Abstract
Aims
To evaluate the effect of lansoprazole, a proton‐pump inhibitor, on the absorption, pharmacokinetics, and safety of neratinib, a pan‐HER tyrosine kinase inhibitor, in healthy subjects.
Methods
This was an open‐label, two‐period, fixed‐sequence study. Fifteen healthy adult subjects received a single oral dose of neratinib 240 mg (Period 1), followed by a washout period, then oral lansoprazole 30 mg once daily for 7 days and a single dose of neratinib 240 mg on Day 5 (Period 2). Pharmacokinetic sampling was performed for 72 h following each neratinib dose. Plasma neratinib concentration–time data were analysed using noncompartmental methods. Geometric mean ratios for AUC0–t, AUC0–inf, and peak plasma concentrations (Cmax) for neratinib plus lansoprazole vs. neratinib were used to assess the magnitude of the drug–drug interaction if the 90% confidence intervals were outside 80.00–125.00%.
Results
Neratinib geometric least‐squares mean (LSM) Cmax was reduced from 84.5 ng ml−1 with neratinib alone to 24.5 ng ml−1 with neratinib plus lansoprazole. The extent of exposure to neratinib was also decreased: geometric LSM AUC0–t was 1478 ng ml−1 h with neratinib vs. 426 ng ml−1 h with neratinib plus lansoprazole, and geometric LSM AUC0–inf was 1557 ng ml−1 h vs. 542 ng ml−1 h, respectively. Mean t½ was similar with both treatments (approximately 14 h). Geometric mean ratios 90% confidence intervals for AUC0–t, AUC0–inf and Cmax fell outside the prespecified equivalence range (80.0–125.0%). Treatment‐emergent adverse events, all mild, were reported by five (33%) subjects.
Conclusions
Coadministration of lansoprazole with neratinib reduced the rate and extent of neratinib exposure in healthy subjects.
Keywords: drug interaction, healthy volunteers, lansoprazole, neratinib, pharmacokinetics, safety
What is Already Known about this Subject
Neratinib is an irreversible oral pan‐HER tyrosine kinase inhibitor that has efficacy in early‐stage and metastatic HER2‐positive breast cancer, and ERBB2‐mutant breast and other solid tumours.
In common with many other tyrosine kinase inhibitors, neratinib has pH‐dependent solubility.
Coadministration with acid‐reducing agents can affect the solubility and absorption of these compounds.
What this Study Adds
Neratinib Cmax was reduced by approximately 70% following coadministration with lansoprazole vs. neratinib alone.
Extent of neratinib exposure (AUC0–t and AUC0–inf) was reduced by approximately 70% and 65%, respectively, with neratinib plus lansoprazole vs. neratinib alone.
Multiple doses of lansoprazole reduced the rate and extent of neratinib exposure.
Tables of Links
| TARGETS |
|---|
| Catalytic receptors 2 |
| Epidermal growth factor receptor |
| HER2 |
| HER4 |
| Transporters 3 |
| H+/K+‐ATPases |
| LIGANDS |
|---|
| Lansoprazole |
| Neratinib |
These Tables lists key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Neratinib (formerly HKI‐272, PB272; Puma Biotechnology Inc., Los Angeles, CA, USA) is a potent small‐molecule tyrosine kinase inhibitor of human epidermal growth factor receptors (HER or ERBB) 1, 2 and 4 4, 5. Unlike most other currently available tyrosine kinase inhibitors, neratinib binds irreversibly to HER kinases and inhibits receptor phosphorylation for many hours after it has been cleared 5. Neratinib is currently being investigated in the treatment of early‐stage and metastatic HER2‐positive breast cancer and ERBB2‐mutant solid tumours as monotherapy and in combination with other agents. In ExteNET, a large randomized, double‐blind, phase III trial, neratinib significantly improved 2‐year invasive disease‐free survival compared with placebo in HER2‐positive early‐stage breast cancer after standard trastuzumab‐based adjuvant therapy 6. In NEfERT‐T, neratinib in combination with paclitaxel had similar efficacy as trastuzumab plus paclitaxel in the first‐line treatment of metastatic HER2‐positive breast cancer, but significantly delayed the onset and reduced the frequency of central nervous system metastases 7. Several phase II trials in patients with ERBB2 mutation–positive breast cancer and other ERBB mutation‐positive or ‐amplified solid tumours are also in progress with encouraging preliminary findings 8.
