Abstract
Rituximab generally is a well-tolerated medication used in a variety of haematological and autoimmune conditions. The safety profile of the medication has been reviewed in the literature. Infusion reactions due to cytokine release are the most common side effects. With the increased use of rituximab, there is an increase incidence of cytopenias, most commonly thrombocytopenia and leucopenia. Coagulopathy is quite rare, reported previously in four cases in the literature. We highlighted the clinical course of a 39-year-old patient with precursor B-cell acute lymphoblastic leukaemia who was started on rituximab infusion. The patient developed a cytokine-release syndrome with haemodynamic instability, followed by rapid-onset cytopenias and disseminated intravascular coagulation abnormalities characterised by coagulopathy with fibrinolysis and mucocutaneous bleeding. The report is followed by a review of the literature. It is important to recognise rituximab-induced coagulopathy early as part of the differential diagnosis of thrombocytopenia and disseminated intravascular coagulation following rituximab administration.
Background
Rituximab is a chimeric anti-CD20 monoclonal antibody that is commonly used to treat different lymphoproliferative diseases and autoimmune conditions. It is relatively very well tolerated. With the increased use of rituximab, an increasing number of serious complications related to rituximab infusions have been noted. These complications encompass an array of presentations and affect different systems. The severity of reactions and side effects of rituximab are reported based on the grading system for reporting adverse effects from chemotherapy. Grade 3–4 represent moderate to severe adverse events and grade 5 represents fatalities.1 The most common reactions (incidence of at least 25%) in patients receiving rituximab monotherapy are infusion reactions.2 The infusion reactions noted are usually mild to moderate (grade 1 or 2); grade 3–4 reactions are rare and dose dependent.3 4 Cytopenias are other complications of rituximab therapy.5 Grade 3 and 4 cytopenias lasting ∼2 weeks have been seen in 48% of patients who had non-Hodgkin lymphoma and who received rituximab monotherapy, lymphopenia being the most common.2 3
Coagulopathy has been rarely described in the literature as a complication of rituximab infusion. A literature review showed four previously reported cases with coagulopathy following rituximab infusion. In this review, we report the case of a patient with precursor B-cell acute lymphoblastic leukaemia who developed a disseminated intravascular coagulation (DIC) picture after rituximab infusion. We will briefly summarise the potential side effects of rituximab infusion and review the previously reported cases with rituximab-associated coagulopathy.
Case presentation
A 39-year-old man with no medical history presented to the emergency room with severe, diffuse body pain. At admission, he endorsed pain in his chest, thighs, legs and back, as well as generalised abdominal discomfort, and he reported many months of drenching night sweats. Presenting vital signs were significant for tachycardia, and physical examination was remarkable only for generalised rib tenderness to moderate palpation and the absence of palpable splenomegaly. Relevant admission laboratory results included leucocytosis of 99 950/mm3 (segmented neutrophils 17%, band neutrophils 8%, blasts 55%), haemoglobin 14.7 g/dL and platelet count 58 000/mm3. Flow cytometry analysis of peripheral blood and bone marrow biopsy were consistent with the diagnosis of precursor B-cell acute lymphoblastic leukaemia (‘pre-B-cell acute lymphoblastic leukaemia (pre-B-ALL)’; negative to weak expression of CD45; positive expression of CD10, CD19, CD20, CD79a, TdT and CD34; aberrant expression of CD33 and CD61; MPO stain negative). Fluorescence in situ hybridisation was positive for the ABL1-BCR fusion gene (Philadelphia chromosome positive). The decision was made to induce the patient on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone), with rituximab 375 mg/m2 prescribed on days one and 11 of the first cycle of chemotherapy. He was maintained on fluids and initiated on routine tumour lysis prophylaxis. Follow-up preinduction blood work was obtained and was remarkable for leucocytosis of 63 910/mm3 (blasts 78%), haemoglobin 13 g/dL and platelet count 14 000/mm3. Coagulation panel showed international normalised ratio (INR) of 1.1, fibrinogen 612 mg/dL and D-dimer 4770 mg/dL. Basic metabolic panel and liver tests were grossly unremarkable (potassium 3.1 mmol/L, phosphorus 2.4 mmol/L), and lactate dehydrogenase (LDH) was 1005 U/L. Uric acid was normal.
