Abstract
Background Patients with chronic hepatitis B virus (HBV) infection are at risk for death from complications of liver disease and development of hepatocellular carcinoma (HCC). To identify the time course and risk factors associated with these events, we conducted a prospective study in chronic hepatitis B patients referred to our clinic.
Methods From January 1989 to March 1998, 400 hepatitis B surface antigen (HBsAg)-positive patients were classified into three categories: inactive carriers (N=110), chronic hepatitis (N=151), and cirrhosis (N=139). These patients were observed at 3- to 6-month intervals with liver tests, alpha-fetoprotein (AFP) levels, and ultrasound examinations. The study endpoints were death from liver disease complications and development of HCC.
Results The patients were followed for a mean time (± SD) of 83.6 ± 39.6 months. During this period, no liver-related deaths or HCC were noted in inactive carriers. However, 38 of 139 (27.3%) patients with cirrhosis died from non-HCC–related liver complications. Multivariate analysis demonstrated that male sex (odds ratio [OR] 5.9; 95% confidence interval [CI], 2.0–22.6; P=.003), decreased initial serum albumin (OR 69.1; 95% CI, 11.5–486.4; P=.0009), low platelet count (OR 8.8; 95% CI, 0.96–92.9; P=.05), and presence of cirrhosis (OR 14.2; 95% CI, 3.4–111.8; P=.0009) were independently associated with increased mortality from chronic hepatitis B. During the same time period, nine of 151 (6.0%) chronic hepatitis patients and 22 of 139 (15.8%) patients with cirrhosis developed HCC. By multivariate analysis, progression to HCC was associated with advanced age (OR 19.7; 95% CI, 1.9–231.9; P=.01) and presence of cirrhosis (OR 3.6; 95% CI, 1.6–8.9; P=.003). Patients positive for hepatitis B early antigen (HBeAg) and HBeAg antibodies experienced liver-related deaths and developed HCC at similar rates.
Conclusions This prospective study from the United States confirms previous observations of the high risk of mortality and development of HCC in patients infected with HBV. To decrease the risk of these complications, antiviral therapy should be initiated early in the course of the disease. In addition, surveillance for HCC must be performed at least every 6 months in patients with chronic hepatitis and cirrhosis.
Keywords: Hepatitis B, inactive carriers, chronic hepatitis, cirrhosis, hepatocellular carcinoma, natural history
Hepatitis B virus (HBV) infection is a significant health problem and ranks as the 10th leading cause of death worldwide. It is estimated that there are 2 billion persons with past or present HBV exposure. Worldwide, up to 400 million people are currently infected with HBV, and complications arising from this chronic viral illness account for over 1 million deaths per year.1 In the United States, there are 1.2 million persons with chronic hepatitis B infection, and this number may rise due to increasing immigration of individuals from countries with high HBV prevalence.2 In these countries the most common modes of HBV acquisition are maternal-infant transmission from hepatitis B surface antigen (HBsAg)-positive carrier mothers and HBV exposure during early childhood.
Chronic infection by HBV can lead to varied clinical outcomes. Some individuals may remain inactive carriers of HBV while others develop chronic hepatitis. Thereafter, a proportion of chronic hepatitis B patients will either progress to cirrhosis leading to death from liver decompensation or eventually develop hepatocellular carcinoma (HCC). It is estimated that 15–40% of patients with hepatitis B will develop cirrhosis, liver failure, or HCC.3 The reasons for the differences in outcome remain unclear. Thus, predictors of eventual progression to death and to HCC need to be identified. Viral factors may play a major role in determining clinical outcome in patients with chronic hepatitis B. A report from Taiwan indicated that hepatitis B early antigen (HBeAg) positivity was associated with an increased risk for HCC.4 During a 9-year follow-up, the relative risk (RR) for HCC was 9.6 among men who were positive for HBsAg alone and 60 among those who were positive for both HBsAg and HBeAg.
In the present study, the long-term follow-up of 400 patients who presented to our clinic with chronic hepatitis B is described. The study endpoints were death from complications of chronic liver disease or progression to HCC. Odds ratios (ORs) were used to identify significant factors leading to these endpoints.
