 0.001; R2 = 0.56; slope = −0.014; 95% CI (−0.028, 0.001) but not D2 dopamine (P = 0.54; R2 = 0.02; slope = −0.003; 95% CI (−0.007, 0.001) receptors.
Conclusions
Using the example of AP‐induced movement disorders, the present study underlines the value of the pharmacoepidemiological–pharmacodynamic method to explore ADR mechanisms in humans and real‐life settings.
Keywords: antipsychotic drugs, drug mechanisms, movement disorders, pharmacoepidemiology, VigiBase
What is Already Known about this Subject
Drug mechanisms of action are mainly studied in vitro during their preclinical development phases.
Pharmacovigilance data are usually used to detect new potential drug safety signals.
What this Study Adds
Using the example of movement disorders induced by antipsychotic drugs, we developed an original method combining both pharmacovigilance and pharmacodynamic data. We term this method ‘the pharmacoepidemiological–pharmacodynamic approach’.
This method could be useful in humans to validate preclinical results on the pharmacological mechanisms of adverse drug reactions.
Tables of Links
| TARGETS |
|---|
| G protein‐coupled receptors [2] |
| Dopaminergic (D2) |
| Muscarinic (M1) |
| Serotoninergic (5‐HT2A) |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, or to the National Library of Medicine's MeSH Index. Entries from the Guide to PHARMACOLOGY are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding and prevention of adverse drug reactions (ADRs) or any other drug‐related problems 3. The first PV programme was established in 1961. Since then, numerous large PV databases have been set up to register reports of ADRs. They contain information about patients suffering from ADRs, and the drugs involved in their occurrence. For these reasons, they are invaluable for detecting potential drug safety signals. Several methods can be used to compare the reporting rate for a reaction of interest for a given drug or drug class with that for other drugs in the database. This disproportionality analysis is termed a ‘case–noncase’ approach, which is based on the case–control study principle 4, 5.
While the identification of ADRs is of primary importance, understanding their mechanism(s) is essential for optimizing prevention and crisis management 6. In fact, drug mechanisms of action are mainly studied in vitro during the preclinical development phases by using cellular and receptor binding models 7, 8, 9. At this stage, the understanding of the pharmacodynamic (PD) properties putatively predicting the occurrence of ADRs is often fairly limited 10. Nevertheless, even in the postmarketing phases, very few methods have been developed in humans to understand the mechanism of ADRs, besides clinical molecular imaging 11, 12. In a recent study, we assessed a method combining the use of PD and PV data 13 to study the potential role of serotonin 5‐HT2C and histamine H1 receptor occupancy in the differences existing across antipsychotic drugs (APs) with regard to the risk of drug‐induced diabetes.
As the mechanism putatively involved in this ADR was a subject for debate 14, 15, 16, we then applied this approach to study an ADR for which the pathophysiology is more consensual. One of the most frequent and well‐known ADRs of APs is the occurrence of movement disorders – i.e. dystonia, Parkinsonism, akathisia or tardive dyskinesia. These disorders mainly result from a direct or indirect blockade of D2 dopamine receptors in the cerebral nigrostriatal pathways 17. Among other hypotheses, the involvement of the antagonist properties of these drugs on M1 muscarinic and/or 5‐HT2A serotonergic receptors in modulating the occurrence of this ADR is suspected 18. If this hypothesis is correct, a proportional association between reports of AP‐induced movement disorders and the antagonist activity of dopamine D2 or serotonin 5‐HT2A or muscarinic M1 would be found in pharmacovigilance databases.
Therefore, we investigated this association between D2, 5‐HT2A and M1 receptor occupancy and the risks of AP‐induced movement disorders by combining the use of both PV and PD data.
Methods
Design
PV evaluation
First, we performed a case–noncase analysis using spontaneous reports from the World Health Organization (WHO) Global Individual Case Safety Report (ICSR) database, VigiBase®. We were thus able to measure the risk of movement disorder reporting among all other ADRs for all marketed APs.
PD evaluation
We then performed a linear regression analysis to explore the association between the estimated risk of reporting for individual drugs and their receptor occupancy properties for D2, 5‐HT2A and M1 receptors. Receptor occupancy was computed by using the classical equation derived from pharmacological receptor theory 19. Hereafter, we refer to this combined pharmacological evaluation as the pharmacoepidemiological (PE)‐PD approach.
Data and data sources
Spontaneous reporting data from VigiBase®
In the framework of the WHO Programme for International Drug Monitoring, the Uppsala Monitoring Center (UMC) has been in charge of the development of PV and drug safety at the international level since 1978. Its main tasks are to collect and analyse reports of ADRs transmitted by the 123 countries contributing to the system worldwide. For this purpose, ICSRs are continuously registered in VigiBase® 20.
Each ICSR a priori includes the available data concerning the reporting country, the reporter's qualification, the patient's demographic characteristics, the drug(s) used and the characteristics of the ADR. The Medical Dictionary for Regulatory Activities (MedDRA) or the WHO Adverse Reaction Terminology (WHO‐ART) are used for coding the types of ADR 21, 22. To help with searching and analysing data related to the 12 million ICSRs currently recorded in VigiBase®, the VigiLyze® tool was developed 23.
All ICSRs entered in VigiBase® between 1 January 1972 (the year of the first report involving an AP) and 31 August 2015 for the 53 APs identified in the database were included in the study [32 first‐generation (FGAPs) and 21 second‐generation (SGAPs) APs; the list is provided in Table S1. We considered only ICSRs for which data on age or gender were recorded, to ascertain a minimal quality of reporting (Figure 1).
Figure 1.