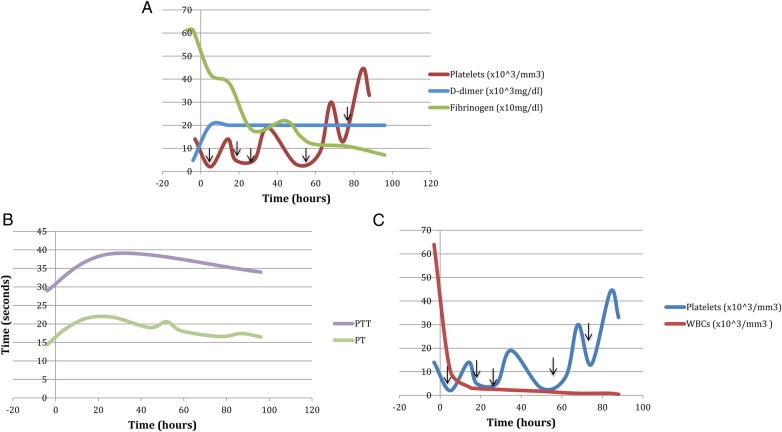
On day one of chemotherapy, ∼150 min after rituximab infusion, the patient evolved fever of 102.4 F with rigors, nausea, vomiting, pronounced sinus tachycardia (approximate rate of 150), tachypnoea and profound hypotension to 82/51, consistent with a severe cytokine-release syndrome. He was haemodynamically stabilised and rituximab infusion was held. Five hours later, significant coagulation abnormalities and relative cytopenias evolved in the setting of clinically significant mucocutaneous bleeding (gingival and nasal bleeding), and in the absence of schistocytes on peripheral blood smear. INR rose to 1.9 from 1.1, D-dimer rose to >20 000 mg/dL from 4770 mg/dL and fibrinogen dropped to 422 mg/dL from 612 mg/dL, with further drop to 71 mg/dL after 96 hours. Platelet and white cell count dropped dramatically (14 000/mm3 to 2000/mm3 and 63 910/mm3 to 10 420/mm3, respectively), while haemoglobin dropped to 11.8 g/dL from 13 g/dL. LDH level rose prominently. Uric acid level remained relatively unchanged, and liver tests rose marginally (aspartate transaminase (AST) to 649 U/L from 23 U/L, alanine transaminase (ALT) to 50 U/L from 14 U/L, alkaline phosphatase to 272 U/L from 102 U/L). Figure 1A–C illustrate the evolution of the patient's coagulopathy over the first 96 hours of chemotherapy (figure 1).
Figure 1.
(A) Coagulation profile during initial rituximab infusion from a few hours prior to the start of infusion until 96 hours after infusion. Time 0 corresponds to the start of rituximab infusion. The black arrows indicate platelet transfusion. (B) Prothrombin time and partial thromboplastin time during initial rituximab infusion from a few hours prior to the start of infusion until 96 hours after infusion. Time 0 corresponds to the start of rituximab infusion. (C) Evolution of rapid-onset relative cytopenias from a few hours prior to the start of infusion until 96 hours after infusion. Time 0 corresponds to the start of rituximab infusion. The black arrows indicate platelet transfusion.
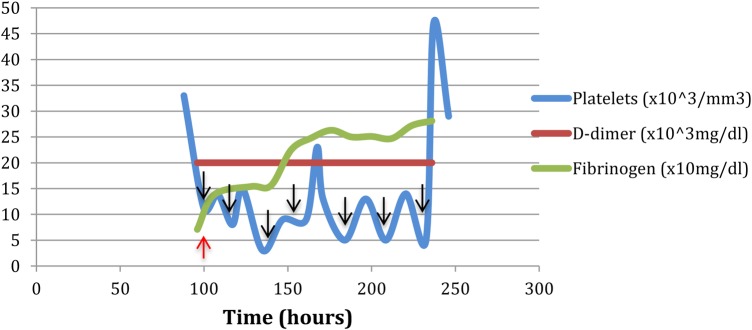
In this setting, the patient was transfused with one pool of cryoprecipitate and with platelets. Fibrinogen levels increased to 138 mg/dL, with subsequent stability; however, he required additional platelet transfusions to maintain stability. After further clinical recovery, the patient resumed his chemotherapy and received his second rituximab infusion on day 11, complicated by recurrent thrombocytopenia (14 000–5000/mm3). However, the postinfusion coagulation profile, including INR, D-dimer and fibrinogen level, was not suggestive of DIC, and there was no objective manifestation of haemodynamically significant cytokine-release syndrome. Figure 2 illustrates the patient's recovery from coagulopathy in the period between the first and second rituximab doses, in the setting of persistent thrombocytopenia (figure 2).