Methods
Patients
From January 1989 to March 1998, 400 HBsAg-positive patients were enrolled in a prospective study. At least 1 year of follow-up was required to remain in the study. Patients who were positive for hepatitis C virus or HIV, or had a history of chronic alcoholism or other chronic liver diseases were excluded. During the initial clinic visit, liver tests and complete blood counts were obtained from all patients. After further evaluation, patients were classified as follows:
Inactive carriers (N=110). These patients had no symptoms or signs of chronic liver disease and had normal liver tests and normal platelet counts. Because of a lack of abnormal findings, liver biopsies were not requested in this group of patients.
Chronic hepatitis (N=151). During the initial visit, these HBsAg-positive patients presented with elevated serum aminotransferase levels. All 151 patients had liver biopsies to determine the histologic grade (1–3) and stage (1–3) of their chronic hepatitis.
Cirrhosis (N=139). These patients presented with abnormal liver tests and had clinical stigmata of chronic liver disease. Liver biopsies were obtained in all 139 patients confirming the presence of cirrhosis (grades 3–4, stage 4).
All liver biopsies were interpreted by hospital pathologists and reviewed by one author (MJT). The scale reported by Ishak and colleagues was used for liver tissue interpretation.5
Laboratory Tests
At baseline and every 3–6 months thereafter, all patients had complete blood counts and liver function tests that included serum albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin. Serum alpha-fetoprotein (AFP) was tested every 12 months in inactive carriers and every 6 months in patients with chronic hepatitis and cirrhosis. HBeAg and HBeAg antibodies (anti-HBe) were measured at baseline using commercially available kits (Abbott Laboratories).
HCC Surveillance
To screen for HCC, abdominal ultrasound examinations were performed every 12 months in inactive carriers and every 6 months in patients with chronic hepatitis and cirrhosis. If serum AFP was elevated or if a lesion was noted on abdominal ultrasound, further studies including computed tomography scan, magnetic resonance imaging, or biopsy of the liver lesion were used to detect HCC.
Follow-up
All patients were followed in our clinic and observed for death or for development of HCC. The last date of follow-up was July 2005. In this study, patients who received liver transplantation either for liver decompensation or for HCC were counted as deaths. In patients who were lost to follow-up, death records from the Liver Cancer Registry at the University of Southern California were reviewed. In addition, mortality records from the State of California were matched for patients who were lost to follow-up. No additional deaths were found in the latter records.
During the late 1980s to the early 1990s, 22 patients—nine with chronic hepatitis and 13 with cirrhosis—received 16-week courses of interferon treatment. Among the nine patients with chronic hepatitis, one patient subsequently died from gastric cancer and the other eight are still alive as of this report. Of the 13 patients with cirrhosis who were treated with 16 weeks of interferon, seven are still alive as of this report and six subsequently died (two developed HCC, one received a liver transplant, and three died from non–HCC-related liver deaths). Thus, the short course of interferon did not appear to influence the course of chronic hepatitis B.
Statistical Analysis
All statistical significance was assessed at the 0.05 level. Baseline data were descriptively summarized, and assessment of differences was completed by using the analysis of variance with post-hoc pair-wise Student t tests for parametric data and chi-squared methods for nonparametric data. Means and standard deviations were computed for all continuous data. Categorical data were summarized by using frequencies. All variables found to be significant by univariate analysis were subjected to multivariate analysis utilizing step-wise logistic regression. Analysis of survival was completed using the Kaplan-Meier analysis. The OR is defined as previously described.6
Results
Baseline Characteristics
The mean follow-up time for all patients was 83.6 ± 39.6 months. There were 282 males (70.5%), and the mean age at recruitment was 48.4 ± 15 years; 314 patients (78.5%) were Asians. Seventy percent of patients were born in Asia and 24% in North America. The baseline laboratory tests in inactive carriers, chronic hepatitis patients, and cirrhosis patients are shown on Table 1. During the initial clinic visit, patients with cirrhosis had significantly higher mean serum bilirubin, AST, and ALT values, and lower mean albumin and platelet levels than inactive carriers (P≤.0003 for all observations). A total of 197 of 396 (49.7%) patients were HBeAg-positive and 189 of 383 (49.3%) patients were anti-HBe. More patients with chronic hepatitis were HBeAg-positive (63.6%), and anti-HBe was detected more frequently in the inactive carriers (75.0%; P<.0001 for both observations). Compared to anti-HBe patients, HBeAg-positive patients were younger (mean age 45.2 ± 15.9 yr vs 49.9 ± 13.7 yr; P=.002), and more males than females were HBeAg-positive (53.9% vs 40.2%; P=.01). HBeAg-positive patients had higher initial serum ALT levels than the anti-HBe patients (63.9 ± 63.4 U/L vs 43.4 ± 63.5; P=.001).
