Abstract
Bisphenol-A (BPA), a widely used synthetic compound in plastics, disrupts endocrine function and interferes with physiological actions of endogenous gonadal hormones. Chronic effects of BPA on reproductive function, learning and memory, brain structure, and social behavior have been intensively investigated. However, less is known about the influence of BPA on long-term potentiation (LTP), one of the major cellular mechanisms that underlie learning and memory. In the present study, for the first time we investigated the effect of different doses of BPA on hippocampal LTP in rat brain slices. We found a biphasic effect of BPA on LTP in the dentate gyrus: exposure to BPA at a low dose (100 nM) enhanced LTP and exposure to BPA at a high dose (1000 nM) inhibited LTP compared with vehicle controls. The rapid facilitatory effect of low-dose BPA on hippocampal LTP required membrane-associated estrogen receptor (ER) and involved activation of the extracellular signal-regulated kinase (ERK) signaling pathway. Coadministration of 17β-estradiol (E2, the primary estrogen hormone) and BPA (100 nM) abolished both the BPA-induced enhancement of LTP and the E2-induced enhancement of baseline fEPSP, suggesting a complex interaction between BPA- and E2-mediated signaling pathways. Our investigation implies that even nanomolar levels of endocrine disrupters (e.g., BPA) can induce significant effects on hippocampal LTP.
1. Introduction
Bisphenol-A (BPA) is a widely used synthetic compound included in polycarbonate plastics and epoxy resins, for example, in food and beverage containers, dental prostheses, compact discs, and baby bottles. It is capable of acting as an endocrine disrupter and interferes with actions of endogenous gonadal hormones (e.g., estrogen or androgen) at low concentrations. BPA can bind to estrogen receptors (ERs) at low concentration and thus affects normal hormonal regulation and endocrine function [1]. A large number of studies have indicated that chronic exposure to the low-dose (nanomolar) BPA during fetal/neonatal stages inhibits sexual differentiation and nonreproductive behaviors of adult animals [2–4].
Although the widespread effects of BPA on reproductive function, brain structure, and social behavior have been investigated, recent studies reported controversial actions of BPA on learning and memory, ranging from deficits to no effect and to enhancements. In rodents, pre- and perinatal exposures to BPA at or below the TDI (tolerable daily intake; ⩽50 μg/kg/day) have resulted in adverse effects on memory processes [4–9]. Adolescent exposure to BPA below the TDI impairs spatial memory in rats [5]. In contrast, other studies have shown that chronic oral exposure to BPA does not alter memory processes of adult male or ovariectomized (OVX) female rats [10, 11]. We previously found that acute exposure to BPA rapidly enhanced short-term passive avoidance memory in the developing rats [12]. The underlying mechanism is unclear. The role of BPA in synaptic remodeling in brain areas involved in learning and memory is also controversial. Adolescent exposure to low-dose BPA inhibited spinogenesis and synaptic modification in hippocampi of rodents [13]. BPA inhibited 17β-estradiol (E2)-induced formation of dendritic spine synapses in hippocampal CA1 area and prefrontal cortex of adult ovariectomized rats or nonhuman primates [14, 15]. However, other studies have shown the facilitatory effects of BPA on synaptic plasticity in neuronal development. Exposure to BPA at low doses (<100 nM) enhanced both dendritic and synaptic development in cultured hypothalamic cells [16, 17]. Exposure to BPA at 10–100 nM for 30 min rapidly increased the spine density dendritic filopodia mobility of the hippocampus [18]. Nanomolar doses of BPA rapidly modulated spinogenesis in adult hippocampal neurons [19]. Our previous study has also identified the facilitatory effect of BPA on dendritic morphogenesis of cultured hippocampal neurons through ER activation [12].
The long-lasting plasticity of synaptic transmission, as long-term potentiation (LTP) or long-term depression (LTD), is thought to be the cellular basis of learning and memory processes. Interestingly, it has been reported that exposure to BPA at low concentrations (10–100 nM) rapidly enhanced LTD in CA1 and CA3 but suppressed LTD in the dentate gyrus of the hippocampus [20, 21]. However, no studies have assessed the potential of BPA to influence LTP, and the underlying mechanisms are yet largely unknown.
The extracellular signal-regulated kinase (ERK) signal pathway is a component of a mitogen-activated protein kinase (MAPK) signaling cascade which regulates a variety of important cellular events. Recently, evidence highlights the ERK-mediated effects of estrogen and xenoestrogens in the brain [22]. Our previous studies have demonstrated that ERK signaling is involved not only in the chronic effect of BPA on dendritic morphogenesis in hippocampal neurons but also in the rapid effect of BPA on passive avoidance memory of young rats [12, 23].