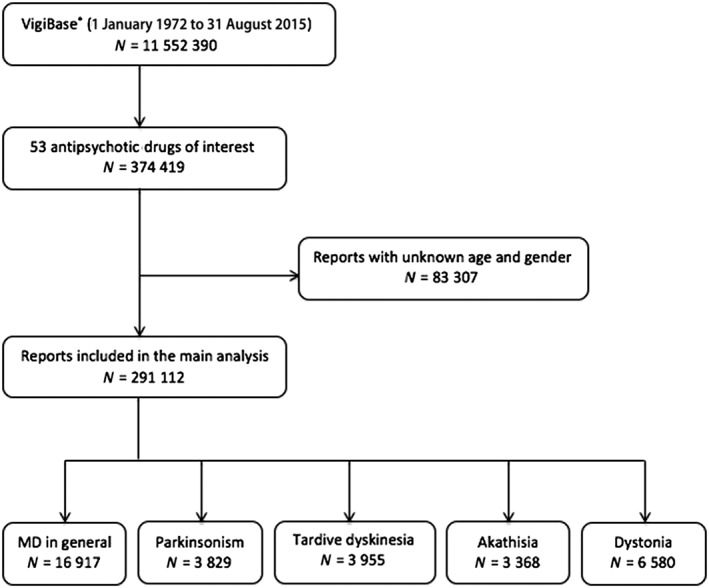
Flowchart for the study protocol. MD, movement disorders; N, number of reports
Among these ICSRs, cases of AP‐induced movement disorders were identified using the MedDRA dictionary specific codes. This is organized into five hierarchical levels, from the least to the most precise: ‘System Organ Class’ (SOC), ‘High‐Level Groups Terms’ (HLGTs), ‘High‐Level Terms’ (HLTs), ‘Preferred Terms’ (PTs) and ‘Lowest Level Terms’ (LLTs) 24. We focused on five subtypes of movement disorders induced by AP drugs: ‘parkinsonism’, ‘dystonia’, ‘akathisia’, ‘tardive dyskinesia’ and ‘movement disorders in general’. The MedDRA terms were selected by three authors (FM, OR, JLM) specialized in neuropsychopharmacology in general, and in movement disorders in particular. The HLT ‘dystonias’ was used to identify cases of dystonia. Cases of parkinsonism were selected by using the following PTs: ‘akinesia’, ‘bradykinesia’, ‘hypokinesia’, ‘masked facies’, ‘Parkinson's disease’, ‘parkinsonian gait’ and ‘parkinsonism’. Cases of akathisia and tardive dyskinesia were selected by using, respectively, the PTs ‘akathisia’ and ‘tardive dyskinesia’. Finally, cases of movement disorders in general were selected by using all of the terms quoted above. All other HLTs, such as ‘choreiform movements’, and PTs, such as ‘vascular parkinsonism’, were excluded from the analysis as they occur infrequently, so the corresponding numbers of recorded ADRs in Vigibase® were too low.
PD: data sources and methods for estimating AP receptor occupancy
For each drug studied, we estimated the degree of receptor occupancy for three receptors of interest (dopamine D2, serotonin5‐HT2A and muscarinic M1 receptors; Table 1) by using an equation derived from pharmacological receptor theory 19. The model derived from this theory is useful for predicting receptor‐mediated pharmacological actions quantitatively 25. It has already been used to evaluate movement disorders induced by the AP drug risperidone 26. The equation used was:
where [Cr] represents the concentration of unbound AP and Ki is a constant which characterizes, for antagonists such as APs, the drug affinity for a given receptor – i.e. its ability to bind to this receptor. [Cr] values were derived from the therapeutic reference ranges reported in the ‘AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry’ 27, and data on plasma protein binding were obtained from pharmacological reference textbooks 28, 29. Values of Ki for the 53 APs studied for the three receptors considered in the present study were obtained by a search on the Integrative navigation in PHarmaCological spacE (iPHACE) project website 30. This tool, which represents a new integrative conceptual framework to navigate in the pharmacological space covered by drugs, contains data related to interactions between drugs and targets extracted from the International Union of Basic and Clinical Pharmacology (IUPHAR) database 31 and the National Institute of Mental Health Psychoactive Drug Screening Program (PDSP) 32. Our approach conformed to the British Journal of Pharmacology's Concise Guide to Pharmacology 2015/2016 33.
Table 1.