At the recommended therapeutic dose of 240 mg 9, neratinib is absorbed relatively slowly with a mean time to maximum concentration (Tmax) of 6 h in healthy adult volunteers 10. Mean peak plasma concentrations (Cmax) and area under the concentration–time curve to 24 h post‐dose were 71.8 ng ml−1 and 891 ng ml−1 h, respectively, with a mean elimination half‐life (t½) of approximately 13 h 10. The mean accumulation ratio (1.14) after repeated doses of neratinib 240 mg/day indicated no major accumulation of the drug 9. The pharmacokinetic profile of neratinib is similar in healthy subjects 10 and patients with cancer 9.
Acid‐reducing agents – proton‐pump inhibitors, H2‐antagonists and antacids – are widely used to alleviate the symptoms of gastroesophageal reflux disease. Many of these agents are available over the counter, and concomitant use of acid‐reducing agents with drugs to treat other diseases is common. For example, a retrospective cross‐sectional analysis of two healthcare databases indicated that 20–33% of cancer patients were receiving acid‐reducing agents 11. Proton pump inhibitors were the most commonly used medication among cancer patients, and were prescribed in 65–79% of patients receiving an acid‐reducing agent 11.
At therapeutic doses, acid‐reducing agents can increase gastric pH from the normal range (1.5–3.0) 12 to values in excess of 6.0 13. For oral drugs with pH‐dependent solubility, increases in gastric pH induced by an acid‐reducing agent may affect their solubility, absorption and systemic exposure. Neratinib, in common with many other tyrosine kinase inhibitors, is a weak base (pKa 7.65 and 4.66) with pH‐dependent solubility. Coadminstration of neratinib with treatments that alter gastric pH may affect its absorption and pharmacokinetics.
The present study, PUMA‐NER‐0101, was designed to evaluate the effects of changes in gastric pH, through the administration of multiple doses of the proton‐pump inhibitor lansoprazole on the absorption, pharmacokinetics' and safety of a single oral dose of neratinib in healthy adults.
Methods
Study design
This was an open‐label, two‐period, fixed‐sequence study conducted at a single centre. Healthy subjects received a single oral dose of neratinib 240 mg alone (Period 1) and in combination with multiple doses of oral lansoprazole 30 mg (Period 2; Figure 1). Pharmacokinetic sampling for neratinib was performed for 72 h following each neratinib dose. The washout period between each neratinib dose was at least 14 days.
Figure 1.
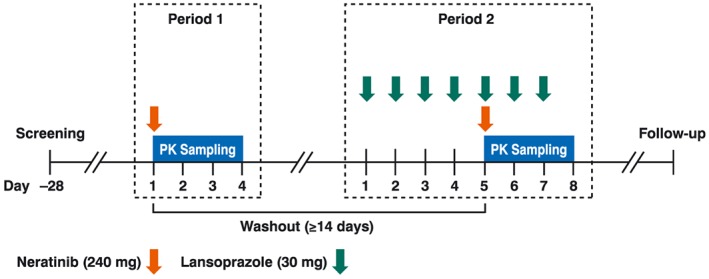
Study design
The primary objective of the study was to evaluate the effect of multiple doses of lansoprazole on the absorption and pharmacokinetics of a single dose of neratinib in healthy subjects. The secondary objective was to assess the safety and tolerability of neratinib when administered with lansoprazole.
The study protocol was approved by Chesapeake Research Review (Columbia, MD, USA) [IRB registration number: IRB#00 000 790]. The study was performed in accordance with the ethical principles of the Declaration of Helsinki (1964) and subsequent amendments. Written informed consent was obtained from all subjects prior to study enrollment. The study is registered on ClinicalTrials.gov (identifier: NCT02334501).
