Abstract
Aims
Anastrozole, an aromatase inhibitor widely used in breast cancer, has recently been indicated to be a P‐glycoprotein (ABCB1) substrate. We have aimed to determine whether ABCB1 single‐nucleotide polymorphisms (SNPs) can affect anastrozole plasma concentrations in these patients. In addition, we assessed the impact of SNPs in CYP19A1 and TCL1A on the development of arthralgia and cancer recurrence in our series.
Methods
This study included 110 postmenopausal women with hormone receptor‐positive breast cancer. Anastrozole plasma levels were determined by a liquid chromatography–electrospray ionization–quadrupole‐time‐of‐flight mass spectrometry system. Patients were genotyped for SNPs in the ABCB1, TCL1A and CYP19A1 genes to search for associations with pharmacokinetic and pharmacodynamics parameters using logistic regression models.
Results
Anastrozole concentrations showed an almost nine‐fold interindividual variability (mean 26.95 ± 11.91 ng ml−1). The ABCB1 2677‐TT genotype was associated with higher plasma levels (32.22 ± 12.82 vs. 25.86 ± 11.56 ng ml−1 for GG/GT subjects; 95% confidence interval: –12.3 to –0.40), whilst the 3435‐TT genotype showed a protective effect on the risk of arthralgia (odds ratio = 0.32 [0.11–0.89]; P = 0.029). The CYP19A1 rs1008805 GG genotype was strongly and inversely associated with arthralgia (odds ratio = 0.24 [0.09–0.65], P = 0.004); however, SNPs near the TCL1A gene were not linked to this adverse effect. None of the patients who had cancer recurrence harboured the CYP19A1 rs727479 AA genotype, which, in contrast, was present in 38% of patients who did not relapse (P for trend = 0.031).
Conclusion
Our findings indicate that variability in anastrozole plasma levels may be attributable to the status of the ABCB1 gene locus. Furthermore, genetic variants in CYP19A1 were associated with arthralgia and cancer recurrence in our patients.
Keywords: anastrozole, arthralgia, breast cancer, single nucleotide polymorphisms
What is Already Known about this Subject
Anastrozole has recently been shown to be a substrate for P‐glycoprotein (ABCB1).
Four single‐nucleotide polymorphisms near the TCL1A gene have been pinpointed in a previous, large genome‐wide association study as putative loci relevant for the development of arthralgia in breast cancer patients on anastrozole.
A number of polymorphisms in the aromatase gene (CYP19A1) have been studied in relation to the clinical response to anastrozole but results have been inconsistent so far.
What this Study Adds
The ABCB1 gene variability affects anastrozole plasma concentrations, which suggests that the present 1 mg standard dose may not be ideal for all patients.
For the first time to our knowledge, we have addressed the validation of four genome‐wide association study‐pinpointed SNPs near TCL1A with regard to their association with arthralgia, but their alleged role could not be confirmed.
SNPs in the aromatase gene (CYP19A1) affect the development of arthralgia in breast cancer patients treated with anastrozole and may modify the incidence of cancer recurrence.
Tables of Links
| TARGETS |
|---|
| Enzymes 2 |
| CYP19A1 http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2635 |
| Transporters 3 |
| ABCB1 |
These Tables lists key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3
.
Introduction
Breast cancer is the most frequently diagnosed cancer in women. Three out of four of the cases occur in postmenopausal women, 80% of whom are hormone‐receptor positive (HR+) 4. Tamoxifen has traditionally been the forefront for the endocrine treatment of this type of breast cancer; however, in recent years, aromatase inhibitors (AIs) have been introduced as an alternate therapy. Anastrozole is a third‐generation, highly‐specific AI that suppresses plasma oestrogen levels in postmenopausal women by inhibiting the enzyme responsible for the conversion of androgens to oestrogens in peripheral tissues. Several clinical trials have shown that anastrozole alone is more effective than tamoxifen at reducing cancer recurrence 5, 6, 7, 8.
A recent study has demonstrated that anastrozole is a substrate for P‐glycoprotein (ABCB1) 9, a member of the ABC (ATP binding cassette) transporter family that functions as an energy‐dependent xenobiotics efflux pump and that is apically expressed in numerous tissues involved in excretion or known to have blood‐tissue barriers. A great body of evidence has fully confirmed the profound effect of the drug‐transporting ABCB1 on the pharmacokinetics of drugs in humans 10. In addition, the encoding ABCB1 gene locus is known to harbour numerous single nucleotide polymorphisms (SNPs) with implications on drug disposition and clinical outcomes 11. Of these mutations, the most studied are a nonsynonymous base change (G > T) at position 2677 in exon 21 and two synonymous transitions (C1236T and C3435T) in exons 12 and 26, respectively 12. Studies reporting on anastrozole plasma concentrations in breast cancer patients have been available just recently 13, 14, 15 but, to our knowledge, none of them have been aimed to determine the impact of the ABCB1 status on anastrozole concentrations.
By contrast, SNPs in the aromatase gene (CYP19A1) have been associated with cancer recurrence in these patients, as well as with the onset of arthralgia 16; a serious adverse effect of AIs caused by oestrogen deprivation that can even lead to discontinuation of therapy 17. In addition, a genome‐wide association study (GWAS) has pinpointed four SNPs near the T‐cell leukaemia 1 A (TCL1A) gene as putative loci also associated with muculoskeletal adverse events in HR+ and/or progesterone receptor + breast cancer patients receiving AIs 18.