Degree of receptor occupancy for antipsychotic drugs and the parameters involved
| Receptor systems | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dopaminergic (D2) | Serotonergic (5‐HT2A) | Muscarinic (M1) | ||||||
| FGAPs & SGAPs | C'T* (ng ml–1) | fu (%) | Ki (nM) | Degree (%) | Ki (nM) | Degree (%) | Ki (nM) | Degree (%) |
| Chlorpromazine | 300 | 4.0 | 50.12 | 42.94 | 7.94 | 82.61 | 30.83 | 55.03 |
| Fluphenazine | 10 | 5.0 | 1.45 | 44.10 | 31.62 | 3.49 | 1093.96 | 0.10 |
| Haloperidol | 10 | 8.0 | 7.94 | 21.17 | 100.00 | 2.09 | >10 000.00 | 0.02 |
| Loxapine | 30 | 3.0 | 7.76 | 28.66 | 7.94 | 28.20 | 105.68 | 2.87 |
| Perphenazine | 2 | 7.0 | 0.60 | 40.99 | 6.31 | 6.19 | 2000.00 | 0.02 |
| Pimozide | 20 | 1.0 | 12.59 | 3.33 | 39.81 | 1.08 | 800.00 | 0.05 |
| Prochlorperazine | 40 | 5.0 | 3.63 | 59.62 | 15.00 | 26.33 | 547.78 | 0.97 |
| Thioridazine | 200 | 5.0 | 14.59 | 64.93 | 19.95 | 57.52 | 6.22 | 81.29 |
| Thiothixene | 15 | 1.0 | 1.40 | 19.47 | 50.12 | 0.67 | 2600.00 | 0.01 |
| Trifluoperazine | 2 | 1.0 | 1.11 | 4.84 | 12.59 | 0.45 | 663.33 | 0.01 |
| AMISULPRIDE | 320 | 84.0 | 12.59 | 98.30 | 8304.00 | 8.06 | >10 000.00 | 6.79 |
| ARIPIPRAZOLE | 500 | 1.0 | 0.79 | 93.40 | 15.85 | 41.37 | 6776.42 | 0.16 |
| ASENAPINE | 5 | 5.0 | 1.20 | 42.22 | 0.06 | 93.60 | 8128.31 | 0.01 |
| CLOZAPINE | 600 | 5.0 | 446.68 | 17.08 | 5.01 | 94.83 | 6.59 | 93.31 |
| ILOPERIDONE | 10 | 5.0 | 5.94 | 16.49 | 0.35 | 77.02 | 4897.79 | 0.02 |
| MELPERONE | 100 | 50.0 | 152.00 | 55.54 | 180.00 | 51.33 | >10 000.00 | 1.86 |
| OLANZAPINE | 80 | 7.0 | 2.09 | 89.57 | 1.78 | 90.97 | 11.38 | 61.19 |
| PALIPERIDONE | 60 | 26.0 | 5.01 | 87.96 | 0.79 | 97.89 | 8800.00 | 0.41 |
| QUETIAPINE | 500 | 17.0 | 63.10 | 77.85 | 199.53 | 52.65 | 240.44 | 47.99 |
| RISPERIDONE | 60 | 11.0 | 0.44 | 97.34 | 0.22 | 98.65 | >10 000.00 | 0.16 |
| SERTINDOLE | 100 | 0.5 | 3.31 | 25.55 | 0.50 | 69.44 | >10 000.00 | 0.01 |
| ZIPRASIDONE | 200 | 0.1 | 2.82 | 14.68 | 0.70 | 40.94 | 6081.35 | 0.01 |
| ZOTEPINE | 150 | 3.0 | 10.97 | 55.34 | 2.51 | 84.41 | 1342.77 | 1.00 |
C'T*, total plasma concentration of a drug; FGAPs, first‐generation antipsychotic drugs; SGAPs, second‐generation antipsychotic drugs (SGAP written in upper case letters); fu, free fraction of a drug in the plasma; Ki, equilibrium dissociation constant
Statistical analysis
Analysis of spontaneous reporting data
In the present study, the parameter chosen for disproportionality analyses was the reporting odds ratio (ROR) of the case–noncase method 34, 35. The ROR is the ratio of the odds of exposure to a given drug in the reports concerning an ADR of interest (cases) compared with the odds of exposure for all other ADR reports present in the database (noncases). In the case–noncase method, the ROR is provided, with its 95% confidence interval (CI).
For the five movement disorders of interest (movement disorders in general, dystonia, parkinsonism, akathisia, tardive dyskinesia), we estimated RORs: (i) for FGAPs vs. SGAPs; and (ii) for each of the 53 APs studied. We compared the differential risks of movement disorders for the two drug classes by splitting the study population according to gender (male and female groups) and age groups. Newborns (0–27 days) were excluded.
PD analysis for movement disorders
Univariate linear regression models were used to investigate the association between the ROR estimates for movement disorders in general and the D2, 5HT2A and M1 receptor occupancies estimated for each individual AP. The ROR for movement disorders in general was the dependent variable and receptor occupancy the explanatory variable. We excluded degrees of receptor occupancy that were below 0.1%. Owing to a lack of data on the affinity for receptors of some APs, linear regressions were performed for 23 products. All analyses were performed using the SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Case–noncase analysis
Between 1 January 1972 and 31 August 2015, 291 112 reports, involving at least one of the 53 APs of interest, were recorded in VigiBase®. The gender ratio for subjects with ADRs was 1.0 and the mean age was 43.5 years (±15.3). Reports originated mainly from the United States (38.1%). The greatest number of reports concerned clozapine (29%), followed by quetiapine (15%) and risperidone (13%). Table 2 presents the characteristics of all reports involving the 53 APs. Movement disorders in general were reported in 16 917 (5.8%) patients; they included 6580 reports of dystonia (2.3%), 3955 reports of tardive dyskinesia (1.4%), 3829 reports of parkinsonism (1.3%) and 3368 reports of akathisia (1.2%).
Table 2.
