Abstract
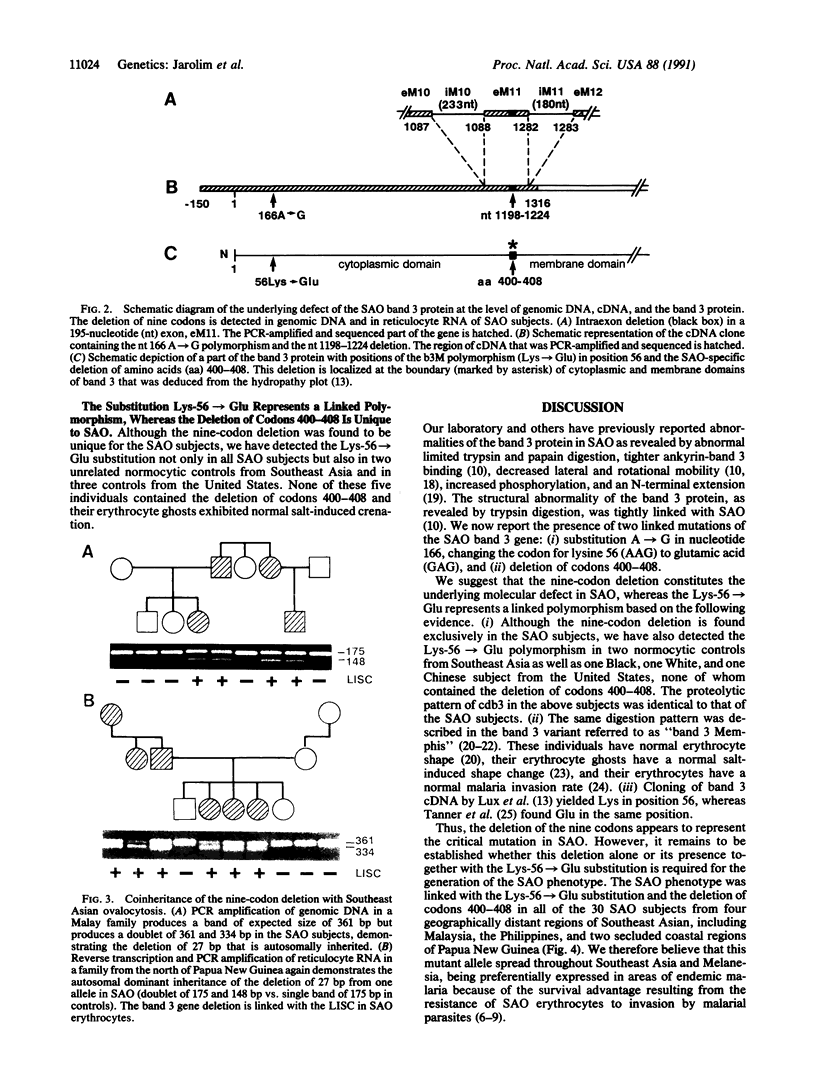
Southeast Asian ovalocytosis (SAO) is a hereditary condition that is widespread in parts of Southeast Asia. The ovalocytic erythrocytes are rigid and resistant to invasion by various malarial parasites. We have previously found that the underlying defect in SAO involves band 3 protein, the major transmembrane protein, which has abnormal structure and function. We now report two linked mutations in the erythrocyte band 3 gene in SAO: (i) a deletion of codons 400-408 and (ii) a substitution, A----G, in the first base of codon 56 leading to substitution of Lys-56 by Glu-56. The first defect leads to a deletion of nine amino acids in the boundary of cytoplasmic and membrane domains of band 3. This defect has been detected in all 30 ovalocytic subjects from Malaysia, the Philippines, and two unrelated coastal regions of Papua New Guinea, whereas it was absent in all 30 controls from Southeast Asia and 20 subjects of different ethnic origin from the United States. The Lys-56----Glu substitution has likewise been found in all SAO subjects. However, it has also been detected in 5 of the 50 control subjects, suggesting that it represents a linked polymorphism. We conclude that the deletion of codons 400-408 in the band 3 gene constitutes the underlying molecular defect in SAO.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato D., Booth P. B. Hereditary ovalocytosis in Melanesians. P N G Med J. 1977 Mar;20(1):26–32. [PubMed] [Google Scholar]
- Atkinson C. T., Aikawa M., Perry G., Fujino T., Bennett V., Davidson E. A., Howard R. J. Ultrastructural localization of erythrocyte cytoskeletal and integral membrane proteins in Plasmodium falciparum-infected erythrocytes. Eur J Cell Biol. 1988 Feb;45(2):192–199. [PubMed] [Google Scholar]
- Booth P. B., Serjeantson S., Woodfield D. G., Amato D. Selective depression of blood group antigens associated with hereditary ovalocytosis among melanesians. Vox Sang. 1977;32(2):99–110. doi: 10.1111/j.1423-0410.1977.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Castelino D., Saul A., Myler P., Kidson C., Thomas H., Cooke R. Ovalocytosis in Papua New Guinea -- dominantly inherited resistance to malaria. Southeast Asian J Trop Med Public Health. 1981 Dec;12(4):549–555. [PubMed] [Google Scholar]
- Cattani J. A., Gibson F. D., Alpers M. P., Crane G. G. Hereditary ovalocytosis and reduced susceptibility to malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1987;81(5):705–709. doi: 10.1016/0035-9203(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Reid M. E., Jensen R. H., Mohandas N. Signal transduction by glycophorin A: role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol. 1988 Oct;107(4):1351–1357. doi: 10.1083/jcb.107.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzewski A. R., Fryer P. R., Griffiths S., Wilson R. J., Gratzer W. B. Red cell membrane protein distribution during malarial invasion. J Cell Sci. 1989 Apr;92(Pt 4):691–699. doi: 10.1242/jcs.92.4.691. [DOI] [PubMed] [Google Scholar]
- Drickamer L. K. Orientation of the band 3 polypeptide from human erythrocyte membranes. Identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem. 1978 Oct 25;253(20):7242–7248. [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Hadley T., Saul A., Lamont G., Hudson D. E., Miller L. H., Kidson C. Resistance of Melanesian elliptocytes (ovalocytes) to invasion by Plasmodium knowlesi and Plasmodium falciparum malaria parasites in vitro. J Clin Invest. 1983 Mar;71(3):780–782. doi: 10.1172/JCI110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain-Chishti A., Ruff P. Malaria and ovalocytosis--molecular mimicry? Biochim Biophys Acta. 1991 Apr 15;1096(3):263–264. doi: 10.1016/0925-4439(91)90014-z. [DOI] [PubMed] [Google Scholar]
- Jones G. L., Edmundson H. M., Wesche D., Saul A. Human erythrocyte Band-3 has an altered N terminus in malaria-resistant Melanesian ovalocytosis. Biochim Biophys Acta. 1990 Nov 14;1096(1):33–40. doi: 10.1016/0925-4439(90)90009-e. [DOI] [PubMed] [Google Scholar]