Table 1.
Baseline Characteristics in Patients With Chronic Hepatitis B Virus Infection
| Inactive Carrier | Chronic Hepatitis | Cirrhosis | P Value* | |
|---|---|---|---|---|
| N | 110 | 151 | 139 | - |
| Mean age, yr | 41.2 ± 16.0 | 45.5 ± 13.7 | 55.2 ± 12.7 | <.0001 |
| Male, n | 51 (46.4%) | 113/151 (74.8%) | 118 (84.9%) | <.0001 |
| Asian, n | 98 (89.1%) | 118/151 (78.1%) | 98 (70.5%) | <.001 |
| Mean follow-up time, mo | 63.6 ± 49.4 | 77.8 ± 69.1 | 52.5 ±45.3 | - |
| Albumin, mg/dL | 4.4 ± 0.4 | 4.2 ± 0.6 | 3.7 ± 0.7 | <.0001 |
| AST, U/L | 21.5 ± 12.0 | 41.6 ± 25.1 | 61.8 ± 95.8 | <.0001 |
| ALT, U/L | 22.4 ± 16.5 | 61.8 ± 60.1 | 68.5 ± 80.3 | <.0001 |
| Total bilirubin, mg/dL | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.7 ± 0.7 | .0003 |
| Platelets, × 103/mm3 | 223 ± 102 | 166 ± 96 | 88 ± 81 | <.0001 |
| HBeAg-positive | 28/106 (26.4%) | 96/151 (63.6%) | 73/138 (52.9%) | <.0001 |
| Anti-HBe | 75/100 (75.0%) | 53/144 (36.8%) | 61/137 (44.5%) | <.0001 |
Patients with cirrhosis versus inactive carriers; χ2 test used for all variables.
ALT = alanine aminotransferase; anti-BHe = antibodies to HBeAg; AST = aspartate aminotransferase; HBeAg = hepatitis B early antigen.
Non–HCC-Related Deaths
During follow-up, there were four deaths from non–liver-related causes (Table 2). One inactive carrier died from pneumonia. In the chronic hepatitis group, one patient died of gastric cancer, one died of colon cancer, and another died from pneumonia. Thirty-eight of 139 (27.3%) patients with cirrhosis died from non-HCC liver-related causes, including liver failure in 27 patients, bleeding esophageal varices in seven, and sepsis in four. In comparing the 38 patients who died from non-HCC liver-related deaths to those who did not, univariate analysis of baseline variables showed that older age, male sex, Asian race, baseline levels of bilirubin, AST, ALT, albumin, and platelets, and presence of cirrhosis were associated with non-HCC liver-related deaths (Table 3). Multivariate analysis showed that sex (OR 6.52, 95% confidence interval [CI], 2.02–63.11; P=.01), baseline albumin level (OR 340; 95% CI, 32.41–5086.2; P=.0001), platelet count (OR 26; 95% CI, 1.34–950.04; P=.04), and cirrhosis (OR 7.34; 95% CI, 1.26–69.56; P=.05) were independent predictors for non-HCC liver-related deaths.
Table 2.
The Clinical Outcomes of 400 Patients With Chronic Hepatitis B Virus Infection
| Inactive Carrier | Chronic Hepatitis | Cirrhosis | Total Deaths | |
|---|---|---|---|---|
| N | 110 | 151 | 139 | - |
| Developed HCC | 0 | 9 | 22 | - |
| HCC deaths | 0 | 9 | 20 | 29 |
| Non-HCC liver-related deaths | 0 | 0 | 39 | 38 |
| Non–liver-related deaths | 1 | 3 | 0 | 4 |
| Total deaths n (%) | 1 (0.9%) | 12 (7.9%) | 59 (42.4%) | 71 (17.8%) |
HCC = hepatocellular carcinoma.