Figure 2.
Coagulation profile between rituximab dose 1 and 2, after transfusion of one pool cryoprecipitate and five units of platelets. Time 0 corresponds to the start of rituximab infusion. The black arrows indicate platelet transfusion and the red arrow indicate platelet transfusion.
Outcome and follow-up
After receiving the second infusion of rituximab, the patient completed his chemotherapy induction without further complications, and was discharged in a good clinical condition to be followed as outpatient.
Discussion
A PubMed database search using the terms: rituximab AND fibrinolysis, rituximab AND coagulation, and rituximab AND thrombocytopenia was conducted. Papers specifically addressing acute haematological adverse effects of rituximab were reviewed. The search yielded one cohort study and 16 case reports.6–22 In total, 22 patients were addressed in these case reports, and 29 rituximab infusions associated with haematological adverse reactions were reported between 2001 and 2013. Patients’ characteristics and clinical courses (not including our patient) are summarised in table 1.
Table 1.
Summary of cases of haematological adverse effects following rituximab
| Pt# | Author (year) | Infusion | G | Age | Haematological disease | Splenomegaly | Preplatelets | Preleukocytes | Pre-Hb | Pre-D-dimer | Pre-fib | Pre-INR | Pre-LDH | Rituximab dose | Hours to ADR | Postplatelets | Postleukocytes | Post-Hb | Post-D-dimer | Post-fib | Post-INR | Post-LDH | CRS | Intravascular fibrinolysis | Shistocytes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rigamonti (2001) | 1 | F | 60 | LPL | Yes | 86 000 | 1100 | 9.7 | NS | NS | NS | NS | 375 mg/m2 | 5 | 7000 | 1100 | 9.7 | Normal | Normal | Normal | 2×up | Yes | No | NS |
| 2 | Shah (2004) | 1 | M | 57 | MCL | Yes | 151 000 | 10 800 | 9.4 | NS | NS | NS | NS | 375 mg/m2 | 24 | 8000 | 6000 | 7.7 | NS | NS | NS | NS | Yes | NS | NS |
| 3 | Pamuk (2005) | 1 | M | 75 | B-cell PLL | Yes | 92 000 | 160 000 | 7.7 | NS | NS | NS | NS | 375 mg/m2 | 4 | 7000 | 12 000 | 7.4 | NS | NS | NS | NS | NS | NS | NS |
| 4 | Otrock (2005) | 1 | M | 41 | HCL | Yes | 85 000 | 2800 | 9.5 | NS | NS | NS | NS | 375 mg/m2 | 24 | 7000 | NS | NS | NS | NS | NS | NS | Yes | NS | NS |
| 5 | Otrock (2005) | 1 | M | 64 | MCL | NS | 90 000 | 90 200 | 10.5 | NS | NS | NS | NS | 375 mg/m2 | 24 | 10 000 | NS | NS | NS | NS | NS | NS | Yes | NS | NS |
| 6 | Thachil (2006) | 1 | M | 44 | HCL | NS | 31 000 | 1400 | 9.4 | NS | NS | NS | NS | NS | 5 | 6000 | NS | NS | 33 020 ng/ml | 410 | 1.2 | NS | No | Yes | NS |
| 7 | Rosado (2007) | 1 | M | 64 | MCL | NS | 175 000 | NS | NS | NS | NS | NS | NS | 375 mg/m2 | 24 | 22 000 | NS | NS | NS | NS | NS | NS | NS | NS | No |
| 7 | Rosada (2007) | 2 | M | 64 | MCL | NS | 135 000 | NS | NS | NS | NS | NS | NS | 375 mg/m2 | 18 | 11 000 | NS | NS | NS | NS | NS | NS | NS | NS | No |
| 8 | Yi (2009) | 1 | M | 58 | MCL-BV | Yes | 132 000 | NS | NS | NS | NS | NS | 994 | 375 mg/m2 | 24 | 24 000 | NS | NS | Normal | Normal | Normal | 3018 | Yes | No | NS |
| 8 | Yi (2009) | 2 | M | 58 | MCL-BV | Yes | 139 000 | NS | NS | NS | NS | NS | NS | 375 mg/m2 | 8 | 48 000 | NS | NS | NS | NS | NS | NS | Yes | NS | NS |
| 9 | Dhand (2008) | 1 | M | 84 | MCL | NS | 121 000 | NS | NS | NS | NS | NS | NS | 375 mg/m2 | 24 | 15 000 | NS | NS | NS | NS | NS | NS | No | NS | NS |