Study participants
Healthy men or women aged 18–55 years with a body mass index within the range of 18.5–32.0 kg m−2 were eligible. Study participants were required to be medically healthy, with no clinically significant medical history based on physical examination, laboratory profiles, vital signs and 12‐lead electrocardiograms (ECGs). Nonsmokers or moderate smokers (up to 10 cigarettes a day for at least 3 months prior to screening) were eligible. Women of childbearing potential were required to use an acceptable method of birth control or be sexually inactive for 14 days prior to the first dose of study drug, and for at least 28 days following the last dose of study drug. Nonvasectomized men were required to use an acceptable form of contraception during the study and until 90 days after the last dose of study drug.
Consumption of foods and beverages containing the following substances was prohibited: xanthines/caffeine (24 h before neratinib administration and until the end of sample collection); alcohol (48 h before Day 1 of each period until the end of sample collection); and grapefruits, Seville oranges or their juice (14 days before Period 1 and throughout the study). Medications (including over‐the‐counter products), herbal products or vitamin supplements were prohibited for 14 days prior to the first dose of study drug (or 28 days for cytochrome P450 and/or P‐glycoprotein inducers) and throughout the study.
Treatment
During Period 1, all subjects received a single oral dose of neratinib 240 mg (six 40 mg tablets; Excella GmbH, Feucht, Germany; lot no: P114127‐0001 L002) following a standard breakfast. During Period 2, all subjects received oral lansoprazole 30 mg (one delayed‐release capsule USP; Natco Pharma Limited, Hyderabad, India; lot no: 404 133) administered approximately every 24 h for 7 days prior to a standard breakfast, and a single oral dose of neratinib 240 mg on the morning of Day 5 following a standard breakfast. All study drugs were administered with approximately 240 ml of water under the supervision of clinic personnel. Subjects were ambulatory or seated upright for 4 h after neratinib administration.
Pharmacokinetics
Noncompartmental pharmacokinetic parameters were calculated from the plasma neratinib concentration–time data using Phoenix® WinNonlin®, Version 6.3 (Certara USA Inc, Princeton, NJ, USA). Actual sample times were used in all calculations.
Cmax was determined directly from the observed plasma concentration data, and tmax was computed from blood draw time and date and medication time and date. Area under the plasma concentration–time curve from time 0 to the last quantifiable nonzero concentration (AUC0–t) was calculated by the linear trapezoidal method with linear interpolation. Area under the plasma concentration–time curve from time 0 extrapolated to infinity (AUC0–inf) was calculated as the sum of AUC0–t plus the ratio of the last quantifiable plasma concentration to the apparent first‐order terminal elimination rate constant (λz). λz was calculated by linear least‐squares regression analysis of the terminal log‐linear phase of the plasma concentration–time curve. The percentage of AUC0–inf extrapolated (AUC%extrap) was calculated as [1 – (AUC0–t/AUC0–inf)] × 100. Apparent first‐order terminal elimination half‐life (t½) was calculated as 0.693/λz. Apparent total plasma clearance after oral (extravascular) administration (CL/F) was calculated as Dose/AUC0–inf. Apparent volume of distribution during the terminal elimination phase after oral (extravascular) administration (Vλz/F) was calculated as Dose/(AUC0–inf x λz).
Sample collection and analytic methods
For quantification of neratinib plasma concentrations, 2‐ml venous blood samples were collected in blood collection tubes containing potassium ethylenediaminetetraacetic acid prior to neratinib dosing and at 0.5, 1, 2, 2.75, 3.5, 4.25, 5, 5.75, 6.5, 7.25, 8, 10, and 12 h (Day 1/Period 1 and Day 5/Period 2), 24 and 32 h (Day 2/Period 1 and Day 6/Period 2), 48 and 60 h (Day 3/Period 1 and Day 7/Period 2), and at 72 h (Day 4/Period 1 and Day 8/Period 2) following neratinib dosing. Collection tubes were centrifuged within 30 min of collection at approximately 5°C. Duplicate plasma aliquots were transferred into labelled storage tubes, and stored within 60 min of collection at approximately −70°C. Samples were shipped to Covance Bioanalytical Services LLC (Indianapolis, IN, USA) for analysis.