Table 3.
Univariate Analysis for Factors Associated With Non-HCC Liver-Related Deaths
| Alive | Non-HCC Liver-related Deaths | P Value | |
|---|---|---|---|
| Mean age, yr | 45.5 ± 14.7 | 57.7 ± 12.3 | <.0001 |
| Male, n | 213/326 (65.3%) | 37/39 (94.9%) | <.0001 |
| Asian, n | 264/326 (81.0%) | 22/39 (54.4%) | <.001 |
| Mean albumin, mg/dL | 4.27 ± 0.45 | 3.41 ± 0.66 | <.0001 |
| Mean AST, U/L | 37.1 ± 36.6 | 83.1 ± 149.7 | <.0001 |
| Mean ALT, U/L | 50.4 ± 58.9 | 77.6 ± 104.1 | .01 |
| Mean bilirubin, mg/dL | 0.54 ± 0.35 | 0.93 ± 0.97 | <.0001 |
| Mean platelets × 103/mm3 | 170.8 ± 106.6 | 69.5 ± 64.9 | <.0001 |
| HBeAg-positive, n/total | 159/183 (86.9%) | 15/178 (8.4%) | .15 |
| Anti-HBe, n/total | 152/167 (91.0%) | 24/180 (13.3%) | .36 |
| Inactive carriers, n/total | 109/110 (99.1%) | 0/110 (0.0%) | <.0001 |
| Chronic hepatitis, n/total | 140/143 (97.9%) | 0/143 (0.0%) | - |
| Cirrhosis, n/total | 77/116 (66.4%) | 39/116 (33.6%) | - |
ALT = alanine aminotransferase; anti-BHe = antibodies to HBeAg; AST = aspartate aminotransferase; HBeAg = hepatitis B early antigen; HCC = hepatocellular carcinoma.
HCC Development
During follow-up, HCC did not appear in the inactive carriers. However, HCC developed in nine of 151 (6%) patients with chronic hepatitis and in 22 of 139 (15.8%) patients with cirrhosis (Table 4). In comparing the 31 patients who developed HCC to those who did not, univariate analysis of baseline variables showed that older age, male sex, serum albumin, platelet counts, and presence of cirrhosis were associated with development of HCC (Table 4). On multivariate analysis, age (OR 19.7; 95% CI, 1.92–231.96; P=.01) and cirrhosis (OR 3.6; 95% CI, 1.6–8.9; P=.003) were independent predictors for HCC development. Patients who were HBeAg-positive or anti-HBe at baseline developed HCC at similar rates.
Table 4.
Univariate Analysis for Factors Associated With Development of HCC
| No HCC | HCC | P Value | |
|---|---|---|---|
| Mean age, yr | 46.9 ± 14.9 | 57.4 ± 14.8 | .0002 |
| Male, n | 254/369 (68.8%) | 28/31 (90.3%) | .006 |
| Asian, n | 288/369 (78.0%) | 26/31 (83.9%) | .002 |
| Mean albumin, mg/dL | 4.16 ± 0.58 | 3.62 ± 1.02 | <.0001 |
| Mean AST, U/L | 42.4 ± 62.9 | 50.9 ± 24.3 | .46 |
| Mean ALT, U/L | 53.6 ± 66.2 | 51.1 ± 22.9 | .84 |
| Mean bilirubin, mg/dL | 0.59 ± 0.48 | 0.74 ± 0.65 | .11 |
| Mean platelets, × 103/mm3 | 158.5 ± 107.8 | 101.7 ± 89.7 | .008 |
| HBeAg-positive, n/total | 185/197 (93.9%) | 12/197 (6.1%) | .26 |
| Anti-HBe, n/total | 170/189 (89.9%) | 19/189 (10.1%) | .11 |
| Inactive carriers, n/total | 110/110 (100%) | 0/110 (0%) | - |
| Chronic hepatitis, n/total | 142/151 (94.0%) | 9/151 (6.0%) | - |
| Cirrhosis, n/total | 117/139 (84.2%) | 22/139 (15.8%) | <.0001 |
ALT = alanine aminotransferase; anti-BHe = antibodies to HBeAg; AST = aspartate aminotransferase; HBeAg = hepatitis B early antigen; HCC = hepatocellular carcinoma.