Characteristics of all reports for the 53 antipsychotic drugs of interest in VigiBase® (n = 291 112)
| Characteristics | n | % |
|---|---|---|
| Age group | ||
| 0–17 | 17 205 | 5.9 |
| 18–44 | 144 845 | 49.8 |
| 45–64 | 87 212 | 30.0 |
| 65–74 | 20 762 | 7.1 |
| ≥75 | 21 088 | 7.2 |
| Female | 142 941 | 49.1 |
| WHO reporting area | ||
| Africa | 1531 | 0.5 |
| Americas | 128 988 | 44.3 |
| Canada | 11 439 | 3.9 |
| United States | 111 002 | 38.1 |
| Others countries | 6547 | 2.2 |
| Asia | 29 537 | 10.2 |
| China | 4688 | 1.6 |
| India | 6614 | 2.3 |
| Japan | 4810 | 1.7 |
| Singapore | 1653 | 0.6 |
| South Korea | 7246 | 2.5 |
| Vietnam | 74 | 0.03 |
| Others countries | 4452 | 1.5 |
| Europe | 113 503 | 39.0 |
| France | 15 839 | 5.4 |
| Germany | 21 127 | 7.3 |
| Italy | 6113 | 2.1 |
| United Kingdom | 39 440 | 13.5 |
| Others countries | 30 984 | 10.6 |
| Oceania | 17 553 | 6.0 |
| Australia | 15 152 | 5.2 |
WHO, World Health Organization
Table 3 shows the results of the disproportionality analyses for FGAPS vs. SGAPs. FGAPs were significantly more associated with the reporting of movement disorders in general, dystonia, parkinsonism, akathisia and tardive dyskinesia, irrespective of gender (except for akathisia in women). The results were less consistent when comparing age categories; no association was found for parkinsonism in patients aged under 23 months and those aged 65–74 years; for akathisia in patients under 17 years and over 65 years; and for tardive dyskinesia in patients under 17 years. RORs were maximal for dystonia, followed by movement disorders and parkinsonism.
Table 3.
Reporting odds ratios (RORs) for first‐generation anti‐psychotic drugs vs. second‐generation anti‐psychotic drugs and their 95% confidence intervals (CIs), calculated according to types of study population for five groups of adverse drug reaction of interest
| ROR (95% CI) | |||||
|---|---|---|---|---|---|
| MD overall | Parkinsonism | Tardive dyskinesia | Akathisia | Dystonia | |
| All patients | 2.7 (2.64, 2.82) | 2.1 (1.98, 2.26) | 1.3 (1.24, 1.44) | 1.2 (1.13, 1.32) | 5.6 (5.31, 5.87) |
| Gender | |||||
| Male | 3.0 (2.88, 3.16) | 2.4 (2.16, 2.61) | 1.6 (1.43, 1.77) | 1.5 (1.30, 1.63) | 5.4 (5.05, 5.79) |
| Female | 2.5 (2.37, 2.59) | 1.9 (1.72, 2.08) | 1.2 (1.04, 1.27) | 1.0 (0.92, 1.15) | 5.8 (5.40, 6.24) |
| Age | |||||
| 28 d to 23 m | 7.4 (2.95, 18.38) | 1.6 (0.10, 26.37) | N/A | N/A | 10.6 (3.60, 31.14) |
| 2–11 y | 2.2 (1.85, 2.63) | 2.7 (1.38, 5.13) | 0.4 (0.23, 0.66) | 0.8 (0.33, 1.75) | 3.3 (2.71, 4.03) |
| 12–17 y | 3.1 (2.77, 3.56) | 1.7 (1.14, 2.54) | 0.4 (0.24, 0.72] | 0.5 (0.29, 0.78) | 5.2 (4.48, 6.02) |
| 18–44 y | 3.5 (3.33, 3.65) | 3.0 (2.65, 3.40) | 1.3 (1.15, 1.46) | 1.3 (1.13, 1.40) | 7.1 (6.60, 7.55) |
| 45–64 y | 2.3 (2.16, 2.47) | 2.4 (2.14, 2.77) | 1.4 (1.27, 1.63) | 1.5 (1.29, 1.73) | 4.6 (4.10, 5.27) |
| 65–74 y | 1.6 (1.40, 1.75) | 1.1 (0.95, 1.33] | 1.5 (1.23, 1.86) | 1.3 (0.90, 1.74) | 3.5 (2.73, 4.41) |
| ≥75 y | 1.5 (1.34, 1.67) | 1.2 (1.02, 1.37) | 1.8 (1.46, 2.29) | 1.4 (0.94, 2.10) | 2.2 (1.76, 2.85) |
d, days of age; m, months of age; MD, movement disorders; N/A, not applicable; y: years of age
Risperidone was the drug most frequently associated with reports of such disorders, followed by haloperidol and aripiprazole. Figure 2 shows the RORs for the 49 APs accounting for more than 10 reports. Clozapine was significantly less associated (ROR = 0.1; 95% CI 0.11, 0.13) with the reporting of movement disorders in general, whereas the ROR was maximal with prochlorperazine (ROR = 5.4; 95% CI 5.09, 5.79).
Figure 2.