| 10 | Ram (2009) | 1 | F | 71 | MCL | NS | 63 000 | 80 000 | 10.5 | NS | NS | NS | NS | 375 mg/m2 | 18 | 10 000 | 1000 | NS | NS | NS | NS | NS | Yes | No | No |
| 11 | Kotsianidis (2009) | 1 | M | 74 | Atypical HCL | Yes | 127 000 | 49 700 | 13.7 | NS | NS | NS | NS | 375 mg/m2 | 24 | 21 000 | 28 000 | NS | 15 470 mg/dL | 127 | 2.43 | 2×ULN | No | Yes | No |
| 12 | Adiyodi (2010) | 1 | F | 83 | HCL | Yes | 74 000 | NS | NS | NS | NS | NS | NS | NS | 12 | 22 000 | NS | NS | High | Normal | Normal | NS | Yes | NS | NS |
| 13 | Adiyodi (2010) | 1 | F | 79 | MZL | Yes | 162 000 | NS | 8.4 | NS | NS | NS | NS | 375 mg/m2 | 24 | 43 000 | NS | NS | NS | NS | NS | NS | Yes | NS | NS |
| 14 | Parajuli (2010) | 1 | F | 73 | MCL | Yes | 85 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 14 000 | NS | NS | NS | NS | NS | NS | Yes | NS | NS |
| 14 | Parajuli (2010) | 2 | F | 73 | MCL | Yes | 100 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 54 000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 14 | Parajuli (2010) | 3 | F | 73 | MCL | Yes | 126 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 27 000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 14 | Parajuli (2010) | 4 | F | 73 | MCL | Yes | 123 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 49 000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 14 | Parajuli (2010) | 5 | F | 73 | MCL | Yes | 103 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 27 000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 15 | Pilorge (2010) | 2 | M | 50 | WM | No | 144 000 | 3800 | 11.8 | NS | 380 | PR=92% | 347 | 375 mg/m2 | 2 | 48 000 | NS | 11.4 | 110 400 ng/L | 50 | PR=50% | NS | Yes | Yes | NS |
| 16 | Novak (2012) | 1 | M | 70 | WM | NS | 171 000 | 2900 | 8.4 | 420 ng/mL | 290 | 1.31 | NS | 375 mg/m2 | 4 | 30 000 | NS | NS | 9670 ng/mL | 180 | 1.78 | NS | No | Yes | NS |
| 17 | El-Osta (2013) | 1 | F | 66 | MCL | NS | 175 000 | 18 400 | NS | NS | NS | NS | Normal | NS | 3 | 16 000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 18 | Sadashiv (2013) | 1 | F | 63 | MCL | Yes | 133 000 | 9990 | 8.6 | NS | NS | NS | NS | NS | 24 | 5000 | 7640 | 7.4 | NS | NS | NS | NS | Yes | NS | NS |
| 18 | Sadashiv (2013) | 2 | F | 63 | MCL | Yes | 149 000 | NS | NS | NS | NS | NS | NS | NS | 24 | 5000 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 19 | Sadashiv (2013) | 1 | M | 72 | MCL | Yes | 33 000 | 26 260 | 9.4 | NS | NS | NS | NS | NS | 24 | 10 000 | 5730 | 9.1 | NS | NS | NS | NS | No | NS | NS |
| 20 | Sadashiv (2013) | 1 | M | 60 | MCL | Yes | 146 000 | 12 320 | 10.1 | NS | NS | NS | NS | NS | 72 | 74 000 | 3860 | 8.8 | NS | NS | NS | NS | NS | NS | NS |
| 21 | Sadashiv (2013) | 1 | F | 64 | MCL | Yes | 91 000 | 13 070 | 8.6 | NS | NS | NS | NS | NS | 24 | 3000 | 6180 | 8.5 | NS | NS | NS | NS | Yes | NS | NS |
| 22 | Sadashiv (2013) | 1 | M | 76 | MCL | Yes | 122 000 | 20 440 | 14.1 | NS | NS | NS | NS | NS | 24 | 26 000 | 8810 | 13.4 | NS | NS | NS | NS | Yes | NS | NS |
2×ULN, two times upper limit of normal; 2×up, twofold increase; ADR, adverse reactions in hours; BV, Blastoid variant; CRS, cytokine-release syndrome; F, female; fib, fibrinogen; G, gender; Hb, haemoglobin; HCL, hairy cell leukaemia; INR, international normalised ratio; LDH, lactate dehydrogenase; LPL, lymphoplasmacytoid lymphoma; M, male; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NS, not specified; PLL, prolymphocytic leukaemia; PR, Prothrombin ratio; Pt#, Patient number; WM, Waldenstrom's macroglobulinemia.