All Deaths
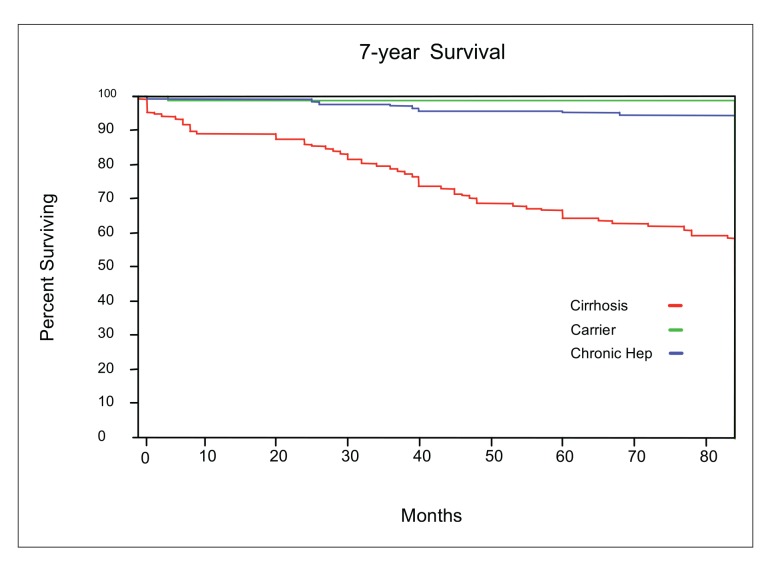
There were 71 deaths during the course of this study. Four patients died of non–liver-related causes, and the remaining patients died either from complications of liver disease (N=38) or from HCC (N=9). In comparing all patients who died with those who were alive, univariate analysis showed that older age, male sex, baseline levels of albumin, AST, ALT, bilirubin, and platelets, and presence of cirrhosis were associated with hepatitis B–related deaths (Table 5). On multivariate analysis, sex (OR 5.9; 95% CI, 2.0–22.6; P=.003), albumin level (OR 69.1; 95% CI, 11.5–486.4; P<.0001), platelet count (OR 8.8; 95% CI, 0.96–92.9; P=.05), and presence of cirrhosis (OR 14.2; 95% CI, 3.4–111.8; P=.0009) were independent predictors of death from chronic hepatitis B infection. HBeAg-positive and anti-HBe patients died at similar rates. The cumulative survival of all patients by clinical category is shown in Figure 1.
Table 5.
Univariate Analysis for Factors Associated With All Deaths
| Alive | Dead | P Value | |
|---|---|---|---|
| Mean age, yr | 45.7 ± 14.8 | 56.8 ± 13.3 | <.0001 |
| Male, n | 216/329 (65.6%) | 66/71 (92.9%) | <.0001 |
| Asian, n | 266/329 (80.8%) | 48/71 (67.6%) | 0.13 |
| Mean albumin, mg/dL | 4.25 ± 0.49 | 3.49 ± 0.84 | <.0001 |
| Mean AST, U/L | 37.2 ± 36.6 | 70.5 ± 117.9 | <.0001 |
| Mean ALT, U/L | 50.3 ± 58.6 | 67.5 ± 82.9 | .04 |
| Mean bilirubin, mg/dL | 0.54 ± 0.35 | 0.87 ± 0.87 | <.0001 |
| Mean platelets, × 103/mm3 | 170 ± 107 | 79 ± 73 | <.0001 |
| HBeAg positive, n/total | 158/197 (80.2%) | 39/197 (19.8%) | .41 |
| Anti-HBe, n/total | 166/198 (83.8%) | 32/198 (16.2%) | .85 |
| Inactive carriers, n/total | 109/110 (99.1%) | 1/110 (0.9%) | - |
| Chronic hepatitis, n/total | 139/151 (92.1%) | 12/151 (7.9%) | - |
| Cirrhosis, n/total | 81/139 (58.3%) | 58/139 (41.7%) | <.0001 |
ALT = alanine aminotransferase; anti-BHe = antibodies to HBeAg; AST = aspartate aminotransferase; HBeAg = hepatitis B early antigen.
