Abstract
Aims
The aim of this systematic review was to assess the efficacy of bisphosphonate therapy as an adjunct to scaling and root planing (SRP) in the management of periodontitis.
Methods
Databases (MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and Cochrane Oral Health Group Trials Register databases) were searched up to and including July 2016. The primary outcome was probing depth (PD), and the secondary outcomes were changes in clinical attachment level (CAL) and bone defect (BD) fill. The mean differences (MD) of outcomes and 95% confidence intervals (CI) for each variable were calculated using random effect model.
Results
Eight clinical studies were included. Seven studies used alendronate as an adjunct to SRP; of these, four studies used topical application and three used oral alendronate. Considering the effects of adjunctive bisphosphonates as compared to SRP alone, a high degree of heterogeneity for PD (Q value = 39.6, P < 0.0001, I2 = 87.38%), CAL (Q value = 13.65, P = 0.008, I2 = 70.71%), and BD fill (Q value = 53.26, P < 0.0001, I2 = 92.49%) was noticed among both the groups. Meta‐analysis showed a statistically significant PD reduction (MD = –1.18, 95% CI = –1.91 to –0.44, P = 0.002), CAL gain (MD = –0.69, 95% CI = –1.20 to –0.18, P = 0.008) and BD fill (MD = –2.36, 95% CI = –3.64 to –1.08, P < 0.001) for SRP + bisphosphonate treatment vs. SRP alone.
Conclusions
Adjunctive bisphosphonate therapy appears to be effective in managing periodontitis, however, due to the potential risk of osteonecrosis of the jaws and short‐term follow‐up of the studies, their clinical application is debatable.
Keywords: Bisphosphonates, scaling and root planning, periodontal disease, systematic review
What is Already Known about this Subject
The influence of bisphosphonates on bone turnover and their ability to inhibit periodontal tissue related proinflammatory cytokines have introduced their use as an adjunct to scaling and root planing in the management of periodontal disease.
What this Study Adds
Delivery of bisphosphonates as an adjunct to scaling and root planing in the management of periodontal disease appears to be effective in the short‐term; however, due to the potential risk of osteonecrosis of the jaws, their long‐term clinical application remains debatable.
Introduction
Reducing bacterial load and inhibiting progression of inflammation are the primary therapeutic goals in the management of periodontal disease 1. Nonsurgical debridement in the form of scaling and root planing (SRP) has been regarded as the traditional treatment regime in the management of periodontal disease. The purpose of SRP is to remove local irritants such as calculus from the surfaces of the teeth and to minimize tooth surface roughness that may facilitate the accumulation of local irritants around the teeth 2. However, manual debridement has physical limitations as it does not allow proper access to debride in deep periodontal pockets, furcations and interproximal areas of malposed teeth 3.
Numerous adjunctive treatments have been proposed to complement SRP, including systemic and localized delivery of antibiotics, antiseptics, lasers, and photodynamic therapy to reduce bacterial counts and improve periodontal parameters in periodontal disease 4, 5, 6, 7, 8. Among these compounds, bisphosphonates have recently been introduced for the treatment of periodontal disease. Bisphosphonates are analogues of pyrophosphate, with high affinity for bone tissue. They inhibit osteoclastic bone activity during high bone turnover, resulting in inhibition of bone resorption 9. Bisphosphonates are leading drugs for the treatment of metabolic bone diseases such as osteoporosis and Paget's disease 10, and they are widely used in the treatment of tumour‐associated osteolysis 11. Research suggests that bisphosphonates not only inhibit osteoclast‐mediated bone resorption, but also induce osteoblast cells to promote early bone formation 12. Furthermore, bisphosphonates may antagonize the action of several matrix metalloproteinase (MMPs) involved in breakdown of structural components of periodontal connective tissue 13, 14. Thus, their action in bone turnover and their ability to inhibit cytokines of periodontal tissue destruction has introduced bisphosphonates as an adjunct to SRP in the management of periodontitis 15.
Recent evidence has shown the benefits of using bisphosphonates as an adjunct to SRP with regards to clinical periodontal outcomes 16, 17. For instance, in a randomized clinical trial (RCT) by Pradeep et al. 16, periodontitis patients treated with local application of alendronate gel as an adjunct to SRP showed significant improvement in clinical periodontal parameters as compared to those treated with SRP alone. Similar results were reported by Sharma and Pradeep 17. Results from these studies 16, 17 suggest that local bisphosphonate delivery is a potential treatment strategy for periodontitis. However, Graziani et al. 18, in a clinical trial evaluating the adjunctive role of bisphosphonates in the periodontal treatment, concluded that periodontitis patients treated with adjunctive bisphosphonate did not show any improvement in clinical outcomes over the use of SRP at follow‐up. Considering the diversity of these results, a systematic review to assess the efficacy of adjunctive bisphosphonate delivery in the management of periodontitis seems desirable. Therefore, the aim of this study was to review systematically the efficacy of bisphosphonate therapy as an adjunct to SRP in the management of periodontitis.
Materials and methods
Based on the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) guidelines 19, a specific question was constructed. The addressed focused question was: “Does bisphosphonates as an adjunct to SRP yield better clinical periodontal outcomes than SRP alone in the treatment of periodontal disease?”
Search strategy
Electronic and manual literature searches were conducted by two independent reviewers (Z.A. and T.A.) in the following databases, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and Cochrane Oral Health Group Trials Register, up to and including July 2016 for articles addressing the focused question. For the PubMed library, combinations of the following MeSH (Medical Subject Headings) and free text words were used: (diphosphonates [MeSH Terms]) OR (alendronate [MeSH Terms]) OR (risedronate sodium [MeSH Terms]) OR (bisphosphonates) OR (neridronic acid) AND (periodontitis [MeSH Terms]) OR (chronic periodontitis [MeSH Terms]) OR (periodontal diseases [MeSH Terms]) OR (periodontal pocket [MeSH Terms]) OR (periodontal attachment loss [MeSH Terms]) OR (tooth mobility [MeSH Terms]) OR (bleeding on probing [MeSH Terms]) AND (dental scaling [MeSH Terms]) OR (root planing [MeSH Terms]) OR (dental prophylaxis [MeSH Terms]).