The present study was intended to achieve two goals. First we aimed to determine whether ABCB1 SNPs can affect anastrozole plasma levels and/or the occurrence of arthralgia in these patients. Second, we also assessed the impact of SNPs in CYP19A1 and TCL1A genes on the onset of this adverse effect and the incidence of cancer recurrence in our series.
Patients and methods
Study sample
This retrospective study included Caucasian postmenopausal female patients with HR+ breast cancer treated with anastrozole (Arimidex®) at the Service of Oncology of Fundación Alcorcón University Hospital (Madrid, Spain).
All participants gave oral and written consent for their participation. The study was approved by the Bioethics Committee of the University of Extremadura (registry number 17/2010), and was conducted in accordance with the Declaration of Helsinki and its subsequent revisions
Determination of anastrozole plasma levels
A peripheral blood sample (10 ml) was obtained from each participant at least 4 weeks after anastrozole treatment was initiated and approximately 24 hours after the last dose in order to measure trough levels 14. Five ml of this sample were immediately processed to separate the plasma. Both plasma and whole blood samples (for DNA purification, see below) were stored at −80°C until analysis. Plasma concentrations of anastrozole were determined by a liquid chromatography–electrospray ionization–quadrupole‐time‐of‐flight mass spectrometry system in positive ionization mode as described previously 19 with minor modifications. Briefly, after plasma samples were subjected to liquid–liquid extraction, anastrozole and verapamil, which was used as internal standard, were separated using a high‐performance liquid chromatography system (Agilent 1260 Series; Agilent Technologies, Santa Clara, CA, USA) equipped with a Zorbax Extend‐C18 rapid resolution analytical column of 2.1 mm × 50 mm and 1.8‐μm particle size (Agilent Technologies). The high‐performance liquid chromatography system was connected to a quadrupole‐time‐of‐flight mass spectrometer (Agilent 6530 Series Accurate Mass QTOFMS; Agilent Technologies) and ions were generated using an electrospray ion source (Dual ESI). Data analysis was carried out with an Agilent Mass Hunter Workstation Software (version B.03.01). A representative chromatogram of anastrozole can be seen in Supplementary Figure S1
Genotyping
Genomic DNA was isolated by using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany) from 5‐ml whole blood samples. Three exonic SNPs in the ABCB1 gene, four polymorphisms in CYP19A1 and four additional SNPs near the TCL1A gene were identified by real‐time PCR using TaqMan SNP Genotype Assays from Life Technologies (Rockville, MD, USA).
The SNPstats platform 20 was used to determine the adequate model of inheritance (additive, dominant or recessive), to provide linkage disequilibrium (LD) data and to estimate the effect of haplotypes by linear regression modelling. Regression parameters pertained to the log odds ratios adjusted by clinical and demographic variables. Frequency threshold for rare haplotypes was set at 0.01.
Outcomes assessment
Demographic and clinical data were abstracted from medical charts and included age, height, weight, time into menopause, date of breast cancer diagnosis, stage at diagnosis, tumour size and grade, hormone receptor status, time on AI therapy, prior/current cancer treatment and cancer recurrence (local, contralateral or metastatic). The presence of arthralgia was recorded by patient report of any joint pain or stiffness in the last week that had either started or worsened after initiating anastrozole therapy 21.
Statistical analyses
Fisher exact or Pearson χ2 test were used for the univariate analysis of the associations between categorical data (e.g. genotypes vs. arthralgia). In order to compare quantitative variables (e.g. anastrozole plasma levels) between the different genotype groups, Student t or anova tests were used depending on the number of groups considered. Multivariate regression analyses were performed to collectively assess the impact of both genetic and nongenetic parameters on the different outcomes. No relevant interactions were observed between the SNPs analysed and any of the covariates included.
Statistical analyses were performed using the SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). In all instances differences were considered to be significant when P values were <0.05.
Results
A total of 110 women with hormone receptor‐positive breast cancer treated with anastrozole were included in the study. Mean therapy time on 31st December 2012 was 53.16 ± 20.45 months. The vast majority of the patients (93.6%) were also taking chemotherapy drugs (three women were on neoadjuvant chemotherapy). Mean age of the patients at the onset of therapy was 56.74 ± 27.88 years. Most of the patients (70.9%) had grade 1 or 2 cancer and were HER2 negative (81.8%). With regard to tumour histology, the most frequent type was infiltrated ductal carcinoma (83.9%), followed by infiltrated lobular carcinoma (8.9%). Other types observed were papillary carcinoma, in situ ductal carcinoma and infiltrated mucinous carcinoma. Additional clinical and demographic characteristics of the patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the postmenopausal women with hormone‐receptor positive breast cancer who were included in the study
| Mean ± SD | n | Range | % | |
|---|---|---|---|---|
| Age at therapy onset (years) | 56.74 ± 27.88 | 39–80 | ||
| Body mass index (kg m –2 ) | 28.69 ± 4.52 | 18.72–40.43 | ||
| Age at menopause (years) | 49.35 ± 4.96 | 30–58 | ||
| Chemotherapy | ||||
| Yes | 100 | 90.9 | ||
| No | 10 | 9.1 | ||
| Grade | ||||
| 1 | 24 | 21.8 | ||
| 2 | 54 | 49.1 | ||
| 3 | 32 | 29.1 | ||
| Stage | ||||
| IA | 41 | 37.3 | ||
| IIA | 32 | 29.1 | ||
| IIB | 17 | 15.5 | ||
| IIIA | 10 | 9.1 | ||
| IIIB | 7 | 6.4 | ||
| IIIC | 3 | 2.7 | ||
| HER2 | ||||
| Negative | 90 | 81.8 | ||
| Positive | 20 | 18.2 | ||
| Ki67 expression | ||||
| < 10 | 34 | 30.9 | ||
| 10–20 | 44 | 40.0 | ||
| > 20 | 32 | 29.1 | ||
Table 2 shows genotyping results for the 11 polymorphisms studied, three in the ABCB1 gene, four in CYP19A1 and four near the TCL1A gene. Minor allele frequencies in the population of study ranged from 0.1 (rs11849538) to 0.495 (rs1128503).