Among the 22 patients, 14 were male patients and eight were female patients. There were 13 mantle cell lymphomas (MCL), four hairy cell leukaemias (HCL), two Waldenstrom's macroglobulinemia, one lymphoplasmacytic lymphoma, one B-cell prolymphocytic leukaemia, and one marginal zone lymphoma. There were no cases of pre-B-ALL. In all studies where the dose of rituximab was reported, the dose infused was 375 mg/m2.
The median time to development of adverse events related to rituximab infusion was 24 hours, the fastest occurring within 2 hours of start of infusion. Clinically evident cytokine-release syndrome was commonly found, occurring in 14 cases (absent in five; not reported on in the remainder), and with similar manifestations to that presented in our case, including fever, chills, severe rigors and tachycardia, but without profound hypotension except in one case where the reaction was severe.19 Postinfusion thrombocytopenia was documented in all cases reviewed (mean preinfusion level of 116 000+/−38 581, and postinfusion level of 22 379+/−18 076). Ten cases reported on post-transfusion leucopenia (mean preinfusion count 31 449+/−43 818 and postinfusion count 8032+/−7767).
Importantly, only eight cases reported on the coagulation profile of their patients, and only four documented intravascular fibrinolysis in a DIC-like fashion.10 15 21 22 In the first of these cases,10 the D-dimer and INR increased to 33 020 mg/dL and 1.2, respectively, and fibrinogen decreased to 410 mg/dL (starting level not specified for any of the three tests), and there was no report on the presence of schistocytes or change in LDH. In the second case,15 the D-dimer and INR increased to 15 470 mg/dL and 2.43, respectively, and fibrinogen decreased to 127 mg/dL (again, starting level not specified for any of the three tests). However, the absence of schistocytes was documented, and LDH level increased to two times above the upper limit of normal. In the two latter cases,21 22 the D-dimer increased from an unspecified starting level and 420 ng/mL to 110 400 ng/L and 9670 ng/mL, respectively; prothrombin ratio (PR) decreased from 92% to 50% in the third case and INR increased from 1.31 to 1.78 in the fourth case; and fibrinogen decreased from 380 mg/dL and 290 mg/dL to 50 mg/dL and 180 mg/dL, respectively. There was again no mention of the presence or absence of schistocytes in neither of these last two cases. Interestingly, in the second case fibrinolysis and thrombocytopenia occurred within hours of rituximab infusion, and fibrinogen continued to decrease in the 96 hours postinfusion (89 mg/dL). Collectively, the findings in these four cases are the most representative of a DIC-like abnormality across all cases reviewed. One study documented a patient with a normal coagulation profile after rituximab infusion despite development of thrombocytopenia and a twofold increase in AST, ALT and LDH,6 and two other studies excluded DIC as the source for post-transfusion thrombocytopenia.12 14 Another study did not comment on the presence of DIC, though an abnormal coagulation profile postinfusion was reported.16
Rituximab is a chimeric monoclonal antibody that targets CD20 and is used in a variety of conditions including lymphoproliferative and autoimmune diseases.23 24 It has a relatively favourable toxicity profile; the most common adverse effects include an acute infusion reaction consisting of fever, chills, rigors, rash and occasionally bronchospasm and hypotension. This effect is known as a ‘cytokine-release syndrome’, and is thought to be due to robust cytokine release,25–27 in the presence of circulating tumour cells.28
Delayed-onset cytopenias are