Selection criteria
Screening and assessment of articles was conducted independently by two reviewers (Z.A. and F.V.). Any disagreement involving the eligibility was resolved through discussion or by consulting a third reviewer (T.A.). Studies, which did not fulfil the inclusion criteria were excluded.
The following eligibility criteria were entailed:
Study design: Only RCTs or randomized split‐mouth clinical trials (a split‐mouth trial is a study design in which anatomical regions of subjects' mouths are divided into homogeneous within‐patient experimental units. Subsequently, each of the treatment modalities is randomly assigned to one within‐patient experimental unit);
Participants: patients diagnosed with periodontitis having at least 10 subjects per group;
Intervention: subjects allocated to experimental and control/placebo group based on having SRP with adjunctive bisphosphonate therapy or SRP alone;
Outcome: reduction in ‘probing depth’ (PD; in mm), which is defined as the distance from the gingival margin to the base of the pocket; ‘clinical attachment level’ (CAL in mm), which is the measurement of the position of the soft tissue in relation to cemento‐enamel junction, and ‘bone defect’ (BD in mm), which is osseous defects in alveolar bone, were included as outcomes;
Follow‐up: the outcome assessment at minimum of 24 weeks
Articles published only in English language.
In‐vitro studies, case series, case reports, animal studies, letters to the editor, opinion articles, abstract, review papers and unpublished articles were excluded.
Screening and selection
Two reviewers (Z.A. and T.A.) independently screened titles and abstracts for eligible papers. If information relevant to the eligibility criteria was not available in the abstract, or if the title was relevant but the abstract was not available, the paper was selected for full reading of the text. Next, full‐text papers that fulfilled the eligibility criteria were identified and included in the review. Reference lists of original studies were hand searched to identify articles that could have been missed during the electronic search. Hand searching of the following journals was performed: Journal of Clinical Periodontology, Journal of Periodontology and Journal of Periodontal Research. Studies that fulfilled the selection criteria were processed for data extraction. Figure 1 describes the screening process according to PRISMA guidelines 19.
Figure 1.
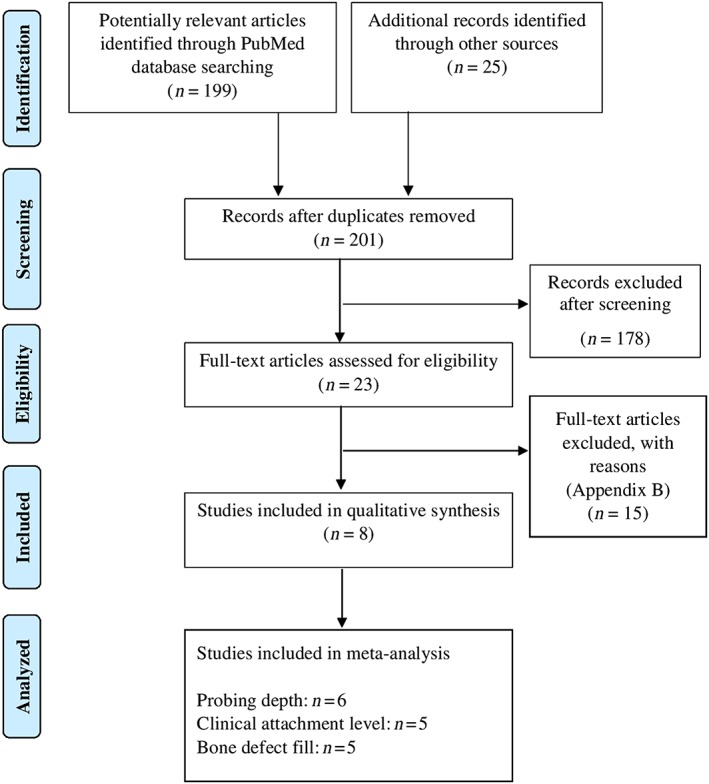
PRISMA (Preferred Reporting Items for Systematic Review and Meta‐Analysis) flow diagram for studies retrieved through the searching and selection process
Data extraction and quality assessment
Two reviewers (Z.A. and F.V.) performed the data extraction independently. The information from the accepted studies was tabulated according to the study designs, subject demographics, drop outs, sex distribution, drug administration, follow‐up period, main outcomes and clinical periodontal parameters. Data collected were based on the focused question outlined for the present systematic review. The reviewers crosschecked all extracted data. Any disagreement was resolved by discussion until consensus was reached. The present systematic review was conducted using a pre‐submission checklist based on the revised recommendations of the Consolidated Standards of Reporting Trials statement 20. The risk of bias was estimated for each selected RCT based on the Cochrane Handbook for Systematic Reviews of Interventions 21: (1) low risk of bias (when all criteria were met); (2) high risk of bias (when ≥1 criterion was not met); and (3) unclear (when ≥1 criterion was partially met). The criteria used are listed in Appendix A.
Outcome measures
In the present review, the primary outcome was the reduction of PD, in mm. Secondary outcomes were changes in CAL in mm and BD fill in mm. All outcome variables to be analysed were measured at 6 months and 12 months of follow‐up.
