Abstract
Aims
Spironolactone is widely used to treat heart failure, hypertension and liver disease with increased usage in recent years. Spironolactone has endocrine effects that could influence cancer risks and historical reports suggest possible links with increased risk of certain types of cancer. The aim of this study was to assess the effect of spironolactone exposure on cancer incidence.
Methods
A pharmacoepidemiological propensity score‐matched cohort study was performed to assess the effect of spironolactone exposure on cancer incidence. Cox proportional hazards models were used to analyse time to first diagnosis of each prespecified cancer and hazard ratios for spironolactone exposure are presented. The setting for the study was UK primary care using the Clinical Practice Research Datalink. The participants were 74 272 patients exposed to spironolactone between 1986 and 2013, matched 1:2 with unexposed controls. The prespecified primary outcomes were the first incidence of ovarian, endometrial, pancreatic, colorectal, prostate, renal cell, pharyngeal and thyroid cancers, and myelomonoblastic/‐cytic leukaemias. Secondary outcomes were the remaining 27 types of cancer.
Results
There was no evidence of an increased risk of any cancer associated with spironolactone use. Spironolactone use was associated with a significantly lower risk of prostate cancer (hazard ratio 0.69; 95% confidence interval 0.60–0.80, P < 0.001).
Conclusions
In this study, spironolactone use was associated with a lower incidence of prostate cancer, the most common cancer in men in the UK. The possible mechanisms and clinical implications merit further investigation.
Keywords: cancer, cohort study, pharmacoepidemiology, prostate, spironolactone
What is Already Known about this Subject
The use of spironolactone has increased in recent years particularly for the treatment of hypertension and heart failure.
Spironolactone has hormonal effects that could potentially influence the incidence of certain types of cancer.
Historical reports have associated spironolactone use with increased risk of certain cancers.
What this Study Adds
Spironolactone exposure was not associated with an increased incidence of cancers.
Our study suggests that spironolactone use may be associated with a lower incidence of prostate cancer (the most common cancer in men).
Further studies are needed to investigate the mechanisms involved and clinical implications of these findings.
Introduction
Spironolactone is an aldosterone antagonist commonly used to treat liver disease and heart failure. Its use in heart failure and hypertension has increased greatly in recent years 1, 2. Although spironolactone has a licence from the Medicines and Healthcare Products Regulatory Agency for other indications, spironolactone is not currently approved for the indication of hypertension in the UK. This approval was withdrawn in 1988 after animal studies suggested an increase in myelomonocytic leukaemia with potassium canrenoate, a similar compound that, like spironolactone, is metabolised to canrenone. The British National Formulary entry for spironolactone carries the caution, ‘potential metabolic products carcinogenic in rodents’ 3. The 2012 International Agency for Research on Cancer monograph for spironolactone summarises the data on possible associations of spironolactone with different cancers and concludes that ‘there is inadequate evidence in humans for the carcinogenicity of spironolactone’ while ‘there is limited evidence in experimental animals for the carcinogenicity of spironolactone’. Overall, it concludes that ‘spironolactone is not classifiable as to its carcinogenicity to humans (Group 3)’ 4.
The metabolism of spironolactone is complicated and not well understood 5. In addition to inhibiting the mineralocorticoid receptor, spironolactone, either directly or via its active metabolites, also has antiandrogenic and progestogenic actions. Therefore, it is plausible that it might increase the risk of hormonally dependent cancers including breast, ovarian, endometrial, pancreatic and colorectal cancers. Spironolactone could potentially either increase or decrease the risk of prostate cancer due to a complex combination of antagonist and partial agonist effects on androgen receptors, possible 5α‐reductase inhibition effects and effects on testosterone metabolism 6, 7.
Previous studies have suggested possible associations of spironolactone use with certain cancers in humans including pharyngeal 8, thyroid 9 and renal cancers 10, 11, 12, 13, 14. However, these have involved very small numbers of cases, or have been confounded due to difficulty in separating spironolactone use from the effects of other drugs or hypertension on renal cancer. There have also been reports of possible associations with breast cancer in some studies 15, 16, 17, 18, 19, 20, 21 but in a large cohort study, we found no association with breast cancer 22. This was confirmed in another recent study that investigated the relationship between spironolactone use and risk of breast and gynaecological cancers 23. Spironolactone, first marketed in 1953 24, is now extensively used. It is therefore important to clarify any association with cancers of all types.
Methods
Primary and secondary objectives
The primary objective was to test the hypothesis that spironolactone exposure influences the development of nine prespecified cancers, either because of biologically plausible mechanisms or because previous observational or animal studies had suggested an association (Table 1). The secondary objective was to screen 27 other types of cancer for an effect of spironolactone exposure.
Table 1.
Prespecified primary outcomes
| Prespecified primary outcomes a | Reasons for prespecifying as primary outcomes |
|---|---|
|
Ovarian cancer
Endometrial cancer
Pancreatic cancer Colorectal cancer |
A higher incidence might be expected on the basis of biologically plausible hormonal effects of spironolactone. |
| Prostate cancer | A higher or lower incidence might be expected on the basis of biologically plausible hormonal effects of spironolactone. |
|
Renal cell cancer
Pharyngeal cancer
Thyroid cancer Myelomonoblastic/myelomonocytic leukaemia |
A higher incidence might be expected on the basis of previously reported associations in other observational or animal studies. |
First incidence during the follow‐up period of each type of cancer
Study design
The study used a cohort design in the UK Clinical Practice Research Datalink (CPRD) database to compare the incidence of cancers in patients exposed and unexposed to spironolactone.