In the present study, we investigated the dose-dependent effect of BPA on hippocampal LTP and explored the downstream intracellular pathways. In addition, we examined the synergistic role of BPA and E2 in hippocampal LTP. Therefore our study provides additional information on possible mechanisms for the effects of BPA on synaptic plasticity in brains.
2. Materials and Methods
2.1. Animal and Drug Treatment
All experiments were carried out on male Wistar rats (Weight 120–140 g, age 5-6 weeks). The use of animals for experimental procedures was carried out in accordance with Guidelines for the Care and Use of the Laboratory Animals of Ningbo University, China.
2.2. Preparation of Slices
All experiments were conducted on transverse slices of the rat hippocampus. The brains were rapidly removed after decapitation and placed in cold oxygenated (95% O2, 5% CO2) artificial cerebral spinal fluid (ACSF). Slices were cut at a sickness of 350 μm using a VT 1000S vibroslicer (Leica, Germany) and placed in a storage chamber containing oxygenated medium at room temperature (20–22°C) for 1 h. The slices were then transferred to a recording chamber and continuously superfused at a rate of 5-6 mL/min at 30–32°C. The ACSF contained (mM) NaCl, 120; KCl 2.5, NaH2PO4, 1.25; NaHCO3 26; MgSO4, 2.0; CaCl2, 2.0; d-glucose 10. All solutions contained 100 μM picrotoxin (Sigma, St Louis, MO, USA) to block GABAa-mediated activity.
2.3. In Vitro Electrophysiological Techniques
The electrophysiological techniques were applied according to our previous reports [24, 25]. Presynaptic stimulation was applied to the medial perforant pathway of the dentate gyrus using a bipolar insulated tungsten wire electrode, and field excitatory postsynaptic potentials (fEPSPs) were recorded at a control test frequency of 0.033 Hz from the middle one-third of the molecular layer of the dentate gyrus with a glass microelectrode. The inner blade of the dentate gyrus was used in all studies. In each experiment, an input-output curve (afferent stimulus intensity versus fEPSP amplitude) was plotted at the test frequency. For all experiments, the amplitude of the test EPSP was adjusted to one-third of maximum (~1.2 mV). LTP was evoked by high-frequency stimulation (HFS) consisting of two trains (each of two stimuli at 100 Hz for 1 s, intertrain interval 15 s) with the stimulation voltage increased during the HFS so as to evoke an initial EPSP of the train of double the normal test EPSP amplitude.
2.4. Statistics
Recordings were analyzed using pCLAMP 10.3 software (Axon Instruments, Foster City, CA, USA). Values are the means ± SEM for n slices. All brain slices in the same group were from different animals. In most experiments, the amplitude of fEPSPs measured 40 min after HFS (post-HFS) was shown, unless indicated otherwise. Two-tailed Student's t-test and one-way ANOVA were used for the detailed statistical analysis where appropriate; p < 0.05 was considered statistically significant.
2.5. Agents
All drugs were applied through the perfusion medium. BPA was purchased from Shanghai Chemical Reagent Research Institute (Shanghai, China). 17β-E2 and U0126 were purchased from Cell Signaling (Boston, MA, USA). ICI182,780 was purchased from Tocris (Ballwin, MO, USA). All reagents were dissolved in dimethyl sulphoxide (DMSO, from Sigma, St. Louis, MO, USA) and then diluted in ACSF (0.05% vehicle). Control levels of LTP were measured on slices perfused with vehicle (DMSO) alone.