- Kidson C., Lamont G., Saul A., Nurse G. T. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5829–5832. doi: 10.1073/pnas.78.9.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. R., Yew N. S., Ansorge W., Voss H., Schwager C., Vennström B., Zenke M., Engel J. D. Two different mRNAs are transcribed from a single genomic locus encoding the chicken erythrocyte anion transport proteins (band 3). Mol Cell Biol. 1988 Oct;8(10):4416–4424. doi: 10.1128/mcb.8.10.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M., Lodish H. F. Structure and organization of the murine band 3 gene. J Biol Chem. 1987 Jun 15;262(17):8035–8040. [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kudrycki K. E., Newman P. R., Shull G. E. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990 Jan 5;265(1):462–471. [PubMed] [Google Scholar]
- Liu S. C., Zhai S., Palek J., Golan D. E., Amato D., Hassan K., Nurse G. T., Babona D., Coetzer T., Jarolim P. Molecular defect of the band 3 protein in southeast Asian ovalocytosis. N Engl J Med. 1990 Nov 29;323(22):1530–1538. doi: 10.1056/NEJM199011293232205. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Lie-Injo L. E., Friedman M., Mak J. W. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984 Jun;63(6):1385–1392. [PubMed] [Google Scholar]
- Mueller T. J., Morrison M. Detection of a variant of protein 3, the major transmembrane protein of the human erythrocyte. J Biol Chem. 1977 Oct 10;252(19):6573–6576. [PubMed] [Google Scholar]
- Palatnik M., Simões M. L., Alves Z. M., Laranjeira N. S. The 60 and 63 kDa proteolytic peptides of the red cell membrane band-3 protein: their prevalence in human and non-human primates. Hum Genet. 1990 Dec;86(2):126–130. doi: 10.1007/BF00197692. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Chasis J. A., Mohandas N., Anstee D. J., Tanner M. J., Merry A. H. Inhibition of malarial parasite invasion by monoclonal antibodies against glycophorin A correlates with reduction in red cell membrane deformability. Blood. 1989 Oct;74(5):1836–1843. [PubMed] [Google Scholar]
- Ranney H. M., Rosenberg G. H., Morrison M., Mueller T. J. Frequencies of Band 3 variants of human red cell membranes in some different populations. Br J Haematol. 1990 Jun;75(2):262–267. doi: 10.1111/j.1365-2141.1990.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Saul A., Lamont G., Sawyer W. H., Kidson C. Decreased membrane deformability in Melanesian ovalocytes from Papua New Guinea. J Cell Biol. 1984 Apr;98(4):1348–1354. doi: 10.1083/jcb.98.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman S., Roth E. F., Jr, Cheng B., Rybicki A. C., Sussman I. I., Wong M., Wang W., Ranney H. M., Nagel R. L., Schwartz R. S. Growth of Plasmodium falciparum in human erythrocytes containing abnormal membrane proteins. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7339–7343. doi: 10.1073/pnas.87.18.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeantson S., Bryson K., Amato D., Babona D. Malaria and hereditary ovalocytosis. Hum Genet. 1977 Jun 30;37(2):161–167. doi: 10.1007/BF00393579. [DOI] [PubMed] [Google Scholar]
- Sykes B. C. DNA in heritable disease. Lancet. 1983 Oct 1;2(8353):787–788. doi: 10.1016/s0140-6736(83)92314-0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley L., Nash G. B., Jones G. L., Sawyer W. H. Decreased rotational diffusion of band 3 in Melanesian ovalocytes from Papua, New Guinea. J Membr Biol. 1991 Apr;121(1):59–66. doi: 10.1007/BF01870651. [DOI] [PubMed] [Google Scholar]
- Yannoukakos D., Vasseur C., Driancourt C., Blouquit Y., Delaunay J., Wajcman H., Bursaux E. Human erythrocyte band 3 polymorphism (band 3 Memphis): characterization of the structural modification (Lys 56----Glu) by protein chemistry methods. Blood. 1991 Aug 15;78(4):1117–1120. [PubMed] [Google Scholar]