Table 2.
Characteristics of the 11 polymorphisms studied and genotyping results in the study sample
| Gene locus | SNP | Chromosome | Location | Position | Genotype | n | % | MAF |
|---|---|---|---|---|---|---|---|---|
| CYP19A1 | rs727479 | 15 | Intron 1 | 49 321 839 | AA | 40 | 36.36 | 0.364 |
| AC | 60 | 54.55 | ||||||
| CC | 10 | 9.09 | ||||||
| CYP19A1 | rs1008805 | 15 | Exon 1 | 49 336 891 | AA | 27 | 24.55 | 0.473 |
| AG | 62 | 56.36 | ||||||
| GG | 21 | 19.09 | ||||||
| CYP19A1 | rs749292 | 15 | Exon 1 | 49 346 023 | AA | 16 | 14.55 | 0.600 |
| AG | 56 | 50.91 | ||||||
| GG | 38 | 34.55 | ||||||
| CYP19A1 | rs730154 | 15 | Exon 1 | 49 378 496 | CC | 31 | 28.18 | 0.368 |
| CT | 77 | 70.00 | ||||||
| TT | 2 | 1.82 | ||||||
| near TCL1A | rs7158782* | 14 | Intronic | 95 238 884 | AA | 85 | 77.27 | 0.118 |
| AG | 24 | 21.82 | ||||||
| GG | 1 | 0.91 | ||||||
| near TCL1A | rs7159713 | 14 | Intronic | 95 239 330 | AA | 84 | 76.36 | 0.123 |
| AG | 25 | 22.73 | ||||||
| GG | 1 | 0.91 | ||||||
| near TCL1A | rs2369049 | 14 | Intronic | 95 241 604 | AA | 85 | 77.27 | 0.118 |
| AG | 24 | 21.82 | ||||||
| GG | 1 | 0.91 | ||||||
| near TCL1A | rs11849538 | 14 | Intronic | 95 245 031 | CC | 89 | 80.91 | 0.100 |
| CG | 20 | 18.18 | ||||||
| GG | 1 | 0.91 | ||||||
| ABCB1 | rs1128503 (C1236T) | 7 | Exon 12 | 87 550 285 | CC | 25 | 22.73 | 0.495 |
| CT | 61 | 55.45 | ||||||
| TT | 24 | 21.82 | ||||||
| ABCB1 | rs2032582 (G2677 T) | 7 | Exon 21 | 87 531 302 | GG | 35 | 31.82 | 0.450 |
| GT | 51 | 46.36 | ||||||
| TT | 24 | 21.82 | ||||||
| ABCB1 | rs1045642 (C3435T) | 7 | Exon 26 | 87 509 329 | CC | 48 | 43.64 | 0.386 |
| CT | 39 | 35.45 | ||||||
| TT | 23 | 20.91 |
In full linkage disequilibrium with rs2369049; MAF, minor allele frequency; SNP, single‐nucleotide polymorphism
A high degree of LD was observed between the four SNPs near the TCL1A gene (r2 ranged from 0.79 to 0.95), with rs7158782 and rs2369049 loci being in complete LD (r2 = 1). All the remaining SNP pairs in the ABCB1 and CYP19A1 genes displayed r2 values lower than 0.80.
Effect of ABCB1 polymorphisms on anastrozole concentrations
Anastrozole concentrations could only be measured in 104 out of the 110 patients because six samples accidently thawed before processing. Plasma levels showed a high variability, over an eight‐fold range (6.60–55.70 ng ml−1), with a mean value of 26.95 ± 11.91 ng ml−1. The distribution of the observed concentrations resulted in a histogram slightly skewed to the left (Figure 1). Lowest limit of quantitation was set at 1.25 ng ml−1. The method revealed excellent linearity for the range 2.5–150 ng ml−1 (y = 0.0153x + 0.0003; r 2 = 0.9983).
Figure 1.