Neratinib and the internal standard (neratinib) were extracted from samples using protein precipitation and analysed using liquid chromatography with tandem mass spectrometric detection. The validated range of the assay was 3.00–250 ng ml−1. The presence of lansoprazole did not affect the quantification of neratinib, and blank samples spiked with lansoprazole did not contain any significant peaks at the retention time of neratinib or the internal standard (data not shown). For the low (9.00 ng ml−1), medium (45.0 ng ml−1) and high (200 ng ml−1) quality control samples, the interassay accuracy was 99.2, 100.2 and 98.5%, respectively, the interassay precision (expressed as relative standard deviation) was 6.7, 4.5 and 4.8%, respectively, and the corresponding biases were −0.8, 0.2 and −1.5%, respectively. All study samples (n = 568) were analysed within the known stability period (703 days) for storage at −60°C to −80°C.
Safety
Safety endpoints included adverse events, physical examinations, vital signs, 12‐lead ECGs, and clinical laboratory tests (i.e. haematology, coagulation/haemolysis, serum chemistry, and urinalysis). Safety was monitored throughout the study by repeated clinical and laboratory evaluations.
All adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA®), version 17.0. A treatment‐emergent adverse event was defined as an event that started or worsened at the time of, or after, study drug administration and up to 14 days after the last dose. For the purposes of reporting adverse events, Period 2 was separated to two parts: lansoprazole alone (i.e. postdose on Day 1 until predose neratinib on Day 5); and lansoprazole plus neratinib (i.e. postdose neratinib on Day 5 until end of study).
Statistical methods
Descriptive statistics were calculated for all pharmacokinetic parameters for neratinib with and without lansoprazole. To assess the effect of lansoprazole on the pharmacokinetics of neratinib, log‐transformed neratinib parameters (i.e. AUC0–t, AUC0–inf, and Cmax) were compared by performing an analysis of variance (anova), which included treatment as a fixed effect and subject as a random effect in each model. The inferential results from these models (i.e. least‐square means, differences between least‐square means and standard errors) were exponentiated to the original scale to obtain geometric least‐square means, geometric mean ratios (GMR), 90% confidence intervals (CIs), and intrasubject coefficient of variations. The GMR point estimates for AUC0–t, AUC0–inf, and Cmax for neratinib plus lansoprazole vs. neratinib were used to assess the magnitude of the drug–drug interaction if the 90% confidence intervals (CIs) were outside the 80.00–125.00% limits.
Allowing for a type I error of 5% with approximately 80% power, and assuming an intrasubject coefficient of variability for Cmax of 16% (as reported in study 3144 A1–1117‐US), and that the true GMR for Cmax was within the range of 95–105%, the estimated sample size required was 13 subjects. An additional two subjects were enrolled to allow for possible drop‐outs, giving a total study population of 15 subjects.
Analyses were performed with Phoenix® WinNonlin®, Version 6.3 and SAS®, Version 9.3.
Results
The study was conducted from 26th August 2014 to 13th October 2014. A total of 15 subjects were enrolled and completed the study; all 15 subjects were included in the pharmacokinetic analysis. Most subjects were Hispanic or Latino (73%) with a median age of 35 (range, 28–54) years and mean body mass index of 27.9 ± 4.1 kg m−2. A summary of baseline characteristics is provided in Table 1.
Table 1.
Demographic and baseline characteristics
| Characteristic | All subjects (n = 15) |
|---|---|
| Age (years) | |
| Median (range) | 35 (28–54) |
| Sex, n (%) | |
| Female | 9 (60) |
| Male | 6 (40) |
| Race, n (%) | |
| White | 14 (93) |
| Black | 1 (7) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 11 (73) |
| Other | 4 (27) |
| Weight (kg) | |
| Mean (SD) | 75.1 (12.6) |
| Height (cm) | |
| Mean (SD) | 164.0 (6.7) |
| Body mass index ( kg m −2 ) | |
| Mean (SD) | 27.9 (4.1) |
SD, standard deviation
Pharmacokinetics
Neratinib plasma concentration–time profiles following a single oral dose of neratinib 240 mg alone (Period 1) or following multiple oral doses of lansoprazole 30 mg (Period 2) are presented in Figure 2. The absorption of neratinib was markedly reduced when coadministered with lansoprazole compared with neratinib alone.
Figure 2.
