Abstract
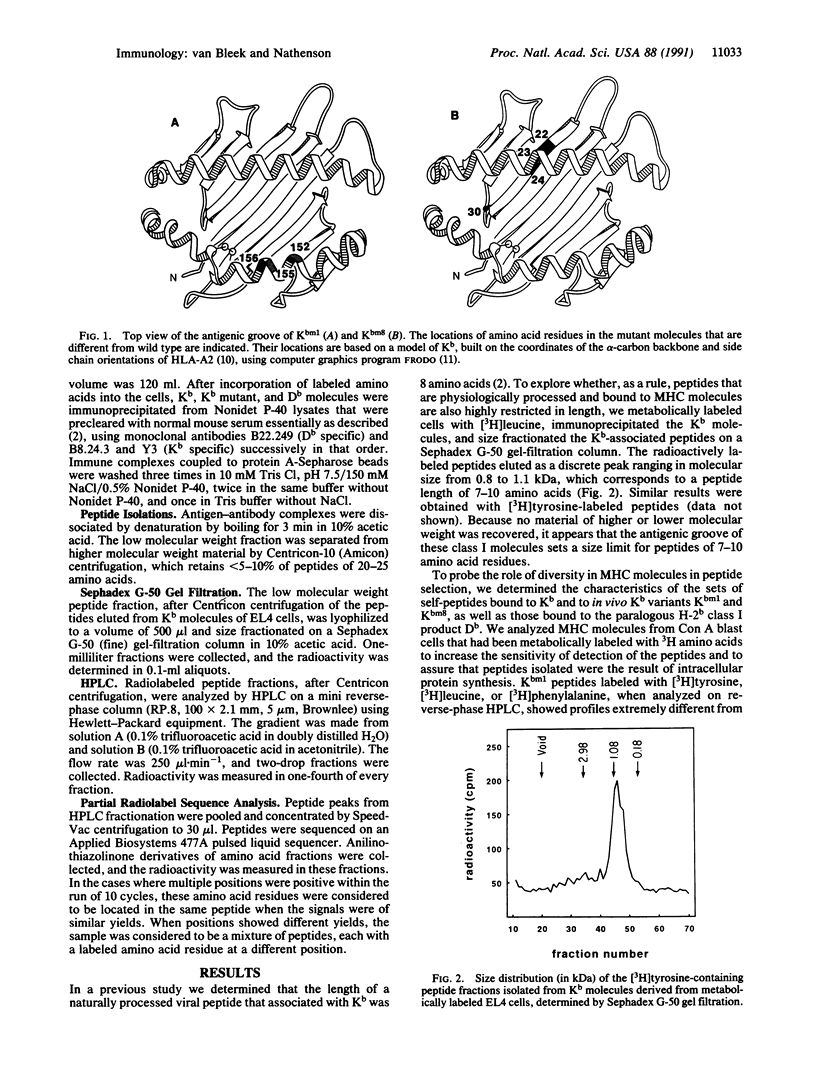
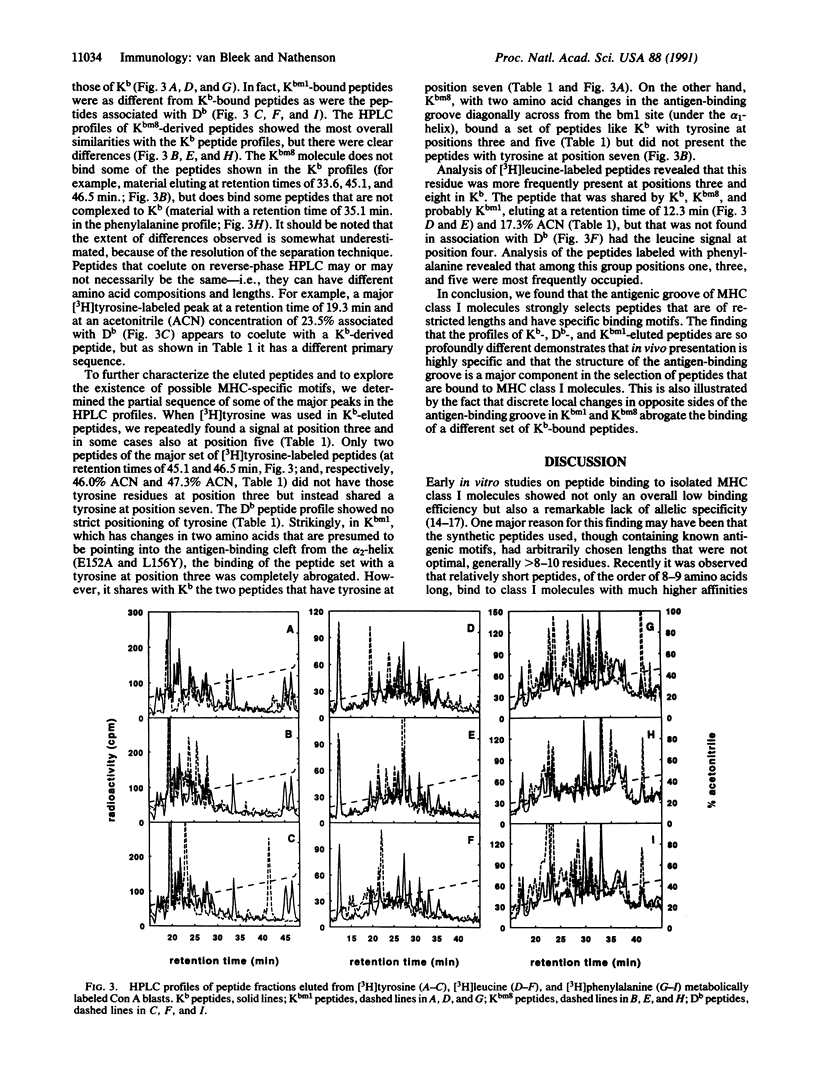
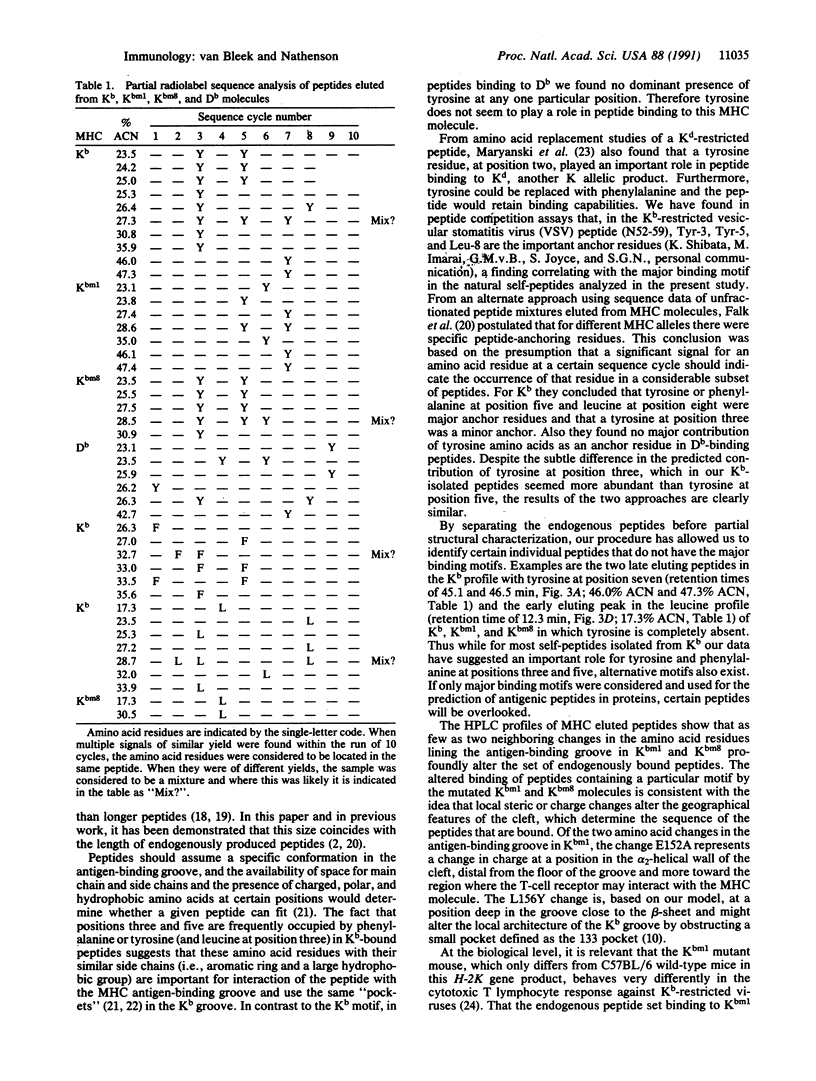
We have examined the effect of diversity in the antigen-binding groove of the Kb, Db, Kbm1, and Kbm8 major histocompatibility complex (MHC) class I molecules on the set of self-peptides they present on the cell surface, by using a procedure we recently developed in our laboratory to isolate endogenously processed peptides bound to MHC class I molecules. We found that such naturally processed peptides are 7-10 amino acids long. A major motif of tyrosine and phenylalanine residues at positions three and five was found for peptides binding to Kb. The availability of Kb mutant molecules Kbm1 and Kbm8, each with localized clustered changes in the antigen-binding cleft, allowed us to probe the effect of such small alterations on peptide selection. We found that such changes in different regions in the antigen-binding groove exert an absolute effect by changing subsets of self-peptides bound to these MHC molecules. In the Kbm1 mutant, the binding of the characteristic major set of Kb-associated peptides with tyrosine at position three or both positions three and five is abrogated, although this MHC molecule still binds peptides with tyrosine at position seven; the latter peptides also bind to Kb. Kbm8 shares the major Tyr-3, Tyr-5 peptide set that binds to Kb but does not bind the peptides with tyrosine at position seven. Thus differences in binding selectivity in Kbm1 and Kbm8 appear to be the major determinant for the observed alterations in in vivo immune responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bouillot M., Choppin J., Cornille F., Martinon F., Papo T., Gomard E., Fournie-Zaluski M. C., Levy J. P. Physical association between MHC class I molecules and immunogenic peptides. Nature. 