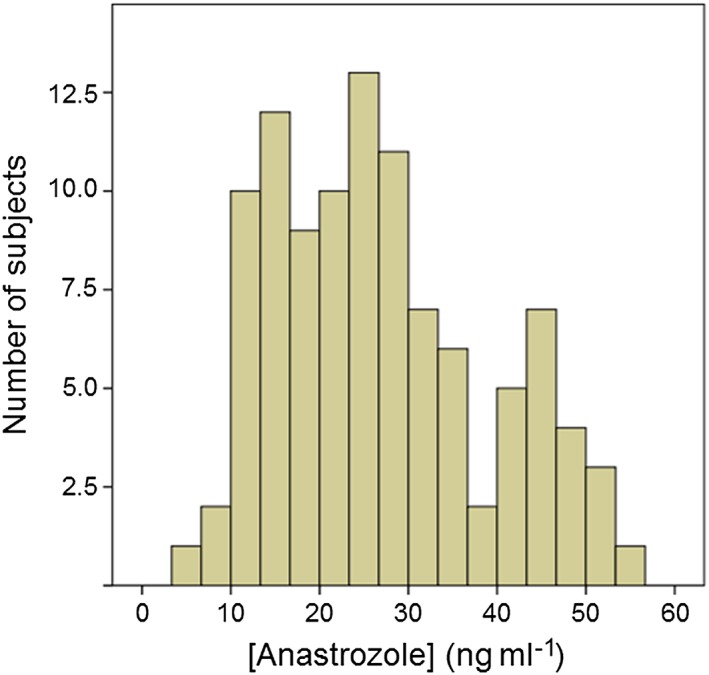
Histogram of the distribution of anastrozole plasma concentrations in the population of study
Figure 2 shows that the mutant homozygous TT genotype of the G2677 T SNP was associated with higher anastrozole plasma concentrations (32.22 ± 12.82 for TT vs. 25.86 ± 11.56 ng ml−1 for the GG/GT genotypes; P = 0.037; 95%CI: −12.3 to –0.40). In contrast, P‐values and 95% confidence intervals for the associations between these levels and the different C1236T (CC: 26.57 ± 14.02; CT: 27.73 ± 11.52 and TT: 27.53 ± 14.06 ng ml−1) or C3435T genotypes (CC: 27.52 ± 12.53; CT: 25.41 ± 12.15 and TT: 30.94 ± 12.95 ng ml−1) were not significant.
Figure 2.
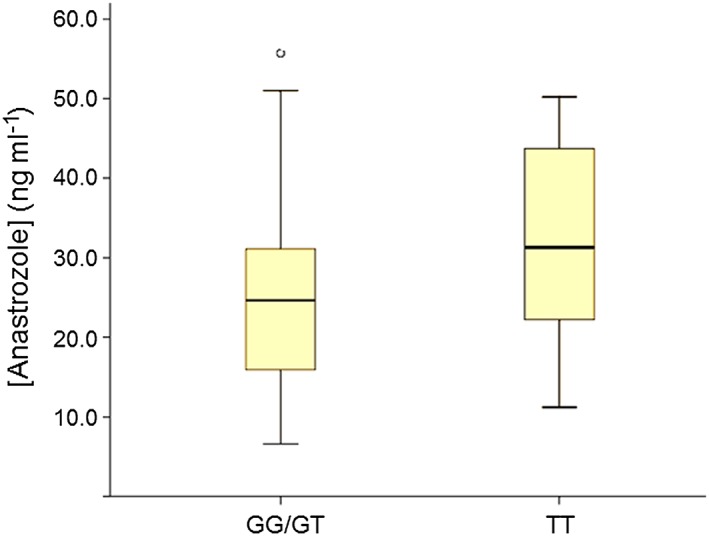
Anastrozole plasma concentrations according to the ABCB1 G2677T genotype
The haplotype study in the three ABCB1 loci considered (1236–2177–3435) showed no relevant differences with regard to anastrozole concentrations between the most frequent allele combination (C–G–C) and the T–T–T mutant homozygous haplotype (mean difference = 1.23 [–2.75–5.22], P = 0.550). Differences regarding anastrozole plasma levels for all the ABCB1 haplotypes identified in the study sample are shown in Supplementary Table S1.
Finally, a comparison between patients carrying mutant genotypes in all three ABCB1 loci (TT–TT–TT; n = 13) and the rest of the patients showed a trend towards higher plasma levels in the former group (32.98 ± 13.35 vs. 26.61 ± 12.25 ng ml−1; P = 0.087). In contrast the difference between patients carrying wild‐type genotypes only (CC–GG–CC; n = 24) and the rest of the population was not significant (26.57 ± 14.01 vs. 27.68 ± 12.09 ng ml−1; P = 0.705)
Associations with clinical outcomes
A total of 78 patients (71%) referred arthralgia after therapy with anastrozole was initiated. The incidence of arthralgia was not related to high levels of the drug. Using the median value as cut‐off point, the odds ratio (OR) for high vs. low concentrations was not significant (0.97 [0.39–2.40], P = 0.565).
We analysed the impact of four SNPs near the TCL1A gene on the development of arthralgia in the population of study; however, none of them were found to be associated with the symptoms in any model of inheritance. Table 3 shows results for the dominant model, as the reduced number of mutant homozygous carriers prevented the analysis of the recessive model in some cases. We also assessed the effect of ABCB1 SNPs, as anastrozole is a known substrate of P‐glycoprotein 9. Given that the 2677 TT mutant genotype had been observed to be associated with anastrozole levels (see above), we used the recessive model for the analyses in this case. The ABCB1 3435 TT genotype showed a significant protective effect on the risk for arthralgia (OR =0.32 [0.11–0.89]; P = 0.029; Table 3). In addition, a statistical trend, also towards a protective effect, was observed for the ABCB1 1236 C/T SNP. Globally, the incidence of arthralgia was far lower in patients who were carriers of a TT genotype in each ABCB1 locus (46.2% vs. 74.4% for patients with other genotypes: OR = 0.295 [0.09–0.98]; P = 0.044). Finally, the CYP19A1 rs1008805 GG genotype was strongly and inversely associated with arthralgia (OR = 0.24 [0.09–0.65], P = 0.004; Table 3).
Table 3.