3. Results
3.1. The Facilitatory Effect of Low-Dose BPA on LTP in the Dentate Gyrus
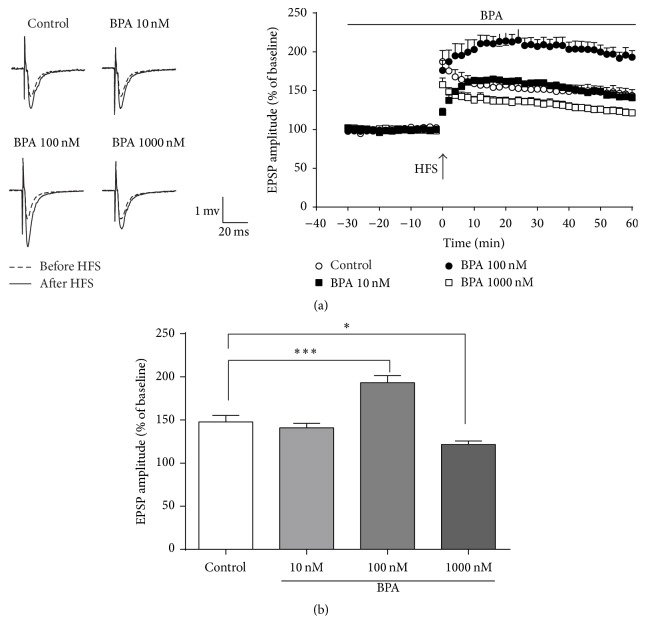
We first investigated the dose-dependent effect of BPA (10, 100, and 1000 nM; added to the ASCF 60 min before HFS) on synaptic plasticity of perforant path-granule cell synapses induced by HFS in the dentate gyrus (DG). We found that application of 10 nM BPA did not have any effect on LTP (140.8 ± 5.2% of baseline, n = 8) compared with vehicle controls (143.7 ± 7.6% of baseline, n = 8, p > 0.05, Figures 1(a) and 1(b)). However, 100 nM BPA increased LTP (193.1 ± 8.3% of baseline, n = 8) compared to control (143.7 ± 7.6% of baseline, n = 8, p < 0.001, Figures 1(a) and 1(b)). In contrast, application of BPA 1000 nM resulted in an inhibition of LTP in DG (121.1 ± 4.0% of baseline, n = 8, p < 0.05, Figure 1(b)), indicating a biphasic effect of low-dose (100 nM) and high-dose (1000 nM) BPA on hippocampal LTP.
Figure 1.
The biphasic effect of BPA on LTP in rat dentate gyrus in vitro. (a) High-frequency stimulation induced LTP in the medial perforant path of the dentate gyrus of acute rat hippocampus slices (open circles, n = 8). Applications of BPA are indicated at concentrations of 10 nM (filled squares, n = 8), 100 nM (filled circles, n = 8), and 1000 nM (open squares, n = 8), respectively. All hippocampal slices were preperfused with ACSF, 30 min before HFS, to obtain baseline EPSP amplitude. (b) Summary of the major experimental outcomes. The average fEPSP amplitudes at 60 min after HFS in separate perfusion of different concentration BPA. Applications of BPA 100 nM and BPA 1000 nM have significant effects on LTP, ∗p < 0.05, ∗∗∗p < 0.001 as compared to controls. Solid and dashed example traces before HFS and after HFS, respectively.
3.2. The BPA-Enhanced LTP Requires Activation of ERs
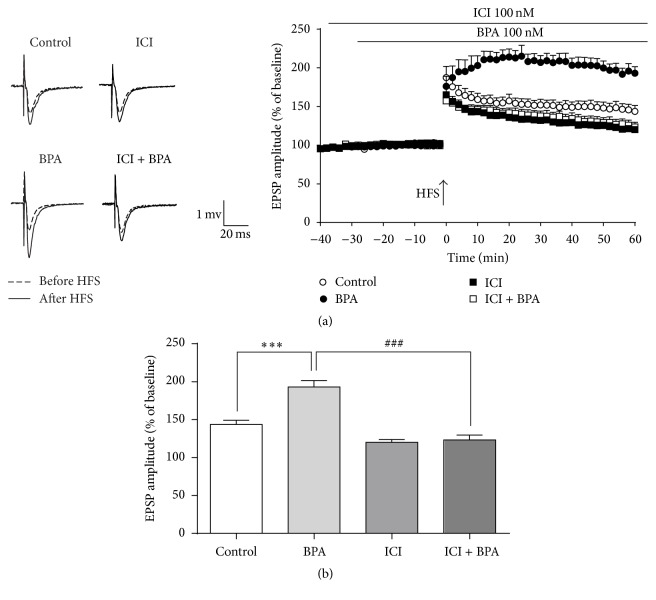
To examine whether the enhancement of LTP by 100 nM BPA involves ERs, we add a high-affinity nonselective ER antagonist ICI 182,780 (100 nM) into bath solution 30 min before BPA application. Application of ICI 182,780 had no effect on LTP (120.6 ± 3.7% of baseline, n = 8, controls: 140.8 ± 5.2% of baseline, n = 8. p > 0.05, Figure 2(b)) but blocked BPA-enhanced LTP (123.4 ± 6.2% of baseline, n = 8, p < 0.001, Figure 2(b)), suggesting that the facilitatory effect of BPA (100 nM) on LTP in hippocampal dentate gyrus requires the activation of ERs.
Figure 2.
The enhancement of BPA on hippocampal LTP was ER-dependent. (a) Administration of ICI 182,780 10 nM (an antagonist of ERs, filled square, n = 8) remarkably decreased the 100 nM BPA-induced enhancement of LTP. Pretreatment with the ERs antagonist ICI 182,780 30 min before BPA 100 nM (open squares, n = 8) application completely blocked BPA-enhanced LTP compared with BPA alone. (b) Figure columns express the average fEPSP amplitudes after HFS in separate perfusion or coperfusion of BPA 100 nM and ICI 182,780 100 nM, ∗∗∗p < 0.001 as compared to the control, ###p < 0.001 as compared to the BPA 100 nM. Solid and dashed example traces before HFS and after HFS, respectively.