1989 Jun 8;339(6224):473–475. doi: 10.1038/339473a0. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Rothbard J., Parham P. Apparent lack of MHC restriction in binding of class I HLA molecules to solid-phase peptides. J Exp Med. 1990 Sep 1;172(3):931–936. doi: 10.1084/jem.172.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. S., Geier S., Nathenson S. G., Forman J. Analysis of associative recognition determinants on class I H-2Kb mutant molecules. Immunogenetics. 1985;22(4):391–397. doi: 10.1007/BF00430922. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Deverson E. V., Gow I. R., Coadwell W. J., Monaco J. J., Butcher G. W., Howard J. C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990 Dec 20;348(6303):738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- Elliott T., Cerundolo V., Elvin J., Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991 May 30;351(6325):402–406. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Frelinger J. A., Gotch F. M., Zweerink H., Wain E., McMichael A. J. Evidence of widespread binding of HLA class I molecules to peptides. J Exp Med. 1990 Sep 1;172(3):827–834. doi: 10.1084/jem.172.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Healy F., Sire J., Gomard E., Yssel H., Jordan B., Levy J. P. A study of functionally active amino acids involved in the interaction of HLA-A2 or HLA-A3 molecules with cytolytic T lymphocytes. J Immunol. 1988 Oct 1;141(7):2487–2496. [PubMed] [Google Scholar]
- Hogan K. T., Clayberger C., Bernhard E. J., Walk S. F., Ridge J. P., Parham P., Krensky A. M., Engelhard V. H. Identification by site-directed mutagenesis of amino acid residues contributing to serologic and CTL-defined epitope differences between HLA-A2.1 and HLA-A2.3. J Immunol. 1988 Oct 1;141(7):2519–2525. [PubMed] [Google Scholar]