Statistical analysis
Meta‐analyses were conducted separately for each of the primary (PD), and secondary outcomes (CAL, BD fill). Heterogeneity among the included studies for each outcome was assessed using the Q‐statistic and I2 statistic 22. Outcome measures for each periodontal parameter were combined with a random‐effects model utilizing the DerSimonian–Laird method due to its robustness in comparison to fixed‐effects models in the case of small sample sizes 23. Forest plots were computed reporting mean difference (MD) of outcomes and 95% confidence intervals (CI). The pooled effect was considered significant if P < 0.05. Data unsuitable for quantitative analysis were assessed descriptively. All above statistical analyses were carried out by a specialized statistical software (MedCalc Software v 15.11.04, Ostend, Belgium).
Results
Study selection
A total of 224 study titles and abstracts were initially identified. After removal of the duplicates (n = 23), initial screening of titles and abstracts was performed, and 178 articles were excluded as irrelevant to the PICO question. A total of 23 papers were selected for full‐text reading. Of these 23 studies, 15 studies were further excluded (Appendix B). After the final stage of selection, 8 studies 16, 17, 18, 24, 25, 26, 27, 28 were included and processed for data extraction (Table 1). All studies 16, 17, 18, 24, 25, 26, 27, 28 were performed at either universities or health care centres. Figure 1 shows the study identification flow chart according to PRISMA 16 with the reasons for exclusion of articles.
Table 1.
General characteristics of included studies
| Investigators; Year; Study design | Sample size; Mean age in years (range) | Number of drop outs | Female (%) | Systemic conditions | Study groups (n) | Drug used (dosage); Route of administration | Follow‐up (weeks) | Study outcome |
|---|---|---|---|---|---|---|---|---|
| Pradeep et al. 16 2013; RCT | 69; Group 1: NA Group 2: NA (30–50) | Group 1: 6 Group 2: 6 | 46.3 | NA | Group 1: 29 SRP + BP Group 2: 28 placebo + SRP | Group 1: 1% ALN gel (10 mg/mL); topical Group2: placebo gel | Up to 48 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
| Sharma and Pradeep 17 2012; RCT | 73; Group 1: NA Group 2: NA (30–50) | Group 1: 5 Group 2: 2 | 46.5 | NA | Group 1: 33 SRP + BP Group 2: 33 placebo + SRP | Group 1: 1% ALN gel (10 mg/mL); topical Group2: placebo gel | Up to 24 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
| Sharma and Pradeep 24 2012; RCT | 20; Group 1: NA Group 2: NA (30–50) | Group 1: 1 Group 2: 2 | 40 | NA | Group 1: 9 SRP + BP Group 2: 8 placebo + SRP | Group 1: 1% ALN gel (10 mg/mL); topical Group2: placebo gel | Up to 24 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
| Pradeep et al. 25 2012; RCT | 43; Group 1: NA Group 2: NA (30–50) | Group 1: 1 Group 2: 1 | 46.5 | Diabetes mellitus | Group 1: 20 SRP + BP Group 2: 21 placebo + SRP | Group 1: 1% ALN gel (10 mg/mL); topical Group2: placebo gel | Up to 24 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
| Graziani et al. 18 2009; RCT | 60; Group 1: 44.7 Group 2: 42.2 (NA) | Group 1: 5 Group 2: 4 | 65 | NA | Group 1: 30 SRP + BP Group 2: 30 SRP | Group 1: Once weekly NE (12.5 mg/intramuscular) / 12 times; systemic | Up to 24 | Improvements in clinical parameters for both groups at follow‐up were comparable |
| Lane et al. 26 2005; RCT | 70; Group 1: 48.2 Group 2: 46.8 (NA) | Group 1: 2 Group 2: 2 | 43.9 | NA | Group 1: 41 SRP + BP Group 2: 25 placebo + SRP | Group 1: ALN (10 mg/day) or RSD 5 mg/day + calcium citrate 1000 mg/day + Vitamin D3; oral Group 2: placebo + calcium citrate 1000 mg/day + Vitamin D3/oral | Up to 48 | Improvements in clinical parameters for both groups at follow‐up were comparable |
| Rocha et al. 27 2004; RCT | 40; Group 1: 57.8 Group 2: 58.0 (55–65) | Group 1: 0 Group 2: 0 | 100 | Obese/overweight; menopause | Group 1: 20 SRP + BP Group 2: 20 placebo + SRP | Group 1: ALN (10 mg/day); oral | Up to 24 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
| Rocha et al. 28 2001; RCT | 40; Group 1: 56.0 Group 2: 55.0 (50–60) | Group 1: 0 Group 2: 0 | 50 | Diabetes mellitus; menopause | Group 1: 20 SRP + BP Group 2: 20 placebo + SRP | Group 1: ALN (10 mg/day); oral Group 2: trivitamin preparation (thiamin 100 mg + pyridoxine 50 mg + cyanocobalamin 250 g) | Up to 24 | Group 1 showed significantly greater improvements in clinical parameters at follow‐up |
RCT, randomized clinical trial; SRP, scaling and root planing; BP, bisphosphonates; ALN, alendronate; RSD, risedronate; NE, neridronic acid; NA, not available.