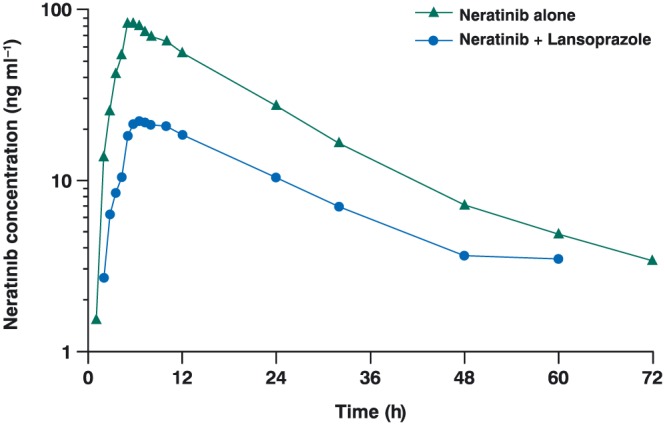
Mean neratinib plasma concentrations over time after administration of a single oral dose of neratinib 240 mg alone or with multiple oral doses of lansoprazole 30 mg in healthy subjects (n = 15)
On Day 5 of Period 2, the interval between lansoprazole and neratinib intake was approximately 35 min; lansoprazole was taken a mean of 5.1 (standard deviation 0.1) min prior to starting breakfast and neratinib was taken a mean of 29.9 (standard deviation 0.1) min after starting breakfast. No measurable plasma neratinib predose concentrations were observed for any subjects in Period 2, indicating that the washout period was adequate.
A descriptive summary of neratinib pharmacokinetic parameters is presented in Table 2. Mean neratinib Cmax was reduced from 84.5 ng ml−1 with neratinib alone to 24.5 ng ml−1 with neratinib plus lansoprazole, and median tmax was delayed by 1.5 h when neratinib was coadministered with lansoprazole. The extent of exposure to neratinib was also decreased following coadministration with lansoprazole; mean AUC0–t decreased from 1478 ng ml−1 h with neratinib to 426 ng ml−1 h with neratinib plus lansoprazole, and mean AUC0–inf decreased from 1557 ng ml−1 h with neratinib to 538 ng ml−1 h with neratinib plus lansoprazole. AUC%extrap was 5.06% with neratinib alone and 16.9% with neratinib plus lansoprazole. Both CL/F (167 vs. 483 L h−1, respectively) and VλZ/F (3333 vs. 9960 L, respectively) increased almost three‐fold when neratinib was administered with lansoprazole compared with neratinib alone. Mean plasma neratinib t½ was similar with both treatments (approximately 14 h).
Table 2.
Neratinib pharmacokinetic parameters in plasma
| Parameter | Neratinib (Period 1) (n = 15) | Neratinib + Lansoprazole (Period 2) (n = 15) d |
|---|---|---|
| C max ( ng ml −1 ) a | 84.5 (49%) | 24.5 (48%) |
| T max (h) b | 5.75 (3.51, 6.50) | 7.25 (5.00, 24.0) |
| AUC 0–t ( ng ml −1 h) a | 1478 (43%) | 426 (57%) |
| AUC 0–inf ( ng ml −1 h) a | 1557 (41%) | 538 (42%) |
| t ½ (h) c | 13.8 ± 2.2 | 13.9 ± 2.3 |
AUC0–inf, area under the plasma concentration–time curve from time 0 extrapolated to infinity; AUC0–t, area under the plasma concentration–time curve, from time 0 to the last measurable nonzero concentration; Cmax, peak plasma concentration; t½, apparent first‐order terminal elimination half‐life; Tmax, time to reach Cmax
Geometric mean (geometric coefficient of variation)
Median (range)
Arithmetic mean ± standard deviation
n = 14 for AUC0‐inf
The 90% CIs around the GMR for AUC0–t (22.68–36.65%), AUC0–inf (28.68–42.18%) and Cmax (22.17–37.87%) all fell outside of the 80.00–125.00% range (Table 3), indicating that multiple doses of lansoprazole may clinically impact the pharmacokinetics of a single dose of neratinib.
Table 3.