Association of TCL1A, ABCB1 and CYP19A1 polymorphisms with the onset of arthralgia in postmenopausal, hormone‐receptor positive breast cancer patients
| Arthralgia | ||||
|---|---|---|---|---|
| TCL1A | No (%) | Yes (%) | OR (95% CI) | P‐value |
| rs7158782–rs2369049 a | ||||
| A/A | 72.0 | 75.6 | Ref. | |
| A/G‐G/G | 28.0 | 24.4 | 0.88 (0.33–2.36) | 0.8 |
| rs7159713 A/G | ||||
| A/A | 72.0 | 74.4 | Ref. | |
| A/G‐G/G | 28.0 | 25.6 | 0.95 (0.36–2.54) | 0.93 |
| rs11849538 C/G | ||||
| C/C | 72.0 | 82.1 | Ref. | |
| C/G‐G/G | 28.0 | 17.9 | 0.61 (0.22–1.70) | 0.35 |
| Arthralgia | ||||
|---|---|---|---|---|
| ABCB1 | No (%) | Yes (%) | OR (95% CI) | P‐value |
| ABCB1 1236 | ||||
| C/C‐C/T | 65.6 | 84.1 | Ref. | |
| T/T | 34.4 | 44.1 | 0.40 (0.15–1.08) | 0.074 |
| ABCB1 2677 | ||||
| G/G‐G/T | 68.8 | 88.1 | Ref. | |
| T/T | 31.3 | 40.1 | 0.52 (0.19–1.41) | 0.2 |
| ABCB 3435 | ||||
| C/C‐C/T | 65.6 | 84.1 | Ref. | |
| T/T | 34.4 | 44.1 | 0.32 (0.11–0.89) | 0.029 |
| Arthralgia | ||||
|---|---|---|---|---|
| CYP19A1 | No (%) | Yes (%) | OR (95% CI) | P‐value |
| rs727479 A/C | ||||
| A/A‐A/C | 93.8 | 91.0 | Ref. | |
| C/C | 6.3 | 9.0 | 1.40 (0.27–7.38) | 0.68 |
| rs1008805 A/G | ||||
| A/A‐A/G | 59.4 | 85.9 | Ref. | |
| G/G | 40.6 | 14.1 | 0.24 (0.09–0.65) | 0.004 |
| rs749292 A/G | ||||
| G/G‐A/G | 90.6 | 83.3 | Ref. | |
| A/A | 9.4 | 16.7 | 1.80 (0.46–6.99) | 0.38 |
| rs730154 C/T | ||||
| C/C‐C/T | 96.9 | 98.7 | Ref. | |
| T/T | 3.1 | 1.3 | 0.45 (0.03–7.38) | 0.58 |
Ref., reference; OR, odds ratio; CI, confidence interval
These two single‐nucleotide polymorphisms were in complete linkage disequilibrium
We then included the two genotypes with significant results (ABCB1 3435 TT and CYP19A1 rs1008805 GG) in logistic regression models including relevant covariates such as age, BMI or years since menopause onset (see P‐values for the individual association of these covariates with arthralgia in supplementary Table S2). The relevant association for the 3435 TT genotype remained so (OR = 0.28 [0.09–0.94]; P = 0.040), and that for the rs1008805 GG genotype increased its significance (OR = 0.20 [0.07–0.59], P = 0.0028). The combined analysis of variants in the ABCB1, TCL1A or CYP19A1 genes (haplotype study) controlling for meaningful variables did not reveal further associations with arthralgia (Supplementary Table S3).
With regard to cancer recurrence, it was experienced by 6% of the patients (seven women). After controlling by relevant demographic and clinical variables, we observed no associations between anastrozole plasma levels and the occurrence of relapse (OR high vs. low concentrations = 0.42 [0.06–3.17], P = 0.404). Table 4 shows OR values for the association between CYP19A1 SNPs and breast cancer recurrence. The small number of both events and subjects carrying the different homozygous CYP19A1 variant genotypes forced an analysis based on a dominant model of inheritance (Table 4). Of note, the rs727479 AA genotype was absent in the group with cancer recurrence but it accounted for roughly 38% of patients who did not relapse (P for trend = 0.031; Table 4). This association remained statistically significant (P = 0.028) after adjusting by age, grade, stage and HER2 status (see P‐values for the individual association of these covariates with the outcome in supplementary Table S2).
Table 4.
Association of CYP19A1 polymorphisms with breast cancer recurrence
| Recurrence | ||||
|---|---|---|---|---|
| No (%) | Yes (%) | OR (95% CI) | P‐value | |
| rs727479 | ||||
| A/A | 37.9 | 0.0 | Ref. | |
| A/C–C/C | 62.1 | 100.0 | a | 0.031 |
| rs1008805 | ||||
| A/A | 25.2 | 14.3 | Ref. | |
| A/G–G/G | 74.8 | 85.7 | 1.67 (0.18–15.01) | 0.543 |
| rs749292 | ||||
| A/A | 15.5 | 0.0 | Ref. | |
| A/G–G/G | 84.5 | 100.0 | a | 0.386 |
| rs730154 | ||||
| C/C | 28.2 | 57.1 | Ref. | |
| C/T–T/T | 71.8 | 42.9 | 0.73 (0.07–1.97) | 0.224 |
Ref., reference; OR, odds ratio; CI, confidence interval
One of the groups analysed had no subjects and hence odds ratio calculations could not be performed
The haplotype study in CYP19A1 adjusted by the same variables did not reveal further associations (Supplementary Table S4).