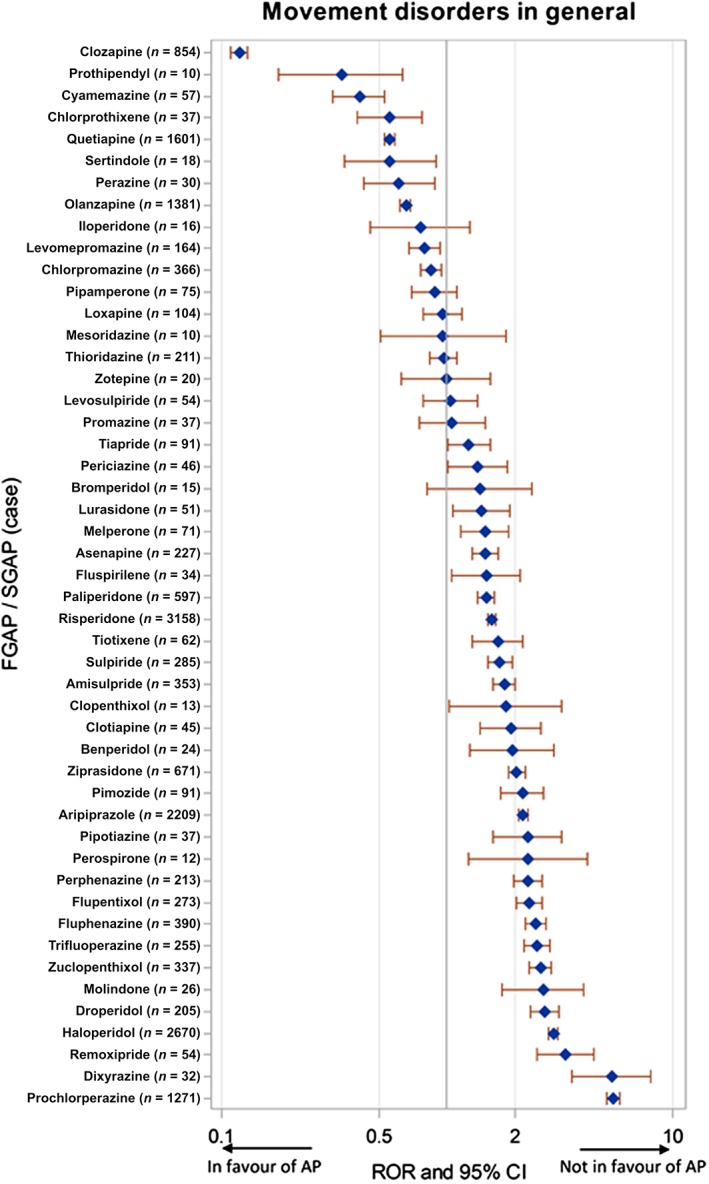
Case–noncase analysis for individual antipsychotic drugs (APs), showing the association between AP exposure and the reporting of movement disorders in general in VigiBase®. Second‐generation APs (SGAP) are written in upper case letters. CI, confidence interval; FGAP, first‐generation AP; ROR, reporting odds ratio
Dystonia was reported in 6580 (2.3%) patients. Haloperidol was the most frequently involved drug, followed by prochlorperazine and risperidone. Figure S1 shows the RORs for the 38 APs accounting for more than 10 reports. Clozapine was significantly less associated (ROR = 0.1; 95% CI 0.08, 0.10) with dystonia reporting, whereas the ROR was maximal with prochlorperazine (ROR = 13.8; 95% CI 12.84, 14.80).
Tardive dyskinesia was reported in 3955 (1.4%) patients. Risperidone was the most frequently involved drug, followed by quetiapine and aripiprazole. Figure S2 shows the RORs for the 29 APs accounting for more than 10 reports. Clozapine was significantly less associated (ROR = 0.1; 95% CI 0.10, 0.13) with tardive dyskinesia reporting, whereas the ROR was maximal with dixyrazine (ROR = 7.5; 95% CI 4.12, 13.54).
Parkinsonism was reported in 3829 (1.3%) patients. Risperidone was the most frequently involved drug, followed by haloperidol and clozapine. Figure S3 shows the RORs for the 35 APs accounting for more than 10 reports. Clozapine was less associated (ROR = 0.3; 95% CI 0.26, 0.32) with parkinsonism reporting, whereas the ROR was maximal with benperidol (ROR = 4.2; 95% CI 2.37, 7.59).
Akathisia was reported in 3368 (1.2%) patients. Aripiprazole was the most frequently involved drug, followed by risperidone and olanzapine. Figure S4 shows the RORs for the 27 APs accounting for more than 10 reports. Clozapine was significantly less associated (ROR = 0.06; 95% CI 0.05, 0.07) with akathisia reporting, whereas the ROR was maximal with aripiprazole (ROR = 3.8; 95% CI 3.49, 4.13) and pipotiazine (ROR = 3.8; 95% CI 2.21, 6.73).
The PE‐PD approach
For the 23 APs considered for analysis, a significant inverse correlation was found between RORs for movement disorders in general and receptor occupancy for 5‐HT2A receptors (P < 0.001; R2 = 0.51; slope = −0.014; 95% CI (−0.029, 0.001) and M1 receptors (P < 0.001; R2 = 0.56; slope = −0.014; 95% CI (−0.028, 0.001), but not with D2 receptors (P = 0.54; R2 = 0.02; slope = −0.003; 95% CI (−0.007, 0.001) (Figure 3).
Figure 3.
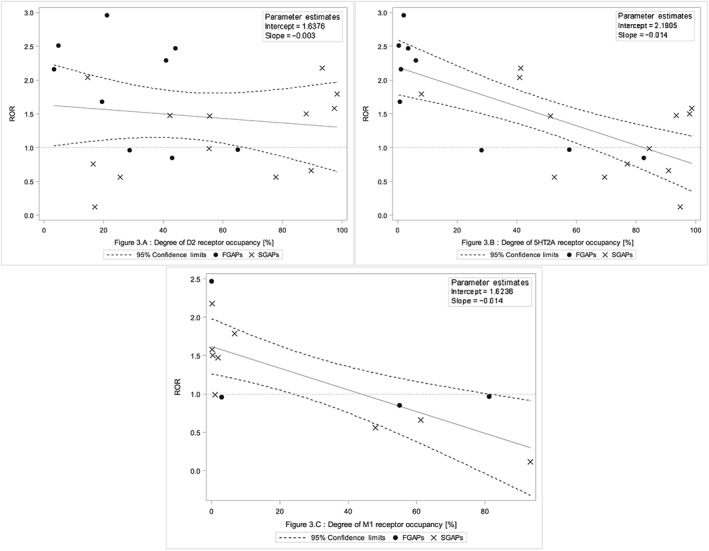
Association between dopamine D2 (A) (P = 0.53), serotonin 5HT2A (B) (P < 0.01) and muscarinic M1 (C) (P < 0.01) receptor occupancy and the reporting odds ratio (ROR) for movement disorders in general. We excluded degrees of receptor occupancy that were below 0.1%. Linear regression was performed with 23 values for antipsychotic drugs (APs) for D2 (A) and 5HT2A (B) receptors, and 14 values for APs for the M1 (C) receptor
Discussion
Main results
The present study aimed to explore the mechanisms of AP‐induced movement disorders using the PE‐PD method – i.e. an original approach combining the case–noncase approach and PD data analysis. We first ranked the investigated APs according to the risk of reporting movement disorders. Clozapine was found to be the least associated with this adverse effect. For each individual AP, we then investigated the mechanism of AP‐induced movement disorders by studying the association between the RORs for movement disorders and 5‐HT2A, M1 and D2 receptor occupancy values. These results support the consistency of our original approach, the PE‐PD method.