Statistical comparison of plasma neratinib pharmacokinetic parameters: neratinib + lansoprazole vs. neratinib
| Geometric LSM a | |||||
|---|---|---|---|---|---|
| Parameter | Neratinib + Lansoprazole (n = 15) b | Neratinib (n = 15) | Geometric mean ratio, c % | 90% confidence intervals | Residual variability, d % |
| C max ( ng ml −1 ) | 24.5 | 84.5 | 29.0 | 22.17–37.87 | 44 |
| AUC 0–t ( ng ml −1 h) | 426 | 1478 | 28.8 | 22.68–36.65 | 39 |
| AUC 0–inf ( ng ml −1 h) | 542 | 1557 | 34.8 | 28.68–42.18 | 30 |
AUC0–inf, area under the plasma concentration–time curve from time 0 extrapolated to infinity; AUC0–t, area under the plasma concentration–time curve, from time 0 to the last measurable nonzero concentration; Cmax, peak plasma concentration; LSM, least‐square mean
Parameters were log‐transformed prior to analysis
Geometric LSMs were calculated by exponentiating the LSMs from the anova
n = 14 for AUC0–inf
Percentage geometric mean ratio = 100 × (test/reference)
100 × √(e [residual var] – 1)
Safety
Fifteen subjects received at least one dose of study drug and were included in the safety analyses. Overall, nine treatment‐emergent adverse events were reported by a total of 5 (33%) subjects (Table 4). All events were mild in severity and all were considered to be drug‐related. The most frequently reported treatment‐emergent adverse events were diarrhoea and flatulence, experienced by three (20%) and two (13%) subjects, respectively, which were considered to be related to neratinib. All remaining adverse events were reported by one (7%) subject each; abdominal pain, fatigue and headache were considered to be related to neratinib, and dizziness was considered to be related to lansoprazole. All treatment‐emergent adverse events resolved without sequelae. There were no deaths, serious adverse events or discontinuations due to adverse events.
Table 4.
Treatment‐emergent adverse events
| Adverse events, n (%) | Neratinib a (n = 15) | Lansoprazole b (n = 15) | Neratinib + Lansoprazole c (n = 15) | Total (n = 15) |
|---|---|---|---|---|
| Subjects with adverse events | 4 (27) | 1 (7) | 2 (13) | 5 (33) |
| Diarrhoea | 1 (7) | 0 | 2 (13) | 3 (20) |
| Flatulence | 2 (13) | 0 | 0 | 2 (13) |
| Abdominal pain | 1 (7) | 0 | 0 | 1 (7) |
| Fatigue | 1 (7) | 0 | 0 | 1 (7) |
| Dizziness | 0 | 1 (7) | 0 | 1 (7) |
| Headache | 1 (7) | 0 | 0 | 1 (7) |
Note: all events were mild
Postdose on Day 1/Period 1 to predose on Day 1/Period 2
Postdose on Day 1/Period 2 to predose neratinib on Day 5/Period 2
Postdose neratinib on Day 5/Period 2 to end of study
Mean serum chemistry, haematology and urinalysis parameters were within the reference range at all study time‐points. Mean systolic and diastolic blood pressure readings, pulse rates and vital signs were also within normal limits at all study time‐points. All individual laboratory abnormalities and ECG abnormalities were not considered to be clinically significant.
Discussion
In the present study, we evaluated the potential effects of multiple doses of lansoprazole on the absorption and pharmacokinetics of a single dose of neratinib. Healthy adult subjects received oral doses of neratinib 240 mg with and without lansoprazole in a fixed‐sequence, two‐period study. As the inhibitory effects of lansoprazole on gastric pH reach a plateau after approximately 3 days of treatment 14, lansoprazole was initiated 4 days prior to neratinib and continued throughout the sampling period to ensure that maximal effects on gastric pH had been attained. A daily dose of lansoprazole 30 mg was selected for the study as it is the highest approved dose for the treatment of erosive esophagitis and gastric ulcers 15. Neratinib 240 mg is the established therapeutic dose of this drug 9.
We observed that mean peak exposure (Cmax) to neratinib was reduced by approximately 70% and Tmax was delayed by 1.5 h when neratinib was administered with lansoprazole compared with neratinib alone. The extent of neratinib exposure (AUC0–t and AUC0–inf) was also reduced by approximately 65–70% following administration of neratinib with lansoprazole compared with neratinib alone. The 90% CIs around the GMR for AUC0–t, AUC0–inf, and Cmax all fell outside of the 80.00–125.00% range, thus indicating that multiple doses of lansoprazole may clinically impact the pharmacokinetics of a single dose of neratinib.