Study population
The study population consisted of patients who were registered with a general practitioner in the CPRD between 1986 and February 2013. CPRD (formerly known as the General Practice Research Database) 25 is a longitudinal primary care database containing details of patients' demographics, medical diagnoses, referrals to consultants and hospitals, and primary care prescriptions from a representative sample of primary care practices in the UK covering about 7% of the UK population. Hospital Episode Statistics (HES) data are available for a subset of CPRD practices (57%), allowing linkage with hospital discharge diagnostic data 26.
Ethical approval
The study protocol was reviewed and approved by the Independent Scientific Advisory Committee of the CPRD.
Follow‐up period
Enrolment started when ‘up‐to‐standard’ data were available for a practice, or when a patient registered after this date. The ‘up‐to‐standard’ date is defined as the date from which the practice data met various quality control criteria set by CPRD. This ensured that we only used data that were considered to be ‘up‐to‐standard’ as defined by CPRD. The last follow‐up date was defined as the practice's last data collection date, or the patient's transfer out date from the practice (includes transfer out due to death) if earlier. In sensitivity analyses we also used a patient's last contact date (last visit to practice, proving that the patient was still contributing to that practice's data) as their last follow‐up date.
Study subjects
Spironolactone cohort
We identified patients who received at least two prescriptions for spironolactone during the study period. These patients formed the spironolactone exposed cohort. We defined an index date for each patient as the date of the second prescription for spironolactone. The first prescription date was not used to avoid introducing immortal time bias. If a patient's index date was at least 1 year after their enrolment date, we classified them as an incident user. Others were classified as prevalent users.
The exposure of each patient was characterised by mean dose and the proportion of days for which drug was prescribed between first and last exposure. Mean dose was calculated as the weighted mean of the prescribed doses, taking account of the days of supply provided by each prescription. The first 180 days following the estimated last exposure date was classified as exposed follow‐up in the statistical models to allow for a lag time in the diagnosis of new cases (Figure 1).
Figure 1.
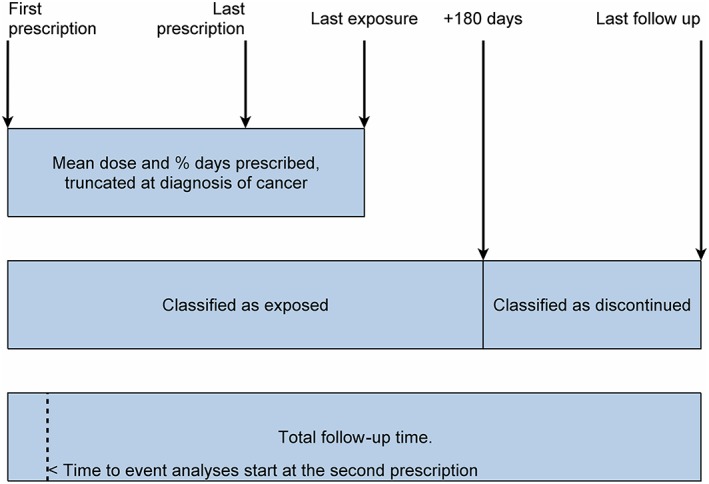
Classification of follow‐up time in exposed patients. Summary of classification of follow‐up time in the spironolactone exposed patients
Comparator cohorts
We constructed a comparator cohort by matching patients on a propensity score, namely the probability of exposure to spironolactone estimated from a logistic regression model. The predictors in this model were age, calendar year of entry to study, use of combined oral contraceptive pills or hormone replacement therapy, smoking status, alcohol intake, body mass index (BMI), family history of cancer, use of drugs that may be protective against cancer (aspirin, metformin, digoxin and angiotensin‐converting‐enzyme inhibitors) and history of hypertension, heart failure, liver disease or diabetes mellitus. The Townsend score for socioeconomic status was available in 57% of practices and was included in the estimation of propensity scores in practices that provided it. If a patient's tobacco consumption, alcohol intake or body mass index was not recorded we classified it as such to ensure that patients with missing information were equally represented in the exposed and control cohorts.
Time‐dependent covariates (age, history of exposure and history of disease) were evaluated on each patient's index date, and in order to do this for potential control patients we assigned index dates randomly by incidence density sampling from the distribution of dates in the exposed cohort. Patients were excluded if they had a history of any cancer or ascites on their index date.
For each exposed patient, two control patients were selected at random from the same practice, matched on deciles of the propensity score and sex (to ensure that the cohorts were well matched in the analyses of sex‐specific cancers).
Study outcomes
Outcomes were defined as the first incidence of cancer during the follow‐up period, classified as one of 36 types (see online [Link]Appendix for code lists). We specified nine of these as primary outcomes in advance: ovarian, endometrial, pancreatic and colorectal cancers (a higher incidence may be expected on the basis of biologically plausible hormonal effects of spironolactone); prostate cancer (biologically plausible hormonal effects may lead to either a higher or lower incidence); renal cell cancer, pharyngeal cancer, thyroid cancer and myelomonoblastic/myelomonocytic leukaemia (previously reported associations in other observational or animal studies suggest a higher incidence). Breast cancer was not specified as a primary outcome in this study because we had previously completed a detailed study in CPRD that showed no association between spironolactone use and breast cancer incidence in women aged over 55 years 22.
Myelomonoblastic/‐cytic leukaemia was treated as a primary outcome despite occurring in only 440 patients in the database, 12% below the arbitrary threshold of 500 specified in the protocol for re‐designating it as a secondary outcome.
The remaining 27 types of cancer were regarded as secondary outcomes. Detailed information on cancer staging was not available in CPRD.