General characteristics of included studies
All studies 16, 17, 18, 24, 25, 26, 27, 28 included in the systematic review were RCTs. In all studies 16, 17, 18, 24, 25, 26, 27, 28, number of subjects ranged between 20 and 73 individuals with mean age ranging between 42.2 and 57.8 years. All studies 16, 17, 18, 24, 25, 26, 27, 28 reported the percentage of female participants, which ranged between 43.9% and 100%. Smokers were included in two studies 18, 26. Periodontal diseases were classified according to PD ≥3 mm in two studies 27, 28 and ≥5 mm in five studies 16, 17, 18, 24, 25, with CAL ≥3 mm in one study 26, ≥4 mm in two studies 17, 25, and ≥5 mm in one study 24. In all studies 16, 17, 18, 24, 25, 26, 27, 28 subjects in the test group received bisphosphonates + SRP. In seven studies 16, 17, 24, 25, 26, 27, 28 control group used SRP with placebo gel, while in one study 18, SRP alone was the treatment. Seven studies 16, 17, 24, 25, 26, 27, 28 used alendronate (10 mg/day), of which four studies 16, 17, 24, 25 used topical application while three studies 26, 27, 28 used oral alendronate. In all the studies 16, 17, 24, 25 that utilized local bisphosphonates, the topical gel was injected into the periodontal pocket using a syringe with a blunt cannula. The topical gel was applied only once in these studies 16, 17, 24, 25. Only one study 18 used intramuscular neridronic acid (12.5 mg) as an adjunct to SRP for treatment of periodontitis. Two studies recruited patients with diabetes mellitus 25, 28 and menopausal females 27, 28, while one study 27 recruited obese/overweight patients. In all studies 16, 17, 18, 24, 25, 26, 27, 28 the follow‐up period ranged from 24–48 weeks (Table 1). All the enrolled participants had complication‐free healing period with no side effects related to bisphosphonates except two studies (with periodontal abscess in three subjects combined) 18, 26.
Quality of the clinical studies
All the included clinical studies 16, 17, 18, 24, 25, 26, 27, 28 in this systematic review were RCTs. Seven RCTs did not estimate the sample size 16, 17, 24, 25, 26, 27, 28. The masking of assessor(s) and methods of allocation concealment was inadequate in three studies 26, 27, 28. All studies 16, 17, 18, 24, 25, 26, 27, 28 presented appropriate statistical analysis and description of withdrawals and dropouts. The risk of bias was considered low in one study 18 and high in the other RCTs assessed 16, 17, 24, 25, 26, 27, 28 (Table 3).
Table 3.
Evaluation of bias risk in the included studies
| Investigators | Sample size calculation | Allocation concealment | Randomization | Losses (withdrawals/dropouts) | Masking of assessor(s) | Appropriate statistical analysis | Estimated risk of bias |
|---|---|---|---|---|---|---|---|
| Pradeep et al. 16 | 0 | 2 | 2 | 1 | 2 | 2 | High |
| Sharma and Pradeep 17 | 0 | 2 | 2 | 1 | 2 | 2 | High |
| Sharma and Pradeep 24 | 0 | 2 | 2 | 1 | 2 | 2 | High |
| Pradeep et al. 25 | 0 | 2 | 2 | 1 | 2 | 2 | High |
| Graziani et al. 18 | 2 | 2 | 2 | 1 | 2 | 2 | Low |
| Lane et al. 26 | 0 | 0 | 2 | 1 | 1 | 2 | High |
| Rocha et al. 27 | 0 | 0 | 2 | 1 | 1 | 2 | High |
| Rocha et al. 28 | 0 | 0 | 2 | 1 | 1 | 2 | High |
Periodontal inflammatory parameters of included studies
The results for clinical periodontal (PD and CAL) and BD fill outcomes are summarized in Table 2. All the studies 16, 17, 18, 24, 25, 26, 27, 28 reported mean PD and CAL, which ranged from 2.8 to 3.96 mm and 2.03 to 5.35 mm, respectively. Six studies 16, 17, 24, 25, 27, 28 reported values of BD fill in mm which ranged from 2.64 to 5.34 mm at follow‐up.
Table 2.
Clinical periodontal outcomes of the included studies
| Investigators; Year | Probing depth (mm) mean ± SD | Clinical attachment level (mm) mean ± SD | Bone defect fill (mm) mean ± SD | |||
|---|---|---|---|---|---|---|
| Pradeep et al. 16 2013 | Group 1:a | Group 2: | Group 1:a | Group 2: | Group 1:a | Group 2: |
| Baseline: 6.93 ± 0.69 | Baseline: 6.77 ± 0.82 | Baseline: 8.07 ± 0.64 | Baseline: 8.03 ± 0.81 | Baseline: 3.94 ± 0.24 | Baseline: 3.94 ± 0.25 | |
| Follow‐up: 3.14 ± 0.71 | Follow‐up: 5.39 ± 0.79 | Follow‐up: 5.00 ± 0.54 | Follow‐up: 7.03 ± 0.79 | Follow‐up: 2.64 ± 0.23 | Follow‐up: 3.84 ± 0.24 | |
| Sharma and Pradeep 17 2012 | Group 1:a | Group 2: | Group 1:a | Group 2: | Group 1:a | Group 2: |
| Baseline: 7.58 ± 2.13 | Baseline: 7.24 ± 2.18 | Baseline: 6.06 ± 1.82 | Baseline: 5.64 ± 1.72 | Baseline: 4.70 ± 1.00 | Baseline: 4.71 ± 1.04 | |
| Follow‐up: 3.09 ± 1.82 | Follow‐up: 5.09 ± 1.54 | Follow‐up: 2.03 ± 1.48 | Follow‐up: 4.03 ± 2.02 | Follow‐up: 2.82 ± 0.87 | Follow‐up: 4.60 ± 1.06 | |
| Sharma and Pradeep 24 2012 | Group 1:a | Group 2: | Group 1:a | Group 2: | Group 1:a | Group 2: |