Figure 1.
The cumulative survival of patients with chronic hepatitis B infection by clinical categories.
Discussion
Several distinct differences were noted in patients with chronic HBV infection who presented to our clinic. The inactive carriers were younger in age than those with cirrhosis and, unlike patients with chronic hepatitis or cirrhosis, in which males predominated, the ratio of male to female patients among the inactive carriers was similar. Also, 75% of the inactive carriers were already anti-HBe positive at the time of presentation. During a mean follow-up of 9 years, these inactive carriers had persistently normal liver tests, spontaneous reactivation of HBV was not detected, and none developed HCC. Even though the risk for HCC is low in inactive carriers, yearly surveillance for development of HCC is still recommended.7 Thus, inactive carriers appear to follow a benign clinical course and have an excellent prognosis.
Patients with chronic hepatitis had the highest HBeAg-positivity rate (64%), and all presented with elevated serum transaminase levels. These findings indicate that these chronic hepatitis patients were in the immune clearance phase of hepatitis B.3 Because of increased hepatic inflammatory activity, patients with chronic hepatitis have a higher probability of progression to cirrhosis and to eventual development of HCC than the inactive carriers. Previous studies have shown that after anti-HBe seroconversion, up to two thirds of patients with chronic hepatitis may revert to the inactive carrier state, while the remainder progress to anti-HBe chronic hepatitis.8,9 In our patients with cirrhosis, 45% already were anti-HBe at presentation to the clinic, and thus may have had liver disease progression as a result of anti-HBe chronic hepatitis. However, the other 53% of the patients were still HBeAg-positive at the first clinic visit, indicating that cirrhosis may develop regardless of HBeAg status.
The highest complication rate occurred in the 139 patients who presented with cirrhosis. During follow-up, 57 (41%) developed serious liver-related complications and 38 (27%) died from liver failure, bleeding esophageal varices, or sepsis. Thus, our annual rate of non-HCC liver-related deaths was 3.9% and is similar to rates (2.4–4%) reported in other studies.10,11 At baseline, these patients already had laboratory tests indicative of poor synthetic function (low albumin), continual inflammation (elevated AST and ALT), and portal hypertension (low platelets). Logistic regression analysis showed that elevated baseline levels of bilirubin and lower platelet counts were independently predictive of liver deaths in patients with cirrhosis. In this analysis, neither initial HBeAg nor anti-HBe status was associated with liver-related deaths. However, a study from Europe indicated that HBeAg positivity was associated with worse survival,12 and the risk of death decreased 2.2-fold after HBeAg seroconversion.10 Nevertheless, chronic inflammation caused by the host immune response to active viral replication appears to be responsible for non-HCC liver-related deaths.
During follow-up, nine of 151 (6%) chronic hepatitis patients developed HCC at an annual rate of 0.9%. In our patients who presented with cirrhosis, 22 of 139 (16%) developed HCC at an annual rate of 2.3% per year. These findings are similar to HCC incidence rates of 1.5–3.8% in Europe12,13 and rates of 0.7–2.2% in Asia.14,15 In a surveillance study reported previously from our clinic, HCC developed in seven of 163 (4.3%) HBsAg-positive patients over a 7-year period.16 In the subset of patients with cirrhosis, seven of 40 (17.5%) developed HCC. Serum AFP was elevated in 74% of HCC patients while abdominal ultrasound detected all the cases of HCC, demonstrating that ultrasound examination is more accurate in detecting HCC. Therefore, because of poor predictive value and low sensitivity, serum AFP should not be used as the only test for screening and surveillance for HCC.