Statistical analysis
The time from index date to first diagnosis of each cancer was analysed using a proportional hazards model. Calendar year and age were treated as time dependent variables. For the exposed cohort, we also included a time dependent flag variable indicating discontinuation of spironolactone for >180 days. All other covariates were evaluated on the index date.
Hazard ratios were estimated for the exposed and discontinued periods in the spironolactone cohort relative to the control cohort, with exposed time in incident and prevalent users classified separately in sensitivity analyses. All the covariates used to estimate the propensity scores were also used in these models. Z‐scores (the estimated hazard ratios divided by their standard errors) were calculated for all 36 types of cancer. Under the null hypothesis that the incidence of all cancers is unaffected by exposure to spironolactone, these scores would have a standard normal distribution. A Q‐Q plot was produced to compare quantiles of the observed distribution with those expected under the null hypothesis.
Confounding by indication may be severe in the analysis of liver cancer because spironolactone is indicated for some its symptoms, but at a higher dose than is generally prescribed for heart failure. Therefore, for liver cancer only, patients who were prescribed >50 mg/day of spironolactone, and their control patients, were excluded from the analyses.
Sensitivity and subgroup analyses
We examined the sensitivity of our results for the primary outcomes to the definition of the follow‐up period, and to the inclusion of HES data. In the primary analyses patients were followed‐up until their practice's last data collection date, unless they transferred out of the practice or died at an earlier date. In one set of sensitivity analyses, follow‐up was censored at the date of last contact with each patient (excluding administrative records). In another, we included outcomes identified from HES as well as the CPRD, but restricted the analyses to the 273 practices with linked HES data (available for April 1997 to March 2012).
Results
We found 74 883 patients with at least two prescriptions for spironolactone and no prior diagnosis of cancer or ascites recorded in the CPRD. The exposed cohort in this study consisted of the 74 272 (99%) of these patients for whom we found at least one matched control. Spironolactone exposure is summarised in Table 2. We classified 52 671 patients (71%) as incident users. Their median follow‐up time was 11.3 years (9.6 years exposed and 1.0 years after discontinuation). The median follow‐up time was 3.1 years for prevalent users. The mean dose was higher in prevalent users (47 mg vs. 25 mg), and the proportion of days for which drug was prescribed between first and last exposure was high in both groups.
Table 2.
Follow‐up time and characteristics of spironolactone exposure
| Median (interquartile range) | |||
|---|---|---|---|
| Overall | Incident users | Prevalent users | |
| Subjects | n = 74 272 | n = 52 671 | n = 21 601 |
| Total follow‐up time (years) | 9.2 (4.2,13.8) | 11.3 (7.1,15.3) | 3.1 (1.1,7.2) |
| Exposed follow‐up time (years) | 6.9 (2.4,12.2) | 9.6 (5.5,13.7) | 1.0 (0.2,3.0) |
| Time after discontinuation (years) | 1.3 (0.2,4.2) | 1.0 (0.2,3.4) | 2.2 (0.3,6.8) |
| Mean daily dose (mg) | 25 (25,50) | 25 (25,50) | 47 (25,75) |
| Coverage (%) | 98 (79,100) | 99 (81,100) | 96 (74,100) |
The median follow‐up time in the control cohort was 11.5 years (interquartile range 6.9–15.5). Baseline characteristics of the control and exposed cohorts are summarised in Table 3. There was a good balance at baseline for most potential risk factors. There was an imbalance in the prevalence of heart failure and liver disease, for which spironolactone may be indicated, and the use of digoxin and angiotensin‐converting‐enzyme inhibitors, also used to treat heart failure, despite matching on a propensity score that included them. Events per 100 000 patient years for the 36 types of cancer defined in this study are given in Table 4. Liver cancer events were higher in the exposed than control cohorts as might be expected due to confounding by indication, but the bias was reduced when we excluded patients on >50 mg/day spironolactone.
Table 3.
Distribution of covariates in the control and exposed cohorts
| Control cohort | Exposed cohort | |||
|---|---|---|---|---|
| n | % | n | % | |
| Total | 147 953 | 100.0 | 74 272 | 100.0 |
| Sex | ||||
| Male | 67 441 | 45.6 | 33 865 | 45.6 |
| Female | 80 512 | 54.4 | 40 407 | 54.4 |
| Age group | ||||
| Under 40 years | 9796 | 6.6 | 4635 | 6.2 |
| 40–49 years | 8994 | 6.1 | 4602 | 6.2 |
| 50–59 years | 16 574 | 11.2 | 8065 | 10.9 |
| 60–69 years | 28 142 | 19.0 | 13 887 | 18.7 |
| 70–79 years | 39 622 | 26.8 | 20 219 | 27.2 |
| 80–89 years | 36 087 | 24.4 | 18 403 | 24.8 |
| ≥90 years | 8738 | 5.9 | 4461 | 6.0 |
| Year of index date | ||||
| 1987–1995 | 13 760 | 9.3 | 6917 | 9.3 |
| 1996–2000 | 19 999 | 13.5 | 10 037 | 13.5 |
| 2001–2005 | 50 809 | 34.3 | 25 508 | 34.3 |
| 2006–2010 | 46 108 | 31.2 | 23 143 | 31.2 |
| 2011–2013 | 17 277 | 11.7 | 8667 | 11.7 |
| History of drug use | ||||
| Oral contraceptives a | 4692 | 3.2 | 2368 | 3.2 |
| Hormone replacement therapy a | 12 942 | 8.7 | 6332 | 8.5 |
| Metformin | 15 068 | 10.2 | 8932 | 12.0 |
| Aspirin | 65 507 | 44.3 | 36 310 | 48.9 |
| Digoxin | 14 381 | 9.7 | 16 781 | 22.6 |
| ACE inhibitors | 69 678 | 47.1 | 41 083 | 55.3 |
| Family history of cancer | 2629 | 1.8 | 1269 | 1.7 |
| Medical history | ||||
| Hypertension | 75 674 | 51.1 | 36 634 | 49.3 |
| Heart failure | 15 546 | 10.5 | 25 552 | 34.4 |
| Liver disease | 3412 | 2.3 | 4773 | 6.4 |
| Diabetes mellitus | 24 957 | 16.9 | 14 457 | 19.5 |
| Abdominal pain | 26 881 | 18.2 | 14 233 | 19.2 |
| BMI 25–30 kg m −2 | 82 386 | 55.7 | 42 121 | 56.7 |
| BMI > 30 kg m −2 | 44 071 | 29.8 | 23 452 | 31.6 |
| > 21 units alcohol/week | 12 086 | 8.2 | 6323 | 8.5 |
| Never smoked | 61 781 | 41.8 | 31 012 | 41.8 |
| > 20 cigarettes/day | 7357 | 5.0 | 3798 | 5.1 |
| 1 | 16 685 | 11.3 | 7998 | 10.8 |
| 2 | 18 496 | 12.5 | 9199 | 12.4 |
| 3 | 18 565 | 12.5 | 9342 | 12.6 |
| 4 | 17 705 | 12.0 | 8909 | 12.0 |
| 5 | 11 835 | 8.0 | 6119 | 8.2 |
| Not available | 64 667 | 43.7 | 32 705 | 44.0 |
Number and percentage of women
ACE = angiotensin‐converting enzyme; BMI = body mass index
Table 4.