These observations are not unique to neratinib. Many drugs are weakly basic, including antiretrovirals, triazole antifungal agents and tyrosine kinase inhibitors, and have the potential for pH‐dependent drug–drug interactions with acid‐reducing agents. Among 231 new molecular entities approved by the US Food and Drug Administration between 2003 and May 2013, dedicated drug–drug interactions studies with acid‐reducing agents were performed for 32 drugs, half of which showed reductions in drug exposure 13. The bioavailability of other HER‐directed tyrosine kinase inhibitors, specifically lapatinib and erlotinib, are also reduced in the presence of acid‐reducing agents 16, 17. Alterations in gastric pH can lead to a shift in the equilibrium between nonionized and ionized forms of weakly basic drugs and explain these interactions, resulting in decreased solubility and drug absorption 18. Other mechanisms may also be involved, including changes in gastric emptying rates, effects on uptake and efflux transporters and changes in metabolic enzymes in the intestine 13.
Treatment‐emergent adverse events observed during both periods of the study were infrequent and mild in severity. No unexpected adverse events were documented with either neratinib or lansoprazole, and there were no serious events or discontinuations because of adverse events. There were also no clinically important findings among the laboratory results, vital signs, ECG or physical examinations. Our findings suggest that a single oral dose of neratinib is safe and well tolerated when given in combination with lansoprazole 30 mg day–1.
Our study had many design strengths and incorporated many of the design considerations recommended by Zhang et al. 13. Lansoprazole, the proton‐pump inhibitor used in our study, does not have clinically significant interactions with other drugs metabolized by the cytochrome P450 system 15 so minimizing the potential for the confounding effects of metabolism‐based drug–drug interactions. We used a fixed‐sequence design to eliminate the risk of confounding carryover effects of residual gastric acid suppression by lansoprazole on the pharmacokinetics of neratinib. Lansoprazole was initiated 4 days before neratinib administration to ensure that steady‐state inhibition of gastric pH had been achieved. Lansoprazole was administered prior to food and neratinib was administered after food to optimize the systemic exposure to both drugs. The timing of drug intake was tightly regulated to ensure minimum variation between subjects.
It is likely that other acid‐reducing agents – H2‐receptor antagonists, antacids and other proton‐pump inhibitors – will also influence the pharmacokinetics of neratinib, although the magnitude of these effects are unknown. As H2‐antagonists and antacids have a shorter duration of action than proton‐pump inhibitors, the effects of these agents may be less than those observed in the present study. In clinical trials, neratinib is coadministered with loperamide prophylaxis for the first one or two cycles of treatment 19; the effect of loperamide together with a proton‐pump inhibitor on the pharmacokinetics of neratinib is also unknown. The implications of the effects observed in our study on the efficacy of neratinib in patients receiving concurrent proton‐pump inhibitors have yet to be investigated.
In conclusion, coadministration of neratinib and lansoprazole reduced the rate and extent of neratinib exposure when compared with neratinib alone in healthy subjects. The effects of other acid‐reducing agents – H2‐antagonists and antacids – on neratinib pharmacokinetics require further study.
Competing Interests
This work was supported by Puma Biotechnology Inc. Kiana Keyvanjah, Daniel DiPrimeo, Ai Li, and Alvin Wong are employees of Puma Biotechnology Inc. Mohammad Obaidi and Dennis Swearingen are employees of Celerion which was contracted by Puma Biotechnology Inc. to conduct this study. Puma Biotechnology Inc. funded the provision of editorial support provided by Lee Miller and Harriet Lamb of Miller Medical Communications.
Contributors
Acquisition, analysis or interpretation of data: All authors; Critical revision of the manuscript for important intellectual content: All authors; Final approval of the version to be published: All authors; Statistical analysis: A.L. and M.O.; Study supervision: A.W., K.K. and D.S.