- Hogan K. T., Clayberger C., Le A. X., Walk S. F., Ridge J. P., Parham P., Krensky A. M., Engelhard V. H. Cytotoxic T lymphocyte-defined epitope differences between HLA-A2.1 and HLA-A2.2 map to two distinct regions of the molecule. J Immunol. 1988 Dec 1;141(11):4005–4011. [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science. 1990 Apr 20;248(4953):367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- Hunt H. D., Pullen J. K., Dick R. F., Bluestone J. A., Pease L. R. Structural basis of Kbm8 alloreactivity. Amino acid substitutions on the beta-pleated floor of the antigen recognition site. J Immunol. 1990 Sep 1;145(5):1456–1462. [PubMed] [Google Scholar]
- Jelachich M. L., Cowan E. P., Turner R. V., Coligan J. E., Biddison W. E. Analysis of the molecular basis of HLA-A3 recognition by cytotoxic T cells using defined mutants of the HLA-A3 molecule. J Immunol. 1988 Aug 15;141(4):1108–1113. [PubMed] [Google Scholar]
- Martinon F., Cornille F., Gomard E., Fournie-Zaluski M. C., Abastado J. P., Roques B. P., Levy J. P. Two epitopes and one agretope map to a single HLA-A2 peptide recognized by H-2-restricted T cells. J Immunol. 1989 May 15;142(10):3489–3494. [PubMed] [Google Scholar]
- Maryanski J. L., Verdini A. S., Weber P. C., Salemme F. R., Corradin G. Competitor analogs for defined T cell antigens: peptides incorporating a putative binding motif and polyproline or polyglycine spacers. Cell. 1990 Jan 12;60(1):63–72. doi: 10.1016/0092-8674(90)90716-r. [DOI] [PubMed] [Google Scholar]