Cases and incidence (events per 100 000 patient–years) for 36 cancer types in the exposed and control cohorts
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposed | Control | Exposed | Control | |||||
| Patient–years follow‐up (10 5 ) | 3.005 | 5.444 | 3.510 | 6.324 | ||||
| n | phtpy | n | phtpy | n | phtpy | n | phtpy | |
|---|---|---|---|---|---|---|---|---|
| Primary outcomes | ||||||||
| Prostate | 450 | 138 | 1424 | 179 | N/A | |||
| Colorectal | 264 | 81 | 647 | 81 | 242 | 63 | 587 | 64 |
| Endometrial | N/A | 69 | 18 | 157 | 17 | |||
| Ovarian | N/A | 64 | 17 | 145 | 16 | |||
| Pancreatic | 42 | 13 | 115 | 14 | 68 | 18 | 136 | 15 |
| Renal | 26 | 8 | 56 | 7 | 25 | 6 | 29 | 3 |
| MML | 14 | 4 | 35 | 4 | 13 | 3 | 22 | 2 |
| Pharyngeal | 8 | 2 | 21 | 3 | 5 | 1 | 9 | 1 |
| Thyroid | 4 | 1 | 5 | 1 | 6 | 2 | 21 | 2 |
| Secondary outcomes | ||||||||
| Minor skin cancers | 655 | 201 | 1635 | 205 | 629 | 163 | 1512 | 166 |
| Breast | N/A | 504 | 131 | 1103 | 121 | |||
| Lung | 314 | 96 | 701 | 88 | 231 | 60 | 485 | 53 |
| Bladder | 132 | 40 | 385 | 48 | 43 | 11 | 137 | 15 |
| Carcinoma in situ | 130 | 40 | 297 | 37 | 305 | 79 | 627 | 69 |
| Oesophagus | 89 | 27 | 185 | 23 | 42 | 11 | 117 | 13 |
| Squamous cell carcinoma of skin | 66 | 20 | 144 | 18 | 42 | 11 | 105 | 12 |
| Stomach | 62 | 19 | 141 | 18 | 38 | 10 | 99 | 11 |
| Leukemia | 37 | 11 | 114 | 14 | 28 | 7 | 62 | 7 |
| Melanoma of skin | 43 | 13 | 103 | 13 | 44 | 11 | 113 | 12 |
| Non Hodgkin lymphoma | 50 | 15 | 99 | 12 | 58 | 15 | 102 | 11 |
| Other malignant neoplasm of skin | 44 | 13 | 84 | 11 | 44 | 11 | 85 | 9 |
| Myeloma | 32 | 10 | 68 | 9 | 25 | 6 | 55 | 6 |
| Mesothelioma | 17 | 5 | 66 | 8 | 4 | 1 | 8 | 1 |
| Other lymphatic and haemopoietic | 28 | 9 | 62 | 8 | 32 | 8 | 71 | 8 |
| Lip and oral cavity | 29 | 9 | 61 | 8 | 18 | 5 | 41 | 5 |
| Other respiratory organs | 19 | 6 | 61 | 8 | 13 | 3 | 18 | 2 |
| Other genitourinary organs | 15 | 5 | 58 | 7 | 38 | 10 | 76 | 8 |
| Other cancers | 31 | 9 | 58 | 7 | 24 | 6 | 59 | 6 |
| Liver | 99 | 30 | 54 | 7 | 38 | 10 | 21 | 2 |
| Liver a | 35 | 14 | 41 | 7 | 8 | 3 | 15 | 3 |
| Kidney | 30 | 9 | 54 | 7 | 18 | 5 | 32 | 4 |
| Other digestive organs | 22 | 7 | 48 | 6 | 28 | 7 | 63 | 7 |
| Nervous system | 13 | 4 | 39 | 5 | 8 | 2 | 31 | 3 |
| Other bone and connective tissue | 11 | 3 | 17 | 2 | 13 | 3 | 27 | 3 |
| Anus | 5 | 2 | 8 | 1 | 3 | 1 | 15 | 2 |
| Hodgkins lymphoma | 5 | 2 | 7 | 1 | 3 | 1 | 11 | 1 |
| Melanoma in situ | 4 | 1 | 4 | 1 | 4 | 1 | 3 | 0 |
N/A Not applicable; MML, myelomonoblastic/myelomonocytic leukaemia’ phtpy, per 100 000 patient–years. We did not analyse a very small number of breast cancer cases in men.