3.3. BPA-Enhanced LTP Involves ERKs
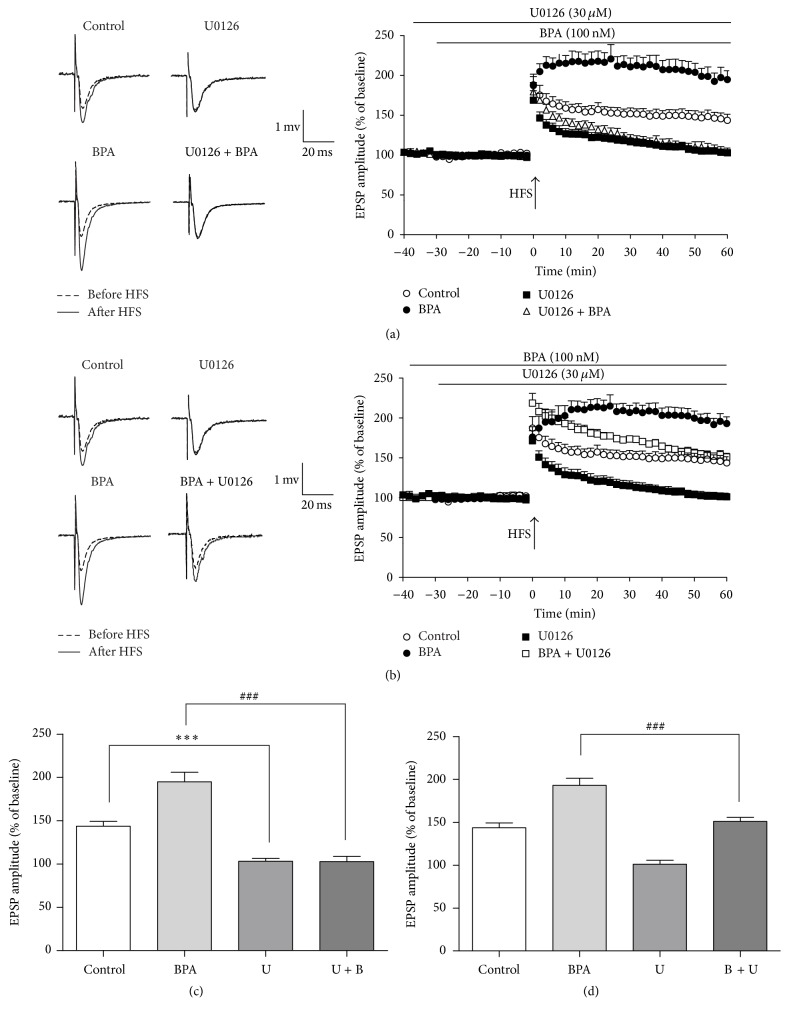
To explore the downstream signaling pathway of the BPA-enhanced LTP in rat hippocampus, we examined whether the ERK pathway is involved. Application of 100 nM U0126 (a MEK1/2 or ERK inhibitor) 60 min before HFS did not alter the baseline fEPSP but inhibited the hippocampus LTP in rat dentate gyrus compared with vehicle controls (103.1 ± 3.5% of baseline, n = 8, p < 0.001, Figures 3(a) and 3(c)). In addition, pretreatment of 100 nM U0126 added 30 min before BPA application completely blocked BPA-enhanced LTP (102.8 ± 6.1% of baseline, n = 8, p < 0.001, Figure 3(c)). However, pretreatment of BPA (added 30 min before U0126 application) resulted in partial inhibition of BPA-enhanced LTP (151.0 ± 4.7% of baseline, n = 8, p < 0.001, Figure 3(d)). These results indicate that activation of ERK pathway is not only required for physiological LTP but also necessary for the facilitatory effect of BPA on LTP in the dentate gyrus.
Figure 3.
ERK signal pathway was involved in BPA-enhanced LTP. Pretreatment with ERK inhibitor U0126 for 30 min before BPA 100 nM (open triangles, n = 8) completely blocked LTP compared with controls. (b) Pretreatment with BPA 100 nM 30 min before the ERK inhibitor (open squares, n = 8) application remarkably decreased the BPA effect as compared with BPA 100 nM alone (open squares). (c, d) Figure columns showing the average fEPSP amplitudes at 60 min after HFS in separate perfusion or coperfusion of BPA 100 nM and U0 126 30 μM, ∗∗∗p < 0.001 as compared to the control, ###p < 0.001 as compared to the BPA 100 nM. Solid and dashed example traces before HFS and after HFS, respectively.