| Baseline: 7.85 ± 2.20 | Baseline: 7.69 ± 2.22 | Baseline: 6.12 ± 1.77 | Baseline: 5.96 ± 1.88 | Baseline: 5.45 ± 1.18 | Baseline: 5.48 ± 0.92 | |
| Follow‐up: 3.96 ± 1.28 | Follow‐up: 6.04 ± 1.68 | Follow‐up: 2.85 ± 1.82 | Follow‐up: 4.54 ± 2.12 | Follow‐up: 2.95 ± 0.86 | Follow‐up: 5.37 ± 0.90 | |
| Pradeep et al. 25 2012 | Group 1:a | Group 2: | Group 1:a | Group 2: | Group 1:a | Group 2: |
| Baseline: 8.41 ± 2.41 | Baseline: 8.31 ± 1.80 | Baseline: 6.24 ± 1.65 | Baseline: 6.53 ± 1.87 | Baseline: 5.40 ± 1.19 | Baseline: 5.36 ± 1.34 | |
| Follow‐up: 3.85 ± 1.89 | Follow‐up: 5.94 ± 1.77 | Follow‐up: 2.65 ± 1.45 | Follow‐up: 4.92 ± 1.93 | Follow‐up: 2.97 ± 0.69 | Follow‐up: 5.22 ± 1.35 | |
| Graziani et al. 18 2009 | Group 1: (mean with interquartile range) | Group 2: (mean with interquartile range) | Group 1: (mean with interquartile range) | Group 2: (mean with interquartile range) | Not available | Not available |
| Baseline: 3.4 (3.2,3.7) | Baseline: 3.5 (3.2,3.7) | Baseline: 4.2 (3.9,4.6) | Baseline: 4.1 (3.6,4.6) | |||
| Change from baseline: 0.7 (0.8,0.9) | Change from baseline: 0.7 (0.5,0.9) | Change from baseline: 0.5 (0.2,0.8) | Change from baseline: 0.6 (0.3,0.9) | |||
| Lane et al. 26 2005 | Group 1:a | Group 2: | Group 1:a | Group 2: | Not available | Not available |
| Baseline: 4.20 ± 1.48 | Baseline: 4.13 ± 1.22 | Baseline: 3.27 ± 1.63 | Baseline: 3.37 ± 1.42 | |||
| Follow‐up: 3.40 ± 0.97 | Follow‐up: 3.68 ± 1.25 | Follow‐up: 2.24 ± 1.29 | Follow‐up: 2.76 ± 1.40 | |||
| Rocha et al. 27 2004 | Group 1:a | Group 2: | Group 1: | Group 2: | Group 1:a | Group 2: |
| Baseline: 3.2 ± 0.6 | Baseline: 3.1 ± 0.4 | Baseline: 4.3 ± 1.0 | Baseline: 4.5 ± 1.0 | Baseline: 3.1 ± 1.4 | Baseline: 3.5 ± 1.7 | |
| Change from baseline: –0.8 ± 0.3 | Change from baseline: –0.4 ± 0.4 | Change from baseline: –0.99 ± 0.8 | Change from baseline: –0.5 ± 0.8 | Change from baseline: –0.4 ± 0.4 | Change from baseline: 0.6 ± 0.5 | |
| Rocha et al. 28 2001 | Group 1: | Group 2: | Group 1: | Group 2: | Group 1:a | Group 2: |
| Baseline: 4.1 ± 1.0 | Baseline: 3.9 ± 0.9 | Baseline: 6.66 ± 1.9 | Baseline: 6.0 ± 2.1 | Baseline: 6.2 ± 2.7 | Baseline: 6.4 ± 2.9 | |
| Follow‐up: 2.8 ± 0.6 | Follow‐up: 3.1 ± 0.8 | Follow‐up: 5.35 ± 1.5 | Follow‐up: 5.2 ± 1.9 | Follow‐up: 5.37 ± 2.3 | Follow‐up: 6.8 ± 2.7 | |
Group 1: Bisphosphonates + scaling and root planing
Group 2: Placebo + scaling and root planing
Significantly different from the other group
Main outcome of the studies
Qualitative analysis
All studies 16, 17, 18, 24, 25, 26, 27, 28 showed that SRP and adjunctive bisphosphonates was effective in the treatment of periodontitis at follow‐up. In six studies 16, 17, 24, 25, 27, 28, periodontal inflammatory parameters (PD, CAL and BD fill) showed significantly higher improvements in test group (bisphosphonate + SRP) as compared to control group (SRP + placebo/SRP alone). However, in two studies 18, 26, improvement in the periodontal parameters for bisphosphonate delivery as an adjunct to SRP and SRP alone were comparable among periodontitis patients. Six studies 16, 17, 24, 25, 27, 28 showed improved BD fill in test group (SRP + bisphosphonate) as compared to control group (SRP + placebo/SRP alone) at follow‐up.
Quantitative analysis
For quantitative data assessment, a meta‐analysis was performed. As significant heterogeneity was observed for PD reduction, CAL gain and BD fill, the random model was employed.
Probing depth
Six studies 16, 17, 24, 25, 26, 28 presented data to be included in the meta‐analysis considering the effects of adjunctive bisphosphonate delivery on PD. Considering the effects of adjunctive bisphosphonates as compared to SRP alone on PD, a high degree of heterogeneity for PD (Q value = 39.6, P < 0.0001, I2 = 87.38%, Figure 2) was noticed among the groups. Significant statistical differences in PD reduction (MD = –1.18, 95% CI = –1.91 to –0.44, P = 0.002) were observed at follow‐up between the test and control groups.
Figure 2.
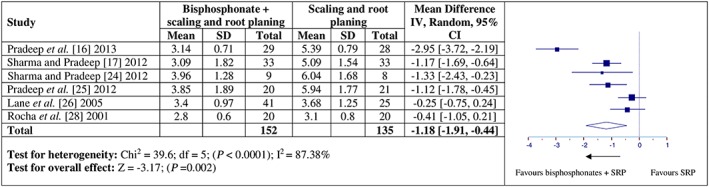
Forest plot presenting post‐therapy probing depth reduction by comparing adjunctive bisphosphonate therapy vs. scaling and root planing (SRP)
Clinical attachment level
Five studies were included in the meta‐analysis for the effect of adjunctive bisphosphonate on CAL 17, 24, 25, 26, 28. Considering the effects of adjunctive bisphosphonates as compared to SRP alone on CAL, a high degree of heterogeneity for CAL (Q value = 13.65, P = 0.008, I2 = 70.71%, Figure 3) was noticed among both the groups. The overall mean difference for CAL gain between adjunctive bisphosphonate and SRP alone groups were significant (CAL: MD = –0.69, 95% CI = –1.20 to –0.18, p = 0.008) at follow‐up.