Excluding patients exposed to >50 mg/day spironolactone and their controls. Events per 100 000 patient–years are rounded to nearest whole number
Z‐scores for the hazard ratios for each type of cancer in three sets of sensitivity analyses are shown in Figure 2. In all three analyses, the z‐score for prostate cancer was negative and highly significant. In the primary analysis, which followed patients to their practice's last data collection date, spironolactone use was associated with a significantly lower risk of prostate cancer (hazard ratio 0.69; 95% confidence interval 0.60–0.80, P < 0.001). In the primary analysis, the hazard ratio for lung cancer was also nominally significant, and in the sensitivity analysis that censored patients at last contact, the hazard ratio for pancreatic cancer was nominally significant. There is a 30% chance of at least one of the remaining 35 tests being significant at P < 0.01 under the null hypothesis that spironolactone has no effect. The hazard ratios relative to controls for each primary outcome during periods of exposure to spironolactone are given in Table 5.
Figure 2.
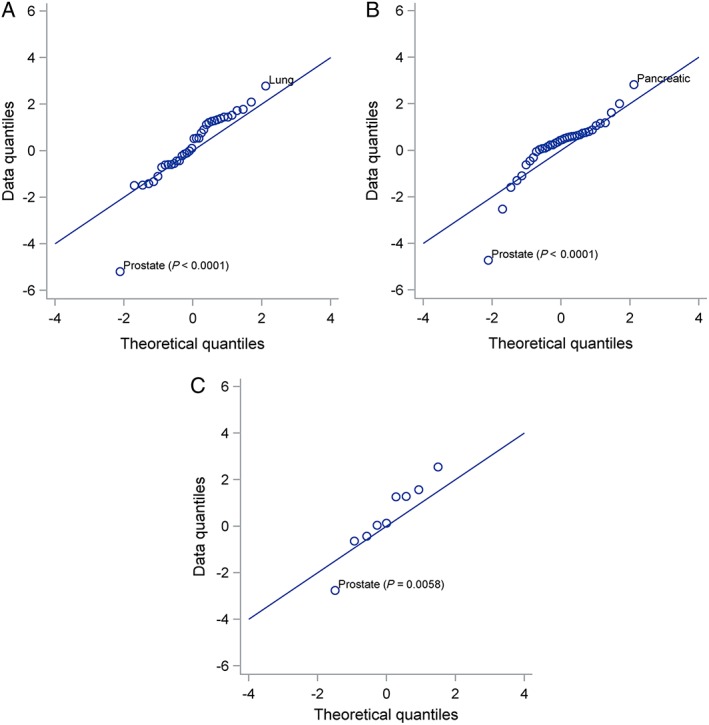
Q‐Q plots for hazard ratios associated with exposure to spironolactone. Three sensitivity analyses are shown: (A) Follow‐up to last data collection; (B) Follow‐up to last contact with patient recorded in the Clinical Practice Research Datalink; (C) Primary outcomes in practices with Hospital Episode Statistics data available. Cancers with hazard ratios that are significant at P < 0.01 are labelled. Prostate cancer is highly significant in all three sensitivity analyses, which use different randomly selected control cohorts. Under the null hypothesis of no effect of exposure to spironolactone, hazard ratios divided by their standard deviations (z scores) would have a standard normal distribution and quantiles of the observed distribution would be close to the theoretical quantiles of the standard normal distribution
Table 5.
Hazard ratios (95% confidence intervals) for primary outcomes
| Follow‐up to last data collection date | Follow‐up to last contact | Subgroup with HES, last data collection date | |
|---|---|---|---|
| Prostate | 0.69 (0.60,0.80), P < 0.001 | 0.72 (0.63,0.82), P < 0.001 | 0.82 (0.71,0.94), P = 0.006 |
| Colorectal | 0.91 (0.80,1.04), P = 0.155 | 0.90 (0.79,1.02), P = 0.111 | 1.01 (0.88,1.15), P = 0.901 |
| Endometrial | 0.90 (0.63,1.30), P = 0.579 | 1.01 (0.71,1.44), P = 0.942 | 1.24 (0.89,1.73), P = 0.201 |
| Ovarian | 1.28 (0.90,1.81), P = 0.168 | 1.14 (0.81,1.60), P = 0.444 | 1.30 (0.94,1.80), P = 0.118 |
| Pancreatic | 1.22 (0.93,1.60), P = 0.150 | 1.48 (1.13,1.94), P = 0.005 | 1.19 (0.91,1.56), P = 0.206 |
| MML | 1.44 (0.84,2.47), P = 0.182 | 1.08 (0.64,1.82), P = 0.765 | 1.90 (1.16,3.11), P = 0.011 |
| Renal | 1.46 (0.95,2.24), P = 0.085 | 1.28 (0.85,1.92), P = 0.236 | 0.94 (0.69,1.27), P = 0.669 |
| Pharyngeal | 0.76 (0.32,1.82), P = 0.540 | 1.07 (0.44,2.63), P = 0.880 | 1.01 (0.47,2.19), P = 0.971 |
| Thyroid | 0.67 (0.23,2.00), P = 0.478 | 0.53 (0.20,1.39), P = 0.196 | 0.70 (0.23,2.11), P = 0.522 |
Hospital Episode Statistics (HES) data are available for a subset of Clinical Practice Research Datalink practices (57 %), allowing linkage with hospital discharge diagnostic data
In the primary analysis for prostate cancer the hazard ratio associated with spironolactone exposure was 0.73 (0.63–0.85) in incident users and 0.58 (0.43–0.78) in prevalent users (Table 6). The hazard ratio for high dose vs. low dose was 0.74 (0.51–1.06), and prevalent users in this study received higher doses of spironolactone than incident users. In patients who discontinued spironolactone, the hazard ratio for prostate cancer was 2.08 (1.48–2.92) relative to their exposed period.