3.4. The Effects of BPA and E2 on Baseline fEPSP and LTP Enhancement
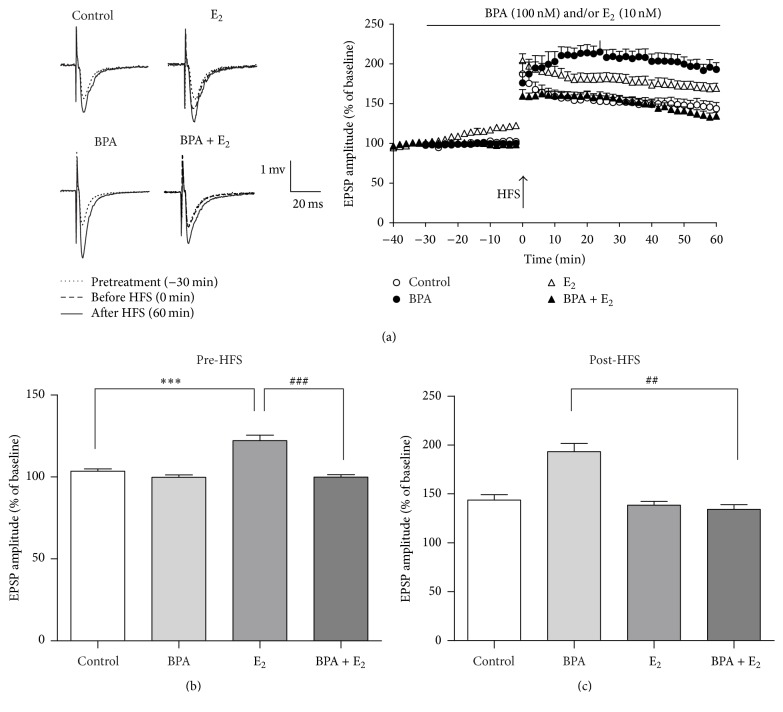
Previous studies have reported the facilitatory effect of E2 on both baseline fEPSP and LTP induction [26]. Here, we applied 10 nM E2 on rat brain slice and observed a significant increase (~20–30%) of baseline fEPSP compared with vehicle controls (Figures 4(a) and 4(b)). However, coapplication of BPA (100 nM) and E2 reversed the enhancement of baseline fEPSP induced by E2 (Figure 4(b)). In terms of LTP enhancement, E2 treatment did not enhance LTP while comparing fEPSP before HFS (at 0 min) and after HFS (at 60 min) in the dentate gyrus (Figures 4(b) and 4(c)). Unexceptionally, coapplication of E2 and BPA blocked both the BPA-induced enhancement of LTP and the E2-induced enhancement of baseline fEPSP (Figures 4(b) and 4(c)).
Figure 4.
The enhancement of BPA on hippocampal LTP was abolished by E2 treatment. Enhanced LTP by E2 (10 nM) in hippocampal area DG (open triangles, n = 8). Coadministration of BPA 100 nM with E2 (filled triangles, n = 8) had no effect on LTP compared with untreated controls. (b) Pretreatment by E2 enhanced the pre-HFS EPSP amplitudes, ∗∗∗p < 0.001 as compared to controls, ###p < 0.001 as compared to the E2 10 nM. (c) Comparison of different groups the fEPSP amplitudes at 60 min. E2 had no effect on LTP compared with untreated controls without baseline increase in EPSP amplitude (before the HFS). ∗∗∗p < 0.001 as compared to the control, ##p < 0.01 as compared to the BPA 100 nM. Solid and dashed traces are examples of before treatment, after treatment, and after HFS, respectively.
4. Discussion
4.1. The Rapid Facilitatory Effect of Low-Dose BPA on Hippocampal LTP Is ER-Dependent and Involves Activation of ERK Pathway
The rapid effect of BPA on synaptic plasticity has been investigated by several studies. It is shown that low-dose BPA (10 nM) increases Ca influx, enhances filopodia flexibility in cultured hippocampal neurons, and rapidly modulates spinogenesis in adult hippocampal slices [18, 19]. These effects have been reported to relate to ERs and MAPK activation [19]. In the terms of memory-related synaptic plasticity (e.g., LTP and LTD), the effect of BPA has been less investigated. Hasegawa et al. [21] have reported the BPA-induced enhancement of LTD in CA1 region of rat hippocampus, but this effect does not require ER activation [21]. However, we here demonstrate that low-dose BPA (100 nM) significantly enhances LTP in rat DG region and this facilitatory effect of BPA on LTP depends on ER activation since E2 antagonist ICI 182,780 completely abolishes the BPA enhancement on LTP.