Case–noncase analysis
First, we found that SGAPs were less frequently associated with movement disorders in general, dystonia, parkinsonism, akathisia and tardive dyskinesia than FGAPs, irrespective of gender (except for akathisia in women). Clozapine was less associated than FGAPs or other SGAPs with movement disorders in general, and with dystonia, parkinsonism, akathisia and tardive dyskinesia in particular. These results, derived from real‐world prescription data, are important as they support the conclusions of several previous studies based upon more conventional approaches 36, 37, including the Leucht meta‐analysis of clinical trials 38. Considering the results obtained with clozapine, we performed a sensitivity analysis to explore its relative influence on the results; after exclusion of clozapine, the difference between FGAPs and SGAPs still persisted for movement disorders (ROR = 1.9; 95% CI 1.80, 1.93). By contrast, among SGAPs, several drugs, such as aripiprazole, ziprasidone, risperidone, paliperidone and lurasidone, were found to be associated (ROR) with more movement disorders than were other APs. In fact, these SGAPs were more involved in akathisia or tardive dyskinesia than with dystonia or parkinsonism, which could explain the observed difference with all movement disorders. Concerning akathisia, our results were in line with previous studies suggesting the risk of this adverse effect does not differ between FGAPs and SGAPs 39.
Movement disorders were more frequently observed with FGAPs than SGAPs, irrespective of age. Similar results were found for dystonia, tardive dyskinesia (except between the ages of 2 years and 17 years) but not akathisia. For parkinsonism, the difference disappeared between 65 years and 74 years of age, and was only marginally significant after 74 years. This might be due to the well‐known susceptibility of elderly people to D2 receptor blockade, irrespective of the AP or its generation 40.
PE‐PD analysis
The main originality of our work was to combine the results of the case–noncase study with PD data for three different receptors (D2, 5‐HT2A, M1), selected owing to their involvement in the pathophysiology of AP‐induced movement disorders. In fact, the respective role of these three receptors in these disorders remains debated, especially 5‐HT2A 18. In the present work, D2 receptor occupancy did not clearly explain the observed disproportionality between the different APs, probably because the dosage levels were selected on the basis of their antidopaminergic activity observed during animal experiments. By contrast, 5HT2A and M1 receptor occupancies were congruent with these differences. Our models suggest that the greater the 5‐HT2A or M1 receptor occupancy, the fewer movement disorders are reported. In fact, our results indicate that blockade of M1 or 5‐HT2A receptors is more involved than the sole blockade of D2 receptors in mitigating the motor effects of APs. Thus, 5‐HT2A antagonist properties have been shown partly to counteract AP‐induced parkinsonism 41. Similarly, dopamine receptor activity in the nigrostriatal pathways is positively balanced by the blockade of muscarinic cholinergic receptors 42.
Our results are in line with previous data obtained in animals and with the few human clinical studies (using molecular imaging) conducted on this topic. However, to our knowledge, our study is the first to demonstrate such a relationship in humans by using data from a PV database.
We deliberately selected ADRs with a well‐established mechanism – i.e. movement disorders – in order to test the validity of the PE‐PD method. The fact that our results were clearly congruent with what was expected highlights the potential of the PE‐PD method for investigating the putative mechanisms associated with the occurrence of various ADRs in real life. In fact, a comparable design was previously used to investigate the association between drug‐induced cardiac arrhythmias and Anti‐human ether‐a‐go‐go‐related gene (HERG) activity 43, and AP‐induced diabetes and 5‐HT2C and H1 receptors 13, two ADRs whose mechanism is currently being debated.
In brief, our findings show that the PE‐PD method shows promise for the analysis of data from PV databases, not only to explore and better understand the mechanisms of ADRs, but also to prevent the use or the development of drugs suspected of inducing a given ADR.
Limitations and strengths
Our work suffers from the inescapable limitations of data‐mining approaches in PV, the first being under‐reporting 44. However, this might not be relevant in the present case as it has previously been shown that under‐reporting is expected to be approximately similar for drugs belonging to the same therapeutic class 45. In fact, under‐reporting might affect the absolute values of ROR but not the comparison between APs for each ADRs studied, which is more relevant for our analysis. Second, we did not take into account the doses used or exposure durations, this information not being systematically recorded in PV databases. For example, under‐reporting might be more important for delayed than for acute ADRs. Moreover, we only considered 23 APs for the analyses as the receptor‐binding properties were not available for the others. However, this list covered most APs used in current practice. Similarly, we did not consider cerebrospinal fluid concentrations of APs as these data are generally not available. Concerning the identification of movement disorders, we used the MedDRA dictionary, which may not always fit with the complex character of movement disorders. Therefore, some clinically atypical disorders may have been misclassified.