Table 6.
Hazard ratios (95% confidence intervals) for prostate cancer by prevalent or incident use of spironolactone and dose
| Hazard ratio (95% CIs) | |
|---|---|
| Incident users vs. controls | 0.73 (0.63,0.85), P < 0.001 |
| Prevalent users vs. controls | 0.58 (0.43,0.78), P < 0.001 |
| Medium vs. low dose | 0.99 (0.78,1.26), P = 0.957 |
| High vs. low dose | 0.74 (0.51,1.06), P = 0.100 |
| Discontinued vs. low dose | 2.08 (1.48,2.92), P < 0.001 |
Low dose <37.5 mg/day, Medium dose 37.5–74.9 mg/day, High dose ≥75 mg/day
Prostate specific antigen (PSA) levels in exposed and control patients in 6 months periods for 2 years before and after their index dates are shown in Figure 3A. Levels were similar in the two cohorts before the index date, but lower in the spironolactone cohort after the index date. PSA levels converged again within 6 months of discontinuation of spironolactone (Figure 3B).
Figure 3.
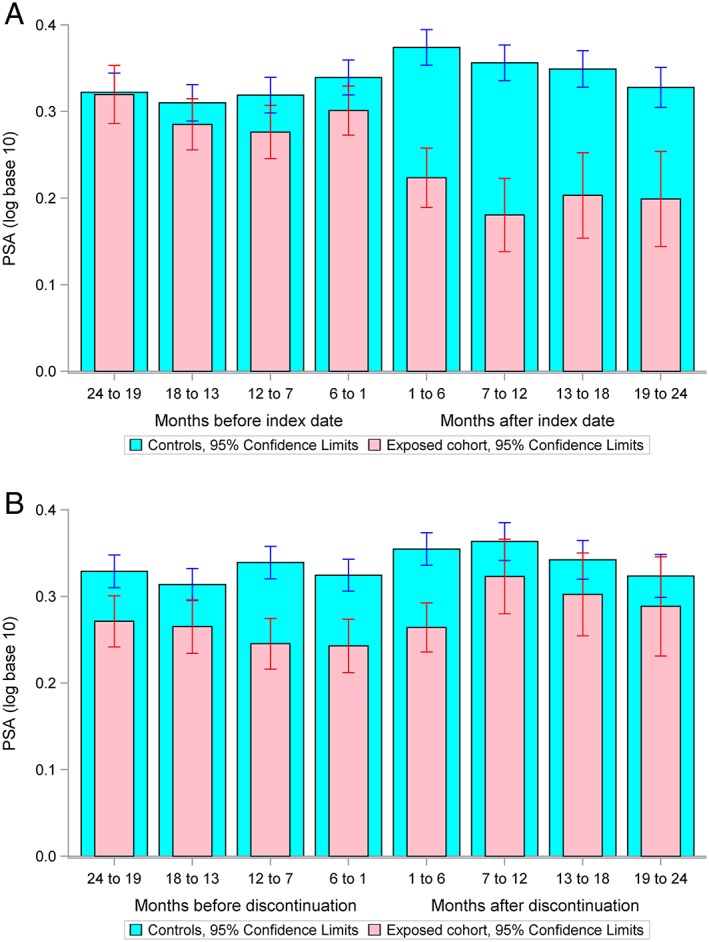
Mean prostate specific antigen (PSA) in exposed and control patients (A) for 2 years before and after their index dates and (B) for 2 years before and after discontinuation of spironolactone. PSA levels were similar in the exposed and control cohorts before the index date, but lower in the spironolactone cohort after the index date suggesting that spironolactone exposure is associated with lower PSA levels. Within 2 months of spironolactone discontinuation, PSA levels in the exposed cohort returned to levels not significantly different to those in control patients
Discussion
Principal findings
This study provided no support for the hypothesis that spironolactone exposure increases the risk of nine prespecified cancers, or 27 other cancers. Two different cancers had nominally significant hazard ratios >1 in separate sensitivity analyses, but this was not an unlikely outcome when 35 cancers (i.e. excluding prostate) were screened. However, for uncommon cancers, the confidence limits of our findings were broad.
The study also found a reduced risk of prostate cancer in a large cohort of patients exposed to spironolactone in three sensitivity analyses using different control cohorts. This is consistent with our a priori hypothesis that spironolactone exposure might either increase or reduce the incidence of prostate cancer, based on its known hormonal effects and the likely partially hormonal basis of this cancer. We found a statistically significant 31% reduction in the incidence of prostate cancer with spironolactone exposure. Prostate cancer is the most common cancer in men (nearly 50 000 new cases per year in the UK) 27 and its treatment causes much morbidity; our finding that the incidence of prostate cancer was reduced in men taking spironolactone suggests that it may have a role in primary prevention of prostate cancer. There was some evidence that the incidence of prostate cancer increased after discontinuation of spironolactone, but a further study matching patients at the time of discontinuation would be required to confirm this.