Figure 3.
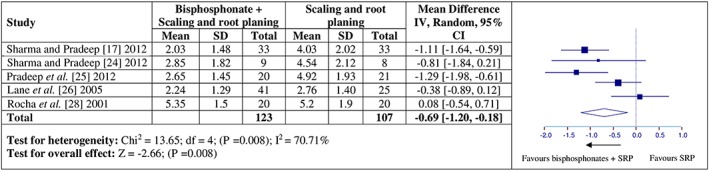
Forest plots presenting post‐therapy clinical attachment level gain by comparing adjunctive bisphosphonate therapy vs. scaling and root planing (SRP)
Bone defect fill
Five studies were included in the meta‐analysis for the effect of adjunctive bisphosphonate on BD fill 16, 17, 24, 25, 28. Similar to PD and CAL, a high degree of heterogeneity for BD fill (Q value = 53.26, P < 0.0001, I2 = 92.49%, Figure 4) was also noticed among both the groups. The overall mean difference for BD fill between adjunctive bisphosphonate and SRP alone groups were significant (BD: MD = –2.36, 95% CI = –3.64 to –1.08, p < 0.001) at follow‐up.
Figure 4.
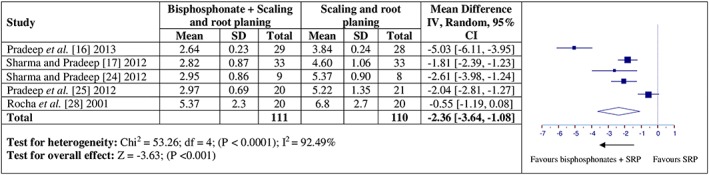
Forest plots presenting post‐therapy bone defect fill by comparing adjunctive bisphosphonate therapy vs. scaling and root planing (SRP)
Grazianin et al. 18 reported mean with interquartile range; Rocha et al. 27 reported change from baseline; while Pradeep et al. 16 did not report mean CAL. Hence these studies were excluded from quantitative synthesis. Two studies 18, 26 did not report BD fill in their findings. The latter authors were contacted in order to collect missing data, but no response was received.
Discussion
The present systematic review was based on the hypothesis that bisphosphonate delivery as an adjunct to SRP improves clinical periodontal parameters and enhances bone formation in periodontal disease conditions. Seventy‐five percent of the studies 16, 17, 24, 25, 27, 28 in the present systematic review supported the aforementioned hypothesis. The efficacy of adjunctive bisphosphonates in improving periodontal parameters may be explained by their role in modulating alveolar bone. Delivery of bisphosphonates in periodontal disease inhibits bone resorption through decrease in osteoclast recruitment and increase in osteoblast differentiation with concomitant reduction in PD and CAL gain 29, 30.
From the studies reviewed, adjunctive bisphosphonates in periodontal disease appear to be effective in improving periodontal inflammatory parameters but several factors such as dosage of drug, frequency of application and route of administration need to be taken into consideration. It is noteworthy that bisphosphonate dosage, frequency of delivery, route of administration and patient follow‐up varied among the included studies 16, 17, 18, 24, 25, 26, 27, 28. For example, in the studies by Sharma and Pradeep 17 and Pradeep et al. 16, local alendronate at a dosage of 10 mg/mL was delivered once throughout the study period, whereas Rocha et al. 27 and Grazaini et al. 18 administered 10 mg of oral alendronate daily for 6 months and 12.5 mg of intramuscular neridronic acid once a week for 12 weeks respectively throughout the study period. Although these clinical studies reported oral and systemic bisphosphonate delivery to enhance bone formation, a precise dosage and frequency of the drug that would yield the most favourable clinical outcome remains unclear. It can be inferred from the results of the metaanalysis that maybe by increasing the dosage and frequency of bisphosphonates administration, the changes in clinical periodontal parameters may have showed good response.
It is well‐recognized that patients who receive bisphosphonate may develop osteonecrosis of the jaw bone (ONJ) as one of the long‐term side effects of bisphosphonate therapy 31, 32 and duration of bisphosphonate therapy is a significant factor associated with an increased likelihood of ONJ 33. In the studies included 16, 17, 18, 24, 25, 26, 27, 28, which evaluated changes in clinical and radiographic parameters of periodontal disease, the follow‐up period ranged from 24–48 weeks. Outcomes based on such short‐term observations are debatable and therefore further long‐term clinical trials should be performed to assess the possible occurrence of ONJ from adjunctive bisphosphonate therapy in periodontal disease. In addition, there are other periodontal therapeutic strategies used as adjunct to SRP such as local antibiotics 34, laser therapy 7, 35, enamel matrix derivative and bone grafts 36, which have fewer side effects. Therefore, such interventions should be compared with local bisphosphonate therapy as an adjunct to SRP to prove their efficacy in improving clinical periodontal outcomes.
Another fact that is worthy of note is that in four studies 18, 26, 27, 28, included in the present review, systemic bisphosphonates as an adjunct to SRP in the management of chronic periodontitis was compared to SRP alone. In two studies 18, 26, delivery of systemic bisphosphonates showed comparable clinical periodontal parameters between test and control groups. This in contrast to the studies 16, 17, 24, 25 that used local bisphosphonates showing significant improvement in the clinical periodontal outcomes as compared to SRP. A possible explanation in this regard may lie in the ability of local drug delivery system to allow high drug concentrations and have controlled long‐term release of the therapeutic agents at target sites 37. Moreover, the results of a study on adult cynomolgus monkeys have indicated that effects of bisphosphonates without SRP on periodontitis showed no change in PD 38. In the present systematic review, the significant change in PD reduction (MD = –1.18, 95% CI = –1.91 to –0.44, p = 0.002) may be explained by the mechanical debridement (SRP) performed in study groups to arrest inflammatory disease process, highlighting the critical role of SRP in the management of periodontal disease.