Discussion
Over the past 15 years it has become clear that the ABCB1 transporter, because of its location in intestine, liver and kidney, greatly contributes to the bioavailability of orally administered drugs. Accordingly, variability in its gene locus has been associated with changes in the disposition, toxicity and response to a wide variety of xenobiotics, including many anticancer compounds 11. The in vitro study by Miyajima et al. 9, which indicates that anastrozole may be a substrate for this transporter, prompted us to carry out the present work in order to test the influence of the ABCB1 gene status in postmenopausal HR+‐breast cancer patients.
First, we observed a high degree of variability (over 8‐fold) of anastrozole plasma concentrations in our series, a range that was similar to that reported recently by other research groups in patients with the same pathology 14, 15. Moreover, we found that a nonsynonymous ABCB1 polymorphism (G2677 T) contributed to this variability; with 2677 TT carriers showing significantly higher drug levels. This same polymorphism, has been associated before with pharmacokinetics and the patient's response to some chemotherapy agents used in breast cancer such as paclitaxel 22, 23 or docetaxel 24, 25. However, to our knowledge, there are no similar studies with anastrozole. The observed higher concentrations in carriers of the 2677 TT genotype would be consistent with a decreased efflux capacity of the mutated transporter, leading to increased blood concentrations. In any case, studies on the specific effect of ACB1 SNPs on protein expression and function have not been conclusive to date 11.
Ingle et al. suggested that the wide range of anastrozole concentrations displayed by breast cancer patients may obey to differences in the drug's metabolism 14. Now, our results indicate that ABCB1‐mediated anastrozole efflux transport, dependent on the ABCB1 genotype (and also subjected to drug interactions), may also play a significant role. We agree with Ingle et al. when they state that the standard 1 mg daily dose of anastrozole may not be optimal for a substantial proportion of women with breast cancer 14.
Also, regarding anastrozole pharmacokinetics, two studies have shown that the correlation between anastrozole plasma concentrations and the effect on oestrogen deprivation in postmenopausal HR+ breast cancer patients is unclear 14, 26. Indeed, these studies report on a significant proportion of patients with high anastrozole concentrations that paradoxically display increased levels of oestrogens. Our results are in line with these findings, as we did not find a correlation between drug levels with either the development of toxicity (arthralgia) or the incidence of cancer recurrence.
Significant musculoskeletal discomfort in anastrozole‐treated patients may lead to suboptimal adherence 21, which is why finding individual risk factors for these adverse effects is crucial if we were to implement personalized medicine in breast cancer patients on AIs. In this regard, the incidence of arthralgia in our patients was in line with that estimated recently (70%) by Nyrop et al. 27, albeit figures are sometimes difficult to compare, given the high variability in the definitions of the phenotype and the self‐reported nature of the adverse effect. The second goal of the study included the validation of the association between AIs‐induced arthralgia and four SNPs, namely rs7158782‐rs2369049, rs7159713 and rs11849538, located near the TCL1A gene; an association that was reported in a previous GWAS 18. We observed a very high LD between these SNPs (which was complete for two of them) in our Spanish Caucasian patients and consequently the results of their association with arthralgia are almost identical (see Table 3). We could not find any evidence of their alleged effect on this adverse event. Indeed, an editorial on the referred GWAS already questioned the clinical significance of its results 28. However, it should be noted that this GWAS used six musculoskeletal adverse events as the phenotype, which included joint pain, muscle pain, bone pain, arthritis, diminished joint function and other musculoskeletal problems, while the present study used arthralgia as the phenotype. Therefore, the discrepant results between our study and the previous one could be due to the different definitions of phenotype.
We also assessed the role of CYP19A1 SNPs in the development of arthralgia, as reports on this association have been contradictory so far 16. We found that the rs1008805 SNP was related to joint stiffening and pain in patients on anastrozole. Consistent with this finding, Park et al. reported that a haplotype containing this variant was related to musculoskeletal adverse events in breast cancer patients on letrozole 29, a similar AI, although there are no data regarding anastrozole‐treated patients. The lack of association with arthralgia displayed for the other CYP19A1 SNPs studied is in agreement with the negative findings reported by Mao et al. 30.
The ABCB1 C3435T SNP was found to be inversely associated to the occurrence of arthralgia. Of note, this is a synonymous polymorphism and therefore one would not expect an impact on protein expression or function; however, Kimchi‐Sarfaty et al. demonstrated that this SNP results in similar mRNA and protein levels but with altered conformations, which affects the substrate specificity of the ABCB1 transporter 31. In any case, the fact that the precise link between anastrozole levels and the onset of musculoskeletal symptoms is yet to be elucidated makes these results hard to interpret. By contrast, the consequences of an altered ABCB1 activity could go beyond its impact on the drug's transport. A recent study has demonstrated that polymorphisms in similar ABC transporters (ABCC2) correlate with the expression of UDP‐glucuronosyltransferase1A4 (UGT1A4) and anastrozole glucuronidation 32, the main elimination pathway for this drug 33.
The last goal of our study was to determine the influence of CYP19A1 SNPs on cancer recurrence. The incidence of recurrence in our series was slightly lower than the 9% reported by Dowsett et al. in a meta‐analysis comprising 9856 breast cancer patients treated with AIs 34. We observed that the rs727479 SNP was significantly related to this event. In agreement with our finding, Miron et al. observed an association of this SNP with reduced local recurrence of the cancer, although the study was carried out in a very small number of patients 35. Conversely, Colomer et al. could not find a statistically significant effect of rs727479 on the clinical response to anastrozole. It should be noted, however, that the primary outcome collected by the authors in this last work was time to progression and not cancer recurrence 36. We should also take into account that rs727479 is an intronic SNP, and therefore it might be in LD with another mutation/s in coding exons that could be the true effector of this association. Longer‐term studies will better clarify the link between CYP19A1 genetic variability and the clinical response to anastrozole and to AIs in general.