There are two types of ERs: one type is nuclear estrogen receptors (nERs), which are members of the nuclear receptor family of intracellular receptors, including ERα and ER; the other type is membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors, including Gq-coupled mER (Gq-ER), GPER1 (formerly GPR30), and ER-X [27]. In the genomic mechanism, E2 binds to ERα and ERβ in the cytoplasm, and then the E2-ER complex translocates into the nucleus, binds to an estrogen response element on the DNA, and finally facilitates gene transcription. The nongenomic mechanism involves actions of mERs at the plasma membrane: ERα and ERβ interact with mERs to rapidly activate extracellular signal-regulated kinase (ERK) cell signaling, which further triggers epigenetic processes, gene expression, and other cell signaling pathways [22]. Although it is not clear which type of ER(s) is involved in the facilitatory effect of BPA on LTP because ICI 182,780 blocks both nERs and mERs, the rapid effect of BPA (within 1 h) indicates a greater contribution of the nongenomic mER signaling to the BPA-induced enhancement of hippocampus LTP. Considering the essential roles of glutamate receptors (AMPA, NMDA, and metabotropic glutamate receptor) in the hippocampal LTP and the interactions between glutamate receptors and mERs, the glutamate receptors may also be involved in BPA-induced enhancement of LTP.
Growing evidence demonstrates that the hippocampal ERK signaling is necessary for E2 to enhance hippocampal memory consolidation [28, 29]. Here our results confirm that ERK activation is also required for the BPA-induced enhancement of LTP. It is interesting that the blockade of ERK pathway did not completely inhibit BPA-enhanced LTP while the slices were preincubated with BPA for 30 min before U0126 treatment. The reason may be due to the rapid effect of BPA on LTP since preincubation of BPA may already launch certain rapid downstream effects to enhance EPSP amplitude after high-frequency stimulation, whereas some slow effects of BPA requiring ERK activation are inhibited by following the application of U0126. These results are consistent with our previous findings on cultured rat hippocampal neurons that exposure to BPA for 30 min rapidly enhances the motility and the density of dendritic filopodia through the ER-mediated pathway [30]. Finally, our results also show that high-dose BPA (1000 nM) could severely inhibit hippocampal LTP, indicating a complex mechanism of BPA actions on neuroplasticity in hippocampi.
4.2. BPA and E2 Differently Influence Hippocampal LTP and There Might Be a Complex Interaction between Them
Estrogen (e.g., E2) is also locally synthesized within the hippocampus in addition to the gonads. Mounting articles demonstrate that E2 influences hippocampal memory [31, 32]. A number of studies have reported rapid effects of E2 on LTP, LTD, and spinogenesis in the hippocampus. Low concertation of E2 (1 nM) rapidly enhances LTD in CA1, CA3, and dentate gyrus of the hippocampus. The density of thin type spines increases in CA1 pyramidal neurons within 2 h after application of 1 nm estradiol and this enhancement of spinogenesis requires ERs and MAPK signals [33]. Vedder et al. have demonstrated that E2-induced enhancements in both spatial memory and LTP occur within a similar time frame, linking E2-induced changes in LTP with hippocampal memory formation [34]. Our previous study also confirms that E2 (10 nM) significantly increases the total dendritic length and enhances motility and density of dendritic filopodia in cultured hippocampal neurons [12]. In the terms of hippocampal LTP, although application of E2 (1–10 nM) does not directly enhance LTP, it induces a baseline increase of the excitatory postsynaptic potential (EPSP) in CA1 neurons [26, 35].
Consistently, in the present study, we have shown a significant increase (~20–30%) of baseline fEPSP induced by application of E2. Molecular mechanisms of modulation through synaptic estrogen receptor (ER) and its downstream signaling are still unknown. It may involve a complex kinase network based on a recent study investigating the induction of LTP by the presence of E2 upon weak theta burst stimulation (a subthreshold stimulation that did not induce full-LTP) in CA1 region of the adult male hippocampus [36]. This E2-induced LTP is ER-dependent and requires activation of multiple kinases including ERK, protein kinase A (PKA), protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), and calcium calmodulin kinase II (CaMKII) [36].