It is well‐known that osteoporosis, besides having a direct association with oestrogen deficiency 39, is also related to chronic hyperglycaemia. Research reports, that persistent hyperglycaemia leads to increased formation of advanced glycation end products in periodontal tissues 40. In addition, advanced glycation end products tend to jeopardize the normal function of osteoblasts and augment inflammatory response in periodontal tissue 41. It is pertinent to mention that subjects with menopause/osteoporosis, diabetes mellitus and obesity were included in the studies reviewed 25, 27, 28. Therefore, the inflammatory burden and compromised immune response due to the systemic diseases 42, 43, 44, could possibly have altered the outcomes in the studies included 16, 17, 18, 24, 25, 26, 27, 28. Therefore, further RCTs with strict inclusion and exclusion criteria should be undertaken to assess the efficacy of adjunctive bisphosphonates in the management of periodontitis.
Findings from the study show that clinical and radiographic parameters of periodontal disease appear to improve by bisphosphonate therapy both qualitatively and quantitatively in the short term but certain heterogeneities (such as dosage, frequency, and route of administration along with patient follow‐up) among the included studies cannot be overruled. This indicates that although bisphosphonates are effective, but clinically it should be used with caution since there is risk of development of ONJ due to systemic and long‐term use of bisphosphonates 33. Therefore, in light of current evidence, to benefit from the antiresorptive effects of bisphosphonates, the authors suggest their localized use as adjunct to SRP rather than systemic administration. Moreover, to assess the risk of ONJ and the role of bisphosphonate therapy as an adjunct to SRP in the management of periodontal disease, further RCTs with standardized dosage and frequency of local drug application, strict inclusion and exclusion criteria and long‐term follow‐up are warranted.
Conclusion
The available evidence suggests that adjunctive bisphosphonate therapy appears to be effective in improving clinical outcomes of periodontitis in the short term. However, due to the potential risk of ONJ and short‐term follow‐up period of the studies, the clinical application of adjunctive bisphosphonate delivery in the management of periodontitis patients is debatable.
Competing Interest
The authors declare that they have no conflict of interest and all authors have read and approved the final draft.
The authors extend their sincere appreciations to Deanship of Scientific Research at King Saud University for its funding this prolific research group (PRG‐1437‐38).
Contributors
Z.A. initiated the idea, researched and authored the topic background, compiled the initial draft, revised late drafts, data analysis and edited the final draft. F.V. provided guidance throughout, reviewed the manuscript, extracted the data, edited the text and helped with data analysis. T. A. and M.I.A.H contributed to the initial conception, were involved in the initial draft, revised late drafts and edited the article. F.J. and S.V.K. provided guidance, contributed to the text, extracted the data, and revised and edited the article.
Akram, Z. , Abduljabbar, T. , Kellesarian, S. V. , Abu Hassan, M. I. , Javed, F. , and Vohra, F. (2017) Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br J Clin Pharmacol, 83: 444–454. doi: 10.1111/bcp.13147.
References
- 1. Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000 2006; 42: 180–218. [DOI] [PubMed] [Google Scholar]
- 2. Waerhaug J. Effect of rough surfaces upon gingival tissues. J Dent Res 1956; 35: 323–325. [DOI] [PubMed] [Google Scholar]
- 3. Cobb CM. Clinical significance of non‐surgical periodontal therapy: an evidence‐based perspective of scaling and root planing. J Clin Periodontol 2002; 29: 22–32. [PubMed] [Google Scholar]
- 4. Vohra F, Akram Z, Safii SH, Vaithilingam RD, Ghanem A, Sergis K, et al. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagnosis Photodyn Ther 2015; 13: 139–147. [DOI] [PubMed] [Google Scholar]
- 5. Akram Z, Al‐Shareef SA, Daood U, Asiri FY, Shah AH, AlQahtani MA, et al. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomed Laser Surg 2016; 34: 137–149. [DOI] [PubMed] [Google Scholar]
- 6. Akram Z, Abduljabbar T, Sauro S, Daood U. Effect of photodynamic therapy and laser alone as adjunct to scaling and root planing on gingival crevicular fluid inflammatory proteins in periodontal disease: a systematic review. Photodiagnosis Photodyn Ther 2016. doi:10.1016/j.pdpdt.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 7. Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci 2016. doi:10.1007/s10103-016-2086-5. [DOI] [PubMed] [Google Scholar]
- 8. Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000 2016; 71: 82–112. [DOI] [PubMed] [Google Scholar]
- 9. Russell R, Watts N, Ebetino F, Rogers M. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008; 19: 733–759. [DOI] [PubMed] [Google Scholar]
- 10. Giger EV, Castagner B, Leroux JC. Biomedical applications of bisphosphonates. J Control Release 2013; 167: 175–188. [DOI] [PubMed] [Google Scholar]
- 11. Dunstan CR, Felsenberg D, Seibel MJ. Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor‐induced bone disease. Nat Clin Pract Oncol 2007; 4: 42–55. [DOI] [PubMed] [Google Scholar]
- 12. Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep 2003; 1: 45–52. [DOI] [PubMed] [Google Scholar]
- 13. Özdemir SP, Kurtis B, Tüter G, Bozkurt S, Gültekin SE, Sengüven B, et al. Effects of low‐dose doxycycline and bisphosphonate clodronate on alveolar bone loss and gingival levels of matrix metalloproteinase‐9 and interleukin‐1β in rats with diabetes: a histomorphometric and immunohistochemical study. J Periodontol 2012; 83: 1172–1182. [DOI] [PubMed] [Google Scholar]
- 14. Sapna G, Gokul S, Bagri‐Manjrekar K. Matrix metalloproteinases and periodontal diseases. Oral Dis 2014; 20: 538–550. [DOI] [PubMed] [Google Scholar]
- 15. Giannobile WV. Host‐response therapeutics for periodontal diseases. J Periodontol 2008; 79: 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pradeep A, Kumari M, Rao NS, Naik SB. 1% alendronate gel as local drug delivery in the treatment of class II furcation defects: a randomized controlled clinical trial. J Periodontol 2013; 84: 307–315. [DOI] [PubMed] [Google Scholar]
- 17. Sharma A, Pradeep A. Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial. J Periodontol 2012; 83: 11–18. [DOI] [PubMed] [Google Scholar]
- 18. Graziani F, Cei S, Guerrero A, La Ferla F, Vano M, Tonetti M, et al. Lack of short‐term adjunctive effect of systemic neridronate in non‐surgical periodontal therapy of advanced generalized chronic periodontitis: an open label‐randomized clinical trial. J Clin Periodontol 2009; 36: 419–427. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63: e1–37. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available at: www.cochrane‐handbook.org (last accessed March 2016).