Strengths and weaknesses of the study
This study was carried out in CPRD, a UK primary care database that is representative of the general UK population. However, because the study was observational, all potentially confounding factors may not have been fully controlled for, despite matching on propensity score and adjusting for covariates. Other limitations include the accuracy of coding for the exposure (for example, spironolactone prescribing from secondary care would not have been detected), outcomes and covariates, missing data, and unrecorded confounding factors. Random errors arising from database coding errors were likely to affect exposed and nonexposed cohorts similarly and would bias the results towards the null. The codes used in the study were cross‐checked by a cancer specialist and two clinical pharmacologists. Some risk factors for cancer such as family history, genetic abnormalities and ethnicity are poorly recorded in CPRD or could not be fully assessed, but we included all relevant covariates for which data were available. We conducted one set of sensitivity analyses that included HES, to assess the effect of missing outcome events in CPRD, and another that censored follow‐up time at last contact instead of last data collection date, to assess the effect of assuming that patients were present until the later date.
There were too few cases of acute myelomonocytic/myelomonoblastic leukaemia to draw firm conclusions on whether there was any association with spironolactone exposure. However, we found no clear evidence of increased risk.
Comparison with other studies
There have been no other recent studies investigating the relationship between spironolactone exposure and prostate cancer incidence. The Prostate Cancer Prevention Trial reported that finasteride, a 5‐α reductase inhibitor, significantly reduced the relative risk of prostate cancer in the general population by 24.8% 28. However, there was more high‐grade prostate cancer in the finasteride group than the placebo group. After 18 years of follow‐up, there was no significant difference in survival rates between the two groups.
A recent high‐throughput chemical screen suggested a potential mechanism by which spironolactone impairs cancer cell survival by inhibiting homology directed repair and suggested that spironolactone might be a new candidate for chemotherapy 29. In addition, recent work has suggested that spironolactone may enhance tumour cell elimination by natural killer cells in multiple colon cancer cell lines by activating the ATM‐Chk2‐mediated checkpoint pathway via actions on the retinoid X receptor γ (RXRγ) 30. However, in the current study, we only found a significant association of spironolactone use with reduced incidence of prostate cancer.
Meaning of the study
The results of this study allay concerns that spironolactone exposure is associated with an increased risk of cancers. In fact, our findings suggest that it is associated with reduced incidence of prostate cancer. Because spironolactone is generally well tolerated and is useful in the treatment of other conditions (hypertension and heart failure) commonly affecting older men at risk of prostate cancer, it may have potential in the primary prevention of prostate cancer, particularly in high‐risk patients with other potential indications for spironolactone therapy. At present, patients considered to be at high risk of prostate cancer include older men, men of African descent and men with a positive family history of prostate cancer in a first‐degree relative.
Future research
Firstly, our findings need to be confirmed independently in observational studies in different populations. If confirmed, further exploration of the possible effects of spironolactone in the primary prevention of prostate cancer and its place, if any, in the treatment of early stage prostate cancer (currently often managed by active surveillance) 31 and effects on overall mortality in prostate cancer in randomised controlled clinical trials would then be needed before its use could be recommended. Investigation of whether it might accelerate the progression of advanced prostate cancer, as reported in a case study in a single patient 32, would also be required. In summary, this study identified no increased risk of cancers in people exposed to spironolactone. Further work is required to establish whether spironolactone has a role in the prevention of prostate cancer.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that S.M. and A.T. have no financial or non‐financial interests that may be relevant to the submitted work; I.M., L.W. and T.M. report a grant from Anonymous Trust during the conduct of the study; I.M. reports personal fees from MSD, grants from Amgen, grants from Menarini, grants from Novartis, personal fees from AstraZeneca, outside the submitted work; T.M. reports grants and personal fees from Novartis, grants and personal fees from Pfizer, grants from Amgen, grants from Ipsen, grants from Teijin, grants and personal fees from Menarini, personal fees from Kaiser Permanente, personal fees from Takeda, personal fees from Servier, personal fees from Shire, personal fees from Astellas, personal fees from Daiicho Sankyo, personal fees from Lundbeck, outside the submitted work.
The study was funded by a grant from the Anonymous Trust.
Role of study sponsor and funder: The study was sponsored by the University of Dundee. The sponsor and funder had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The researchers are independent from the study funder.
Contributors
I.S.M. and T.M.M. had the original idea for the study. All authors contributed to design of the protocol and writing and revising the paper. Statistical analysis was performed by S.V.M. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The guarantor for the study is I.S.M.
Supporting information
Appendix S1 Code lists for outcomes
Supporting info item
Mackenzie, I. S. , Morant, S. V. , Wei, L. , Thompson, A. M. , and MacDonald, T. M. (2017) Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol, 83: 653–663. doi: 10.1111/bcp.13152.
References
- 1. Wei L, Struthers AD, Fahey T, Watson AD, MacDonald TM. A population‐based longitudinal analysis of spironolactone use and renal toxicity. BMJ 2010; 340: c1768. [DOI] [PubMed] [Google Scholar]
- 2. NICE Clinical Guideline CG127: Hypertension: Clinical management of primary hypertension in adults. National Institute for Health and Care Excellence 2011. Available at http://publications.nice.org.uk/hypertension‐cg127# (last accessed 08/04/2014).
- 3. Spironolactone . British National Formulary April 2014. Available at http://www.medicinescomplete.com/mc/bnf/current/PHP834‐spironolactone.htm (last accessed 08/04/2014).
- 4. IARC Monographs, Volume 79: Spironolactone. P319–337. Available at http://monographs.iarc.fr/ENG/Monographs/vol79/mono79‐13.pdf (last accessed 17th March 2014).