In terms of the statistical analysis, we performed univariate linear regressions to graph potential correlation between occupancy and ROR. However, PD effects (degree of occupancy) often have a nonlinear shape, thus limiting the graphical analyses. In fact, in PE‐PD methods, others models should be used, such as logistic regression; however, for a graphical representation, linear regression models are adapted. Estimates of the receptor occupancies should also be discussed. Indeed, receptor occupancy for the three receptors considered was calculated according to the model derived from pharmacological receptor theory, which has several limitations, already described by Kenakin 19. One could postulate that receptor occupancy does not directly reflect the intrinsic activity of a drug. For example, some SGAPs also act as inverse agonists at 5‐HT2A receptors, and aripiprazole acts as a D2 receptor partial agonist. However, it is well known that the action of APs is explained in clinical practice by their antagonist activity at the level of 5HT2A and M1 receptors. In addition, we considered only three receptors as there is a consensus about their involvement in the pharmacological mechanism of AP‐induced movement disorders. The putative role of other receptors, such as serotonergic, glutamatergic or adrenergic receptors, has been also evoked but, as their role is still debated, they were not investigated in the present study. Finally, it was not possible to take into account the pharmacogenetic factors putatively involved in the occurrence of these AP‐induced ADRs as this information was lacking in the database used.
Despite these limitations, the present work had several strengths. First, it was conducted using the largest PV database available, Vigibase®, which includes more than 12 million reports worldwide, which clearly minimizes the risk of a bias specific to a given country. Furthermore, almost 300 000 reports were included in the study, which attests to its statistical power. The material also represents a unique source of data for studying the effects of drugs in real‐life settings and adds to knowledge acquired from clinical trials.
Conclusion
Using the example of AP‐induced movement disorders, the present study supports the value of the PE‐PD method for investigating the mechanisms of ADRs recorded in PV databases. By using this method, we found an inverse proportional correlation between serotonergic 5‐HT2A or muscarinic M1 receptor occupancies and reports of movement disorders involving APs. This new tool could be used to validate the results of pharmacological preclinical studies in humans. Finally, we found that PK databases, which are used pragmatically to detect new potential signals of drug safety, can also be used for explanatory purposes – i.e. to explain the putative mechanisms of ADRs using a PD approach.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The authors would like to thank the Uppsala Monitoring Centre (UMC), which provided, and gave permission to use, the data analysed in the present study. The authors are indebted to the French pharmacovigilance centres that contributed data. The opinions and conclusions expressed in this paper do not necessarily reflect those of the various centres or of the WHO. The authors also thank Mr Ray Cooke for revising the English.
Supporting information
Table S1 List of the 53 antipsychotic drugs (APs) of interest, ranked by type of AP and alphabetical order
Figure S1 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of dystonia in general in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S2 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of tardive dyskinesia in general in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S3 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of parkinsonism in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S4 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of akathisia in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Nguyen, T. T. H. , Pariente, A. , Montastruc, J.‐L. , Lapeyre‐Mestre, M. , Rousseau, V. , Rascol, O. , Bégaud, B. , and Montastruc, F. (2017) An original pharmacoepidemiological–pharmacodynamic method: application to antipsychotic‐induced movement disorders. Br J Clin Pharmacol, 83: 612–622. doi: 10.1111/bcp.13145.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Pharmacovigilance [online]. Available at http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/ (last accessed 26 February 2016).
- 4. Moore N, Thiessard F, Begaud B. The history of disproportionality measures (reporting odds ratio, proportional reporting rates) in spontaneous reporting of adverse drug reactions. Pharmacoepidemiol Drug Saf 2005; 14: 285–286. [DOI] [PubMed] [Google Scholar]
- 5. Montastruc F, Scotto S, Vaz IR, Guerra LN, Escudero A, Sáinz M, et al. Hepatotoxicity related to agomelatine and other new antidepressants: a case/noncase approach with information from the Portuguese, French, Spanish, and Italian pharmacovigilance systems. J Clin Psychopharmacol 2014; 34: 327–330. [DOI] [PubMed] [Google Scholar]
- 6. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 7. Bender A, Scheiber J, Glick M, Davies JW, Azzaoui K, Hamon J, et al. Analysis of pharmacology data and the prediction of adverse drug reactions and off‐target effects from chemical structure. ChemMedChem 2007; 2: 861–873. [DOI] [PubMed] [Google Scholar]
- 8. Kuhlmann PJ, Mück W. Clinical‐pharmacological strategies to assess drug interaction potential during drug development. Drug Saf 2012; 24: 715–725. [DOI] [PubMed] [Google Scholar]
- 9. Schenone M, Dančík V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol 2013; 9: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rogers AS. Adverse drug events: identification and attribution. Drug Intell Clin Pharm 1987; 21: 915–920. [DOI] [PubMed] [Google Scholar]
- 11. Uchida H, Takeuchi H, Graff‐Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol 2011; 31: 497–502. [DOI] [PubMed] [Google Scholar]
- 12. Jeraj R, Bradshaw T, Simončič U. Molecular imaging to plan radiotherapy and evaluate its efficacy. J Nucl Med 2015; 56: 1752–1765. [DOI] [PubMed] [Google Scholar]
- 13. Montastruc F, Palmaro A, Bagheri H, Schmitt L, Montastruc J‐L, Lapeyre‐Mestre M. Role of serotonin 5‐HT2C and histamine H1 receptors in antipsychotic‐induced diabetes: a pharmacoepidemiological‐pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol 2015; 25: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 14. Chebane L, Tavassoli N, Bagheri H, Montastruc J‐L, Centres Régionaux de Pharmacovigilance Français . Drug‐induced hyperglycemia: a study in the French pharmacovigilance database. Therapie 2010; 65: 447–458. [DOI] [PubMed] [Google Scholar]
- 15. Fathallah N, Slim R, Larif S, Hmouda H, Ben SC. Drug‐induced hyperglycaemia and diabetes. Drug Saf 2015; 38: 1153–1168. [DOI] [PubMed] [Google Scholar]
- 16. Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment –pharmacological mechanisms. Pharmacol Ther 2010; 125: 169–179. [DOI] [PubMed] [Google Scholar]
- 17. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 18. Stahl SM. What makes an antipsychotic atypical? J Clin Psychiatry 1998; 59: 403–404. [DOI] [PubMed] [Google Scholar]
- 19. Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci 2004; 25: 186–192. [DOI] [PubMed] [Google Scholar]
- 20. Lindquist M. VigiBase, the WHO Global ICSR Database System: basic facts. Drug Inf J 2008; 42: 409–419. [Google Scholar]
- 21. Lindquist M, Edwards IR. The WHO Programme for International Drug Monitoring, its database, and the technical support of the Uppsala Monitoring Center. J Rheumatol 2001; 28: 1180–1187. [PubMed] [Google Scholar]
- 22. Brown EG, Wood L. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999; 20: 109–117. [DOI] [PubMed] [Google Scholar]
- 23. Uppsala Monitoring Centre . VigiLyze [online]. Available at http://who‐umc.org/DynPage.aspx?7252&mn3=7254&mn4=7695 (last accessed 9 March 2016).