A limitation of this work was the relatively low sample size available, which along with the small number of carriers of some allelic variants precluded the analysis of the data with some genetic models. However, this limited sample also allowed for all the patients to be diagnosed and treated by the same clinicians in the same facilities, and resulted in all patients having the same ethnicity, which altogether increases the homogeneity of our analyses and reduces the chance that the findings may be due to population structure. Another limitation was that no survival analyses could be carried out (particularly in relation to CYP19A1 SNPs), since no deaths were recorded over the period of study. Finally, oestrogen concentrations were not measured in our patients, which could have been informative given that anastrozole plasma levels did not explain arthralgia or cancer recurrence.
In summary, the results of the present work reveal a substantial variability of anastrozole plasma concentrations in postmenopausal women with hormone receptor‐positive breast cancer. Our findings indicate that this variability may be, at least in part, attributable to the status of the ABCB1 gene locus. Furthermore, genetic variants in the CYP19A1 gene were associated with arthralgia and cancer recurrence in our patients. These results taken together indicate that anastrozole, a commonly used agent in breast cancer, makes an ideal candidate for multicentric, pharmacogenomic studies that can include other possibly relevant genes such as UGT1A4 and larger number of patients in long follow‐up periods.
Competing Interests
The authors declare no competing interests. We would like to acknowledge the technical and human support provided by the Service of Elemental and Molecular Analysis and the Service of Bioscience‐Applied Techniques at SAIUEx (financed by University of Extremadura, Junta de Extremadura, MICINN, FEDER and FSE). We also thank the patients who participated in this study.
This work has been supported in part by a grant from Fundación de Investigación Médica Mutua Madrileña, Madrid, Spain; grant PI15/00804 from Instituto de Salud Carlos III, Ministry of Health, Madrid, Spain; and grant GR15012 from Junta de Extremadura, Consejeria de Economia, Comercio e Innovacion, Merida, Spain.
Contributors
G.G. performed the genetic association studies and drafted the paper; J.A.C. carried out the pharmacokinetic analyses and designed the study along with C.J.; N.R., C.O. and R.M. collected demographic and clinical parameters from clinical records.
Supporting information
Figure S1 (A) Representative chromatograms of anastrozole (0.8 min, channel 1) and verapamil (1.3 min, channel 2) in human plasma samples. (B) Blank plasma spiked with 75 ng ml−1 anastrozole (channel 1) and 10.9 ng ml−1 verapamil (channel 2).
Table S1 Differences regarding anastrozole plasma levels for ABCB1 haplotypes. Frequency threshold for haplotype identification was set at 0.01.
Table S2 Univariate analysis of the association between covariates considered clinically relevant that were included in the final regression models and the two clinical outcomes studied. Median values were used as cut‐off points for age, body mass index (BMI) and time since menopause. Nearly all patients underwent previous chemotherapy and therefore this variable was not included in the models.
Table S3 Differences regarding anastrozole plasma levels for ABCB1 haplotypes. Frequency threshold for haplotype identification was set at 0.01.
Table S4 Haplotype study of the four polymorphisms in the CYP19A1 gene with regard to their association with breast cancer recurrence. Frequency threshold for haplotype identification was set at 0.01.
Figure S1. Supporting info item
Table S1. Supporting info item
Gervasini, G. , Jara, C. , Olier, C. , Romero, N. , Martínez, R. , and Carrillo, J. A. (2017) Polymorphisms in ABCB1 and CYP19A1 genes affect anastrozole plasma concentrations and clinical outcomes in postmenopausal breast cancer patients. Br J Clin Pharmacol, 83: 562–571. doi: 10.1111/bcp.13130.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat 2002; 76: 27–36. [DOI] [PubMed] [Google Scholar]
- 5. Needleman SJ, Tobias JS. Review of the ATAC study: tamoxifen versus anastrozole in early‐stage breast cancer. Expert Rev Anticancer Ther 2008; 8: 1871–1881. [DOI] [PubMed] [Google Scholar]
- 6. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 2005; 365: 60–62. [DOI] [PubMed] [Google Scholar]
- 7. Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early‐stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003; 98: 1802–1810. [DOI] [PubMed] [Google Scholar]
- 8. Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002; 359: 2131–2139. [DOI] [PubMed] [Google Scholar]
- 9. Miyajima M, Kusuhara H, Takahashi K, Takashima T, Hosoya T, Watanabe Y, et al. Investigation of the effect of active efflux at the blood–brain barrier on the distribution of nonsteroidal aromatase inhibitors in the central nervous system. J Pharm Sci 2013; 102: 3309–3319. [DOI] [PubMed] [Google Scholar]
- 10. Borst P, Schinkel AH. P‐glycoprotein ABCB1: a major player in drug handling by mammals. J Clin Invest 2013; 123: 4131–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT. Impact of genetic polymorphisms of ABCB1 (MDR1, P‐glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin Pharmacokinet 2015; 54: 709–735. [DOI] [PubMed] [Google Scholar]
- 12. Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P‐glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther 2004; 75: 13–33. [DOI] [PubMed] [Google Scholar]