Keyvanjah, K. , DiPrimeo, D. , Li, A. , Obaidi, M. , Swearingen, D. , and Wong, A. (2017) Pharmacokinetics of neratinib during coadministration with lansoprazole in healthy subjects. Br J Clin Pharmacol, 83: 554–561. doi: 10.1111/bcp.13132.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 2015; 172: 5980–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabindran SK. Antitumor activity of HER‐2 inhibitors. Cancer Lett 2005; 227: 9–23. [DOI] [PubMed] [Google Scholar]
- 5. Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI‐272, an orally active, irreversible inhibitor of the HER‐2 tyrosine kinase. Cancer Res 2004; 64: 3958–3965. [DOI] [PubMed] [Google Scholar]
- 6. Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab‐based adjuvant therapy in patients with HER2‐positive breast cancer (ExteNET): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2016; 17: 367–377. [DOI] [PubMed] [Google Scholar]
- 7. Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs. trastuzumab plus paclitaxel in previously untreated metastatic HER2‐positive breast cancer: the NEfERT‐T randomized clinical trial. JAMA Oncol 2016. doi:10.1001/jamaoncol.2016.0237. [DOI] [PubMed] [Google Scholar]
- 8. Hyman DM, Piha‐Paul SA, Rodón J, Saura C, Puzanov I, Arteaga C, et al Neratinib for ERBB2 mutant, HER2 non‐amplified, metastatic breast cancer: preliminary analysis from a multicenter, open‐label, multi‐histology phase II basket trial. 38th Annual San Antonio Breast Cancer Symposium 8–12 December 2015, poster PD5–05.
- 9. Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, et al. A phase I study with neratinib (HKI‐272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 2009; 15: 2552–2558. [DOI] [PubMed] [Google Scholar]
- 10. Abbas R, Hug BA, Leister C, Sonnichsen D. A double‐blind, randomized, multiple‐dose, parallel‐group study to characterize the occurrence of diarrhea following two different dosing regimens of neratinib, an irreversible pan‐ErbB receptor tyrosine kinase inhibitor. Cancer Chemother Pharmacol 2012; 70: 191–199. [DOI] [PubMed] [Google Scholar]
- 11. Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, et al. Prevalence of acid‐reducing agents (ARA) in cancer populations and ARA drug–drug interaction potential for molecular targeted agents in clinical development. Mol Pharm 2013; 10: 4055–4062. [DOI] [PubMed] [Google Scholar]
- 12. Hochman J, Tang C, Prueksaritanont T. Drug–drug interactions related to altered absorption and plasma protein binding: theoretical and regulatory considerations, and an industry perspective. J Pharm Sci 2015; 104: 916–929. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH‐dependent drug–drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther 2014; 96: 266–277. [DOI] [PubMed] [Google Scholar]
- 14. Bell NJ, Hunt RH. Time to maximum effect of lansoprazole on gastric pH in normal male volunteers. Aliment Pharmacol Ther 1996; 10: 897–904. [DOI] [PubMed] [Google Scholar]
- 15. Takeda Pharmaceuticals America, Inc . PREVACID (lansoprazole) delayed‐release capsules, PREVACID SoluTab (lansoprazole) delayed‐release orally disintegrating tablets. Revised September 2012. Available at http://general.takedapharm.com/content/file.aspx?filetypecode=PREVACIDPI&cacheRandomizer=95b4680a‐7f48‐40f4‐8a45‐3c58a8ce78dc (last accessed 2 May 2016).
- 16. Kletzl H, Giraudon M, Ducray PS, Abt M, Hamilton M, Lum BL. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs 2015; 26: 565–572. [DOI] [PubMed] [Google Scholar]
- 17. Koch KM, Im YH, Kim SB, Urruticoechea Ribate A, Stephenson J, Botbyl J, et al. Effects of esomeprazole on the pharmacokinetics of lapatinib in breast cancer patients. Clin Pharmacol Drug Dev 2013; 2: 336–341. [DOI] [PubMed] [Google Scholar]
- 18. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug–drug interactions with tyrosine‐kinase inhibitors: a clinical perspective. Lancet Oncol 2014; 15: e315–e326. [DOI] [PubMed] [Google Scholar]
- 19. Ustaris F, Saura C, Di Palma J, Bryce R, Moran S, Neuman L, et al. Effective management and prevention of neratinib‐induced diarrhea. Am J Hematol/Oncol 2015; 11: 13–22. [Google Scholar]