- 24. MedDRA . [online]. Available at http://www.meddra.org/ (last accessed 9 March 2016).
- 25. Ayuhara H, Takayanagi R, Okuyama K, Yoshimoto K, Ozeki T, Yokoyama H, et al. Receptor occupancy theory‐based analysis of interindividual differences in antiemetic effects of 5‐HT3 receptor antagonists. Int J Clin Oncol 2009; 14: 518–524. [DOI] [PubMed] [Google Scholar]
- 26. Yamada Y, Ohno Y, Nakashima Y, Fukuda M, Takayanagi R, Sato H, et al. Prediction and assessment of extrapyramidal side effects induced by risperidone based on dopamine D(2) receptor occupancy. Synapse 2002; 46: 32–37. [DOI] [PubMed] [Google Scholar]
- 27. Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 2011; 44: 195–235. [DOI] [PubMed] [Google Scholar]
- 28. Brunton L, Chabner BA, Knollman B, eds. Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th edn. New York, NY: McGraw‐Hill Medical, 2011. [Google Scholar]
- 29. Martindale BA. The Complete Drug Reference (38th revised edn). London: Pharmaceutical Press, 2014. [Google Scholar]
- 30. Garcia‐Serna R, Ursu O, Oprea TI, Mestres J. iPHACE: integrative navigation in pharmacological space. Bioinformatics 2010; 26: 985–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. IUPHAR – International Union of Basic and Clinical Pharmacology website [online]. Available at http://www.iuphar.org/ (last accessed 9 May 2013).
- 32. PDSP . Home page [online]. Available at https://pdspdb.unc.edu/pdspWeb/ (last accessed 9 March 2016).
- 33. Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al. The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 2015; 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004; 13: 519–523. [DOI] [PubMed] [Google Scholar]
- 35. Montastruc J‐L, Sommet A, Bagheri H, Lapeyre‐Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 2011; 72: 905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin 2011; 29: 127–148, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naber D, Lambert M. The CATIE and CUtLASS studies in schizophrenia: results and implications for clinicians. CNS Drugs 2009; 23: 649–659. [DOI] [PubMed] [Google Scholar]
- 38. Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. Lancet 2013; 382: 951–962. [DOI] [PubMed] [Google Scholar]
- 39. Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: extrapyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry 2006; 14: 152–164. [DOI] [PubMed] [Google Scholar]
- 40. Uchida H, Kapur S, Mulsant BH, Graff‐Guerrero A, Pollock BG, Mamo DC. Sensitivity of older patients to antipsychotic motor side effects: a PET study examining potential mechanisms. Am J Geriatr Psychiatry 2009; 17: 255–263. [DOI] [PubMed] [Google Scholar]
- 41. Reynolds GP. Antipsychotic drug mechanisms and neurotransmitter systems in schizophrenia. Acta Psychiatr Scand Suppl 1994; 380: 36–40. [DOI] [PubMed] [Google Scholar]
- 42. Snyder S, Greenberg D, Yamamura HI. Antischizophrenic drugs and brain cholinergic receptors. Affinity for muscarinic sites predicts extrapyramidal effects. Arch Gen Psychiatry 1974; 31: 58–61. [DOI] [PubMed] [Google Scholar]
- 43. De Bruin ML, Pettersson M, Meyboom RHB, Hoes AW, Leufkens HGM. Anti‐HERG activity and the risk of drug‐induced arrhythmias and sudden death. Eur Heart J 2005; 26: 590–597. [DOI] [PubMed] [Google Scholar]
- 44. Hazell L, Shakir SAW. Under‐reporting of adverse drug reactions : a systematic review. Drug Saf 2006; 29: 385–396. [DOI] [PubMed] [Google Scholar]
- 45. Pierfitte C, Bégaud B, Lagnaoui R, Moore ND. Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol 1999; 47: 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of the 53 antipsychotic drugs (APs) of interest, ranked by type of AP and alphabetical order
Figure S1 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of dystonia in general in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S2 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of tardive dyskinesia in general in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S3 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of parkinsonism in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Figure S4 Case–noncase analysis for individual antipsychotic drugs (APs): association between AP exposure and reporting of akathisia in VigiBase® [second‐generation APs (SGAP) are written in upper case]
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