Asian Americans comprised up to 80% of the patients who were referred to our liver clinic, and over 70% of these patients were immigrants to the United States. The prevalence of HBV markers in Asian Americans include rates of 5–15% for HBsAg positivity and 43–66% for presence of HBV antibodies.17 In an earlier hepatitis B study from the United States initiated in the 1970s, only 13% of patients were of Asian origin,18 reflecting the fact that the Asian American population was smaller at that time. HBV-related death rates were similar to the report herein, but development of HCC appeared to be significantly less frequent.19
The limitations in this study include the small number of liver biopsies in the 110 inactive carriers. Seven of these patients presented with liver biopsies performed prior to referral to our clinic, and all showed minimal or no liver damage. Because these patients had normal liver tests and were without signs or symptoms of liver disease at presentation, we did not feel it was ethical to request liver biopsies. In support of this, the inactive carriers in our study had consistently normal liver tests and platelet counts during follow-up, and none developed liver complications or HCC. Also, this study described only laboratory tests performed at the time of presentation to our clinic. It is well known that patients with chronic hepatitis B experience exacerbations and remissions, which are usually accompanied by abnormal liver tests. However, our primary endpoints in this study were either death from liver disease or development of HCC, and our aim was to identify commonly measured tests at the time of presentation to the clinic which may predict these serious outcomes. Recently, other hepatitis B viral factors have been described, such as HBV genotypes, precore and core promoter mutants, and serum HBV DNA levels. These viral factors may play important roles in the natural history of chronic hepatitis B. Such studies are now in progress in our clinic.
In summary, this study of hepatitis B patients showed that death from non-HCC liver-related complications only occurred in patients with cirrhosis, while HCC developed both in patients with chronic hepatitis or cirrhosis. Therefore, surveillance for HCC with AFP and abdominal ultrasound must be performed in patients with chronic hepatitis or cirrhosis at least every 6 months.19 Since HCC seldom occurs in inactive carriers, surveillance should be less often but still should be conducted annually.
Acknowledgment
The authors wish to appreciate the following individuals for their effort in contributing to this study: Ruth Co, Agnes Baronowski, Yoon Sin Kim, DO, Andrew Lu, MD, and Jason Cheng, MD.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 3.Lok ASF. Chronic hepatitis B. N Engl J Med. 2002;346:168–173. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 4.Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 5.Ishak K, Baptista A, Bianchi L, Callea F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 6.Friis RH, Stellers TA. Epidemiology for Public Health Practice. Gaithersburg, Md: Aspen Publishers; 1996. [Google Scholar]
- 7.McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24:17–21. doi: 10.1055/s-2004-828674. [DOI] [PubMed] [Google Scholar]
- 8.Chan HLY, Leung NWY, Hussain M, Wong ML, Lok ASF. Hepatitis B e antigen-negative chronic hepatitis B in Hong Kong. Hepatology. 2000;31:763–768. doi: 10.1002/hep.510310330. [DOI] [PubMed] [Google Scholar]
- 9.Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 10.De Jongh FE, Janssen HLA, De Man RA, Hop WCJ, Schalm SW, Van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630–1635. doi: 10.1016/0016-5085(92)91188-a. [DOI] [PubMed] [Google Scholar]
- 11.Di Marco V, Lo Iacono O, Camma C, et al. The long-term course of chronic hepatitis B. Hepatology. 1999;30:257–264. doi: 10.1002/hep.510300109. [DOI] [PubMed] [Google Scholar]
- 12.Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. J Hepatol. 1994;21:656–656. doi: 10.1016/s0168-8278(94)80115-0. [DOI] [PubMed] [Google Scholar]
- 13.Fattovich G. Natural course and prognosis of chronic hepatitis type B. J Viral Hepat. 1996;2:263–276. [Google Scholar]
- 14.Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493–496. doi: 10.1002/hep.1840080310. [DOI] [PubMed] [Google Scholar]
- 15.Lo KJ, Tong MJ, Chien MC, et al. The natural course of hepatitis B surface antigen-positive chronic active hepatitis in Taiwan. J Infect Dis. 1982;146:205–210. doi: 10.1093/infdis/146.2.205. [DOI] [PubMed] [Google Scholar]
- 16.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–555. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, Hwang SH. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am. 1994;23:523–536. [PubMed] [Google Scholar]
- 18.Cardenas CL, Soetikno R, Robinson WS, et al. Long-term follow-up of patients with chronic hepatitis B: a 25 year prospective study. Hepatology. 1999;30(4 part 2):300A–559. [Google Scholar]
- 19.Weissberg JI, Andres LL, Smith CI, et al. Survival in chronic hepatitis B. Ann Intern Med. 1984;101:613–616. doi: 10.7326/0003-4819-101-5-613. [DOI] [PubMed] [Google Scholar]