It is worth noting that although exposure to either low-dose BPA or E2 alone enhances LTP suppression of these effects is observed when low-dose BPA and low-dose E2 are administrated together. Our findings are consistent with a previous in vivo study showing that the E2-induced increase in synapse density is inhibited by the simultaneous application of BPA (40 ug/kg) and E2 (60 ug/kg) in ovariectomized rats for 30 min [37]. The underlying mechanism is still unclear and requires further exploration. A possible explanation might be the influence of the allosteric effect of BPA on ERs. Binding of BPA to ERs may change the structure of E2 binding sites and affect the affinity of E2 to ERs. However, recent studies highlight another possibility that fluctuations of local E2 levels during a learning event may be a key factor in learning and memory [32]. A study in adult nonhuman primates reported that elevated E2 level by applying exogenous E2 interferes with a cognitive function on the delayed response task in female monkeys [38]. Nevertheless, a study in finches has found that dynamic suppression of E2 synthesis during a learning event may be a critical component of learning processes [39]. Possibly, low-dose BPA alone may act as the ER modulator and has estrogen-like effects on synaptic plasticity in the hippocampus, whereas high-dose BPA alone may act as the ER disrupter and impair hippocampal LTP, LTD, and spinogenesis. However, in physiological states, if we take into account the locally synthesized E2 in the hippocampus and the importance of fluctuations of local E2 levels in cognitive circuits, a small amount of BPA could disturb the subtle regulation of E2 level and then influence hippocampal LTP.
5. Conclusions
In summary, we demonstrated biphasic effects of BPA on LTP in DG region of rat hippocampus: exposure to BPA at a low dose (100 nM) enhances LTP while to a high dose BPA (1000 nM) inhibits LTP. The rapid facilitatory effect of low-dose BPA on hippocampal LTP requires membrane-associated ER and involves activation of ERK signaling pathway. Coadministration of E2 and BPA (100 nM) abolishes BPA-induced enhancement of LTP and E2-induced enhancement of baseline fEPSP, suggesting a complex interaction between BPA- and E2-mediated downstream pathways. Our investigation about hippocampal LTP implies that even nanomolar low doses of endocrine disrupters (e.g., BPA) could induce significant effects on hippocampal synaptic plasticity.
Acknowledgments
This work was supported by National Natural Science Foundation of China (U1503223, 81671089, 81472935, and 81172627), Zhejiang Provincial Natural Science Foundation of China (nos. LY15H090011, LY14H090004, and Z2090955), Ningbo Natural Science Foundation (2016A610086), and Ningbo municipal innovation team of life science and health (2015C110026) and sponsored by K. C. Wong Magna Fund in Ningbo University.
Competing Interests
All authors declare that they have no conflicts of interests.
Authors' Contributions
Xiaowei Chen and Yu Wang contributed equally to this study.
References
- 1.Richter C. A., Birnbaum L. S., Farabollini F., et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neuroscience Research. 2003;45(3):345–356. doi: 10.1016/S0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves C. R., Cunha R. W., Barros D. M., Martínez P. E. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environmental Toxicology and Pharmacology. 2010;30(2):195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Xu X.-H., Zhang J., Wang Y.-M., Ye Y.-P., Luo Q.-Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-d-aspartate receptors of hippocampus in male offspring mice. Hormones and Behavior. 2010;58(2):326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Diaz Weinstein S., Villafane J. J., Juliano N., Bowman R. E. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Research. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D., Kumar Thakur M. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0110482.e110482 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Sadowski R. N., Wise L. M., Park P. Y., Schantz S. L., Juraska J. M. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C., Niu R., Zhu Y., et al. Changes in memory and synaptic plasticity induced in male rats after maternal exposure to bisphenol A. Toxicology. 2014;322:51–60. doi: 10.1016/j.tox.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q., Xu X., Li T., et al. Exposure to bisphenol-A affects fear memory and histone acetylation of the hippocampus in adult mice. Hormones and Behavior. 2014;65(2):106–113. doi: 10.1016/j.yhbeh.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Neese S. L., Bandara S. B., Schantz S. L. Working memory in bisphenol-A treated middle-aged ovariectomized rats. Neurotoxicology and Teratology. 2013;35(1):46–53. doi: 10.1016/j.ntt.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwahara R., Kawaguchi S., Kohara Y., Jojima T., Yamashita K. Bisphenol A does not affect memory performance in adult male rats. Cellular and Molecular Neurobiology. 2014;34(3):333–342. doi: 10.1007/s10571-013-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Lu Y., Zhang G., et al. Bisphenol A promotes dendritic morphogenesis of hippocampal neurons through estrogen receptor-mediated ERK1/2 signal pathway. Chemosphere. 2014;96:129–137. doi: 10.1016/j.chemosphere.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Bowman R. E., Luine V., Khandaker H., Villafane J. J., Frankfurt M. Adolescent bisphenol-A exposure decreases dendritic spine density: role of sex and age. Synapse. 2014;68(11):498–507. doi: 10.1002/syn.