- 5. Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, et al. Spironolactone metabolism: steady‐state serum levels of the sulfur‐containing metabolites. J Clin Pharmacol 1989; 29: 342–347. [DOI] [PubMed] [Google Scholar]
- 6. Luthy IA, Begin DJ, Labrie F. Antiandrogenic activity of synthetic progestins and spironolactone in androgen‐sensitive mouse mammary carcinoma (Shionigi) cells in culture. J Steroid Biochem 1988; 31: 845–852. [DOI] [PubMed] [Google Scholar]
- 7. Terouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, et al. A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects. Mol Cell Endocrinol 2000; 160: 39–49. [DOI] [PubMed] [Google Scholar]
- 8. Selby JV, Friedman GD, Fireman BH. Screening prescription drugs for possible carcinogenicity: eleven to fifteen years of follow‐up. Cancer Res 1989; 49: 5736–5747. [PubMed] [Google Scholar]
- 9. Ron E, Kleinerman RA, Boice JD Jr, LiVolsi VA, Flannery JT, Fraumeni JF Jr. A population‐based case–control study of thyroid cancer. J Natl Cancer Inst 1987; 79: 1–12. [PubMed] [Google Scholar]
- 10. McLaughlin JK, Chow WH, Mandel JS, Mellemgaard A, McCredie M, Lindblad P, et al. Int J Cancer 1995; 63: 216–221. [DOI] [PubMed] [Google Scholar]
- 11. Mellemgaard A, Niwa S, Mehl ES, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark: role of medication and medical history. Int J Epidemiol 1994; 23: 923–930. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro JA, Williams MA, Weiss NS, Stergachis A, LaCroix AZ, Barlow WE. Hypertension, antihypertensive medication use, and risk of renal cell carcinoma. Am J Epidemiol 1999; 149: 521–530. [DOI] [PubMed] [Google Scholar]
- 13. Weinman S, Glass AG, Weiss NS, Psaty BM, Siscovick DS, White E. Use of diuretics and other antihypertensive medications in relation to the risk of renal cell cancer. Am J Epidemiol 1994; 140: 792–804. [DOI] [PubMed] [Google Scholar]
- 14. Yuan J‐M, Castelao JE, Gago‐Dominguez M, Ross RK, Yu MC. Hypertension, obesity and their medications in relation to renal cell carcinoma. Cancer 1998; 77: 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stierer M, Spoula H, Rosen HR. Breast cancer in the male – a retrospective analysis of 15 cases. Onkologie 1990; 13: 128–131. [Article in German, English abstract] [DOI] [PubMed] [Google Scholar]
- 16. Loube SD, Quirk RA. Breast cancer associated with administration of spironolactone. Lancet 1975; 1: 1428–1429. [DOI] [PubMed] [Google Scholar]
- 17. Jick H, Armstrong B. Breast cancer and spironolactone. Lancet 1975; 2: 368–369. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong B, Stevens N, Doll R. Retrospective study of the association between use of rauwolfia derivatives and breast cancer in English women. Lancet 1974; 2: 672–675. [DOI] [PubMed] [Google Scholar]
- 19. Boston Collaborative Drug Surveillance Program . Reserpine and breast cancer. Lancet 1974; 2: 669–671.4142955 [Google Scholar]
- 20. Danielson DA, Jick H, Hunter JR, Stergachis A, Madsen S. Nonestrogenic drugs and breast cancer. Am J Epidemiol 1982; 116: 329–332. [DOI] [PubMed] [Google Scholar]
- 21. Shaw JC, White LE. Long‐term safety of spironolactone in acne: results of an 8‐year followup study. J Cutan Med Surg 2002; 6: 541–545. [DOI] [PubMed] [Google Scholar]
- 22. Mackenzie IS, MacDonald TM, Thompson A, Morant S, Wei L. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. Br Med J 2012; 345: e4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biggar RJ, Andersen EW, Wohlfahrt J, Melbye M. Spironolactone use and the risk of breast and gynaecologic cancers. Cancer Epidemiol 2013; 37: 870–875. [DOI] [PubMed] [Google Scholar]
- 24. Futterman LG, Lemberg L. The resurrection of spironolactone on its golden anniversary. Am J Crit Care 2004; 13: 162–165. [PubMed] [Google Scholar]
- 25. Walley T, Mantgani A. The, UK General Practice Research Database. Lancet 1997; 350: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 26. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancer Research UK . Prostate cancer statistics. Available at http://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/prostate‐cancer#heading‐Zero (last accessed 22/04/2016)
- 28. Thompson IM, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long‐term survival of participants in the Prostate Cancer Prevention Trial. N Engl J Med 2013; 369: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shahar OD, Kalousi A, Eini L, Fisher B, Weiss A, Darr J, et al. A high‐throughput chemical screen with FDA approved drugs reveals that the antihypertensive drug spironolactone impairs cancer cell survival by inhibiting homology directed repair. Nucleic Acids Res 2014; 42: 5689–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leung WH, Vong QP, Lin W, Janke L, Chen T, Leung W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J Exp Med 2013; 210: 2675–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. NICE Clinical Guideline CG175: Prostate cancer: diagnosis and treatment. National Institute for Health and Care Excellence 2014. Available at guidance.nice.org.uk/cg175 (last accessed 08/04/2014).
- 32. Sundar S, Dickinson PD. Spironolactone, a possible selective androgen receptor modulator, should be used with caution in patients with metastatic carcinoma of the prostate. BMJ Case Rep 2012. doi:10.1136/bcr.11.2011.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Code lists for outcomes
Supporting info item