- Mattson D. H., Shimojo N., Cowan E. P., Baskin J. J., Turner R. V., Shvetsky B. D., Coligan J. E., Maloy W. L., Biddison W. E. Differential effects of amino acid substitutions in the beta-sheet floor and alpha-2 helix of HLA-A2 on recognition by alloreactive viral peptide-specific cytotoxic T lymphocytes. J Immunol. 1989 Aug 15;143(4):1101–1107. [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. N., Morrison L. A., Gorka J., Braciale V. L., Braciale T. J. The recognition of a viral antigenic moiety by class I MHC-restricted cytolytic T lymphocytes is limited by the availability of the endogenously processed antigen. J Immunol. 1990 Nov 15;145(10):3188–3193. [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Perkins D. L., Berriz G., Kamradt T., Smith J. A., Gefter M. L. Immunodominance: intramolecular competition between T cell epitopes. J Immunol. 1991 Apr 1;146(7):2137–2144. [PubMed] [Google Scholar]
- Pfaffenbach G. M., Uehara H., Geliebter J., Nathenson S. G., Schulze D. H. Analysis of the H-2Kbm8 mutant: correlation of structure with function. Mol Immunol. 1991 Jul;28(7):697–701. doi: 10.1016/0161-5890(91)90111-v. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Wallny H. J., Faath S., Rammensee H. G. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990 Jul 20;249(4966):283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T. N., De Bruijn M. L., Vernie L. N., Kast W. M., Melief C. J., Neefjes J. J., Ploegh H. L. Peptide selection by MHC class I molecules. Nature. 1991 Apr 25;350(6320):703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991 May 23;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Winter C. C., Carreno B. M., Turner R. V., Koenig S., Biddison W. E. The 45 pocket of HLA-A2.1 plays a role in presentation of influenza virus matrix peptide and alloantigens. J Immunol. 1991 May 15;146(10):3508–3512. [PubMed] [Google Scholar]