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLusky N. J., Luine V. N., Hajszan T., Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the Ca1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146(1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 15.Hajszan T., Leranth C. Bisphenol A interferes with synaptic remodeling. Frontiers in Neuroendocrinology. 2010;31(4):519–530. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokosuka M., Ohtani-Kaneko R., Yamashita K., Muraoka D., Kuroda Y., Watanabe C. Estrogen and environmental estrogenic chemicals exert developmental effects on rat hypothalamic neurons and glias. Toxicology in Vitro. 2008;22(1):1–9. doi: 10.1016/j.tiv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Iwakura T., Iwafuchi M., Muraoka D., et al. In vitro effects of bisphenol A on developing hypothalamic neurons. Toxicology. 2010;272(1-3):52–58. doi: 10.1016/j.tox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe N., Kimoto T., Kawato S. Rapid Ca(2+) signaling induced by Bisphenol A in cultured rat hippocampal neurons. Neuroendocrinology Letters. 2006;27(1-2):97–104. [PubMed] [Google Scholar]
- 19.Tanabe N., Yoshino H., Kimoto T., et al. Nanomolar dose of bisphenol A rapidly modulates spinogenesis in adult hippocampal neurons. Molecular and Cellular Endocrinology. 2012;351(2):317–325. doi: 10.1016/j.mce.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Ogiue-Ikeda M., Tanabe N., Mukai H., et al. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Research Reviews. 2008;57(2):363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa Y., Ogiue-Ikeda M., Tanabe N., et al. Bisphenol a significantly modulates long-term depression in the hippocampus as observed by multi-electrode system. Neuroendocrinology Letters. 2013;34(2):129–134. [PubMed] [Google Scholar]
- 22.Frick K. M. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Hormones and Behavior. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X., Li T., Luo Q., Hong X., Xie L., Tian D. Bisphenol-A rapidly enhanced passive avoidance memory and phosphorylation of NMDA receptor subunits in hippocampus of young rats. Toxicology and Applied Pharmacology. 2011;255(2):221–228. doi: 10.1016/j.taap.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Klyubin I., Wang Q., Reed M. N., et al. Protection against Aβ-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiology of Aging. 2011;32(4):614–623. doi: 10.1016/j.neurobiolaging.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Lin R., Chang L., et al. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience. 2013;253:435–443. doi: 10.1016/j.neuroscience.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Foy M. R., Xu J., Xie X., Brinton R. D., Thompson R. F., Berger T. W. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. Journal of Neurophysiology. 1999;81(2):925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 27.Vahaba D. M., Remage-Healey L. Brain estrogen production and the encoding of recent experience. Current Opinion in Behavioral Sciences. 2015;6:148–153. doi: 10.1016/j.cobeha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez S. M., Lewis M. C., Pechenino A. S., et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. The Journal of Neuroscience. 2008;28(35):8660–8667. doi: 10.1523/jneurosci.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortress A. M., Lu F., Orr P. T., Zhao Z., Frick K. M. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learning & Memory. 2013;20(3):147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X., Ye Y., Li T., et al. Bisphenol-A rapidly promotes dynamic changes in hippocampal dendritic morphology through estrogen receptor-mediated pathway by concomitant phosphorylation of NMDA receptor subunit NR2B. Toxicology and Applied Pharmacology. 2010;249(2):188–196. doi: 10.1016/j.taap.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Luine V. N. Estradiol and cognitive function: past, present and future. Hormones and Behavior. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korol D. L., Pisani S. L. Estrogens and cognition: friends or foes?. An evaluation of the opposing effects of estrogens on learning and memory. Hormones and Behavior. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukai H., Tsurugizawa T., Murakami G., et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. Journal of Neurochemistry. 2007;100(4):950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 34.Vedder L. C., Smith C. C., Flannigan A. E., Mcmahon L. L. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23(1):108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawato S. Endocrine disrupters as disrupters of brain function: a neurosteroid viewpoint. Environmental sciences : an international journal of environmental physiology and toxicology. 2004;11(1):1–14. [PubMed] [Google Scholar]
- 36.Hasegawa Y., Hojo Y., Kojima H., et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: involvement of kinase networks. Brain Research. 2015;1621:147–161. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 37.MacLusky N. J., Hajszan T., Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environmental Health Perspectives. 2005;113(6):675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacreuse A., Chang J., Metevier C. M., Laclair M., Meyer J. S., Ferris C. M. Oestradiol modulation of cognition in adult female marmosets (Callithrix jacchus) Journal of Neuroendocrinology. 2014;26(5):296–309. doi: 10.1111/jne.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao A., Paon A., Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Developmental Neurobiology. 2015;75(3):271–286. doi: 10.1002/dneu.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]