- 13. Hertz DL, Barlow WE, Kidwell KM, Albain KS, Vandenberg TA, Dakhil SR, et al. Fulvestrant decreases anastrozole drug concentrations when taken concurrently by patients with metastatic breast cancer treated on SWOG study S0226. Br J Clin Pharmacol 2016; 81: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids 2015; 99: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hubalek M, Oberguggenberger A, Beer B, Meraner V, Sztankay M, Oberacher H, et al. Does obesity interfere with anastrozole treatment? Positive association between body mass index and anastrozole plasma levels. Clin Breast Cancer 2014; 14: 291–296. [DOI] [PubMed] [Google Scholar]
- 16. Artigalas O, Vanni T, Hutz MH, Ashton‐Prolla P, Schwartz IV. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: a systematic review and meta‐analysis. BMC Med 2015; 13: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bryce J, Bauer M, Hadji P. Managing arthralgia in a postmenopausal woman taking an aromatase inhibitor for hormonesensitive early breast cancer: a case study. Cancer Manag Res 2012; 4: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ingle JN, Schaid DJ, Goss PE, Liu M, Mushiroda T, Chapman JA, et al. Genome‐wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 2010; 28: 4674–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu J, He J, Zhang Y, Qin F, Xiong Z, Li F. An ultraperformance liquid chromatography–tandem mass spectrometry method for determination of anastrozole in human plasma and its application to a pharmacokinetic study. Biomed Chromatogr 2011; 25: 511–516. [DOI] [PubMed] [Google Scholar]
- 20. Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22: 1928–1929. [DOI] [PubMed] [Google Scholar]
- 21. Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early‐stage breast cancer. J Clin Oncol 2007; 25: 3877–3883. [DOI] [PubMed] [Google Scholar]
- 22. Fransson MN, Green H, Litton JE, Friberg LE. Influence of Cremophor EL and genetic polymorphisms on the pharmacokinetics of paclitaxel and its metabolites using a mechanism‐based model. Drug Metab Dispos 2011; 39: 247–255. [DOI] [PubMed] [Google Scholar]
- 23. Chang H, Rha SY, Jeung HC, Im CK, Ahn JB, Kwon WS, et al. Association of the ABCB1 gene polymorphisms 2677G > T/A and 3435C > T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol 2009; 20: 272–277. [DOI] [PubMed] [Google Scholar]
- 24. Tsai SM, Lin CY, Wu SH, Hou LA, Ma H, Tsai LY, et al. Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta 2009; 404: 160–165. [DOI] [PubMed] [Google Scholar]
- 25. Choi JR, Kim JO, Kang DR, Shin JY, Zhang XH, Oh JE, et al. Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat 2015; 47: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res 2010; 70: 3278–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nyrop KA, Callahan LF, Rini C, Altpeter M, Hackney B, DePue A, et al. Aromatase inhibitor associated arthralgia: the importance of oncology provider‐patient communication about side effects and potential management through physical activity. Support Care Cancer 2016; 24: 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Offit K, Robson ME. New pharmacogenomic paradigm in breast cancer treatment. J Clin Oncol 2010; 28: 4665–4666. [DOI] [PubMed] [Google Scholar]
- 29. Park IH, Lee YS, Lee KS, Kim SY, Hong SH, Jeong J, et al. Single nucleotide polymorphisms of CYP19A1 predict clinical outcomes and adverse events associated with letrozole in patients with metastatic breast cancer. Cancer Chemother Pharmacol 2011; 68: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 30. Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res 2011; 13: R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimchi‐Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007; 315: 525–528. [DOI] [PubMed] [Google Scholar]
- 32. Edavana VK, Penney RB, Yao‐Borengasser A, Starlard‐Davenport A, Dhakal IB, Kadlubar S. Effect of MRP2 and MRP3 polymorphisms on anastrozole glucuronidation and MRP2 and MRP3 gene expression in normal liver samples. Int J Cancer Res Mol Mech 2015; 1. doi: 10.16966/2381-3318.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamdem LK, Liu Y, Stearns V, Kadlubar SA, Ramirez J, Jeter S, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol 2010; 70: 854–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta‐analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010; 28: 509–518. [DOI] [PubMed] [Google Scholar]
- 35. Miron L, Negura L, Peptanariu D, Marinca M. Research on aromatase gene (CYP19A1) polymorphisms as a predictor of endocrine therapy effectiveness in breast cancer. Rev Med Chir Soc Med Nat Iasi 2012; 116: 997–1004. [PubMed] [Google Scholar]
- 36. Colomer R, Monzo M, Tusquets I, Rifa J, Baena JM, Barnadas A, et al. A single‐nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clin Cancer Res 2008; 14: 811–816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) Representative chromatograms of anastrozole (0.8 min, channel 1) and verapamil (1.3 min, channel 2) in human plasma samples. (B) Blank plasma spiked with 75 ng ml−1 anastrozole (channel 1) and 10.9 ng ml−1 verapamil (channel 2).
Table S1 Differences regarding anastrozole plasma levels for ABCB1 haplotypes. Frequency threshold for haplotype identification was set at 0.01.
Table S2 Univariate analysis of the association between covariates considered clinically relevant that were included in the final regression models and the two clinical outcomes studied. Median values were used as cut‐off points for age, body mass index (BMI) and time since menopause. Nearly all patients underwent previous chemotherapy and therefore this variable was not included in the models.
Table S3 Differences regarding anastrozole plasma levels for ABCB1 haplotypes. Frequency threshold for haplotype identification was set at 0.01.
Table S4 Haplotype study of the four polymorphisms in the CYP19A1 gene with regard to their association with breast cancer recurrence. Frequency threshold for haplotype identification was set at 0.01.
Figure S1. Supporting info item
Table S1. Supporting info item