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 24. Sharma A, Pradeep A. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol 2012; 83: 19–26. [DOI] [PubMed] [Google Scholar]
- 25. Pradeep A, Sharma A, Rao NS, Bajaj P, Naik SB, Kumari M. Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double‐masked controlled clinical trial. J Periodontol 2012; 83: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 26. Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12‐month, randomized, placebo‐controlled study. J Periodontol 2005; 76: 1113–1122. [DOI] [PubMed] [Google Scholar]
- 27. Rocha ML, Malacara JM, Sánchez‐Marin FJ, de la Torre CJV, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo‐controlled trial. J Periodontol 2004; 75: 1579–1585. [DOI] [PubMed] [Google Scholar]
- 28. Rocha M, Nava LE, de la Torre CV, Sánchez‐Marín F, Garay‐Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo‐controlled trial. J Periodontol 2001; 72: 204–209. [DOI] [PubMed] [Google Scholar]
- 29. Hughes D, MacDonald B, Russell R, Gowen M. Inhibition of osteoclast‐like cell formation by bisphosphonates in long‐term cultures of human bone marrow. J Clin Investig 1989; 83: 1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vitte C, Fleisch H, Guenther HL. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast‐mediated resorption. Endocrinol 1996; 137: 2324–2333. [DOI] [PubMed] [Google Scholar]
- 31. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate‐induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005; 63: 1567–1575. [DOI] [PubMed] [Google Scholar]
- 32. Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc 2005; 136: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 33. Estilo CL, Van Poznak CH, Wiliams T, Bohle GC, Lwin PT, Zhou Q, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist 2008; 13: 911–920. [DOI] [PubMed] [Google Scholar]
- 34. Matesanz‐Pérez P, García‐Gargallo M, Figuero E, Bascones‐Martínez A, Sanz M, Herrera D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol 2013; 40: 227–241. [DOI] [PubMed] [Google Scholar]
- 35. Campanile VSM, Giannopoulou C, Campanile G, Cancela JA, Mombelli A. Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: clinical, microbiological, and local biological effects. Lasers Med Sci 2015; 30: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koop R, Merheb J, Quirynen M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol 2012; 83: 707–720. [DOI] [PubMed] [Google Scholar]
- 37. Greenstein G. Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results. J Periodontol 2006; 77: 565–578. [DOI] [PubMed] [Google Scholar]
- 38. Brunsvold MA, Chaves ES, Kornman KS, Aufdemorte TB, Wood R. Effects of a bisphosphonate on experimental periodontitis in monkeys. J Periodontol 1992; 63: 825–830. [DOI] [PubMed] [Google Scholar]
- 39. Brennan M, Gleeson J, O'Brien F, McNamara L. Effects of ageing, prolonged estrogen deficiency and zoledronate on bone tissue mineral distribution. J Mech Behav Biomed Mater 2014; 29: 161–170. [DOI] [PubMed] [Google Scholar]
- 40. Katz J, Bhattacharyya I, Farkhondeh‐Kish F, Perez F, Caudle R, Heft M. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT‐PCR. J Clin Periodontol 2005; 32: 40–44. [DOI] [PubMed] [Google Scholar]
- 41. Zizzi A, Tirabassi G, Aspriello S, Piemontese M, Rubini C, Lucarini G. Gingival advanced glycation end‐products in diabetes mellitus‐associated chronic periodontitis: an immunohistochemical study. J Periodontal Res 2013; 48: 293–301. [DOI] [PubMed] [Google Scholar]
- 42. Akram Z, Abduljabbar T, Abu‐Hassan MI, Javed F, Vohra F. Cytokine profile in chronic periodontitis patients with and without obesity: a systematic review and meta‐analysis. Dis Markers 2016. doi:10.1155/2016/4801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akram Z, Safii SH, Vaithilingam RD, Baharuddin NA, Javed F, Vohra F. Efficacy of non‐surgical periodontal therapy in the management of chronic periodontitis among obese and non‐obese patients: a systematic review and meta‐analysis. Clin Oral Investig 2016; 20: 903–914. [DOI] [PubMed] [Google Scholar]
- 44. Akram Z, Rahim ZH, Taiyeb‐Ali TB, Shahdan MS, Baharuddin NA, Vaithilingam RD, et al. Resistin as potential biomarker for chronic periodontitis: a systematic review and meta‐analysis. Arch Oral Biol 2016. doi:10.1016/j.archoralbio.2016.08.016. [DOI] [PubMed] [Google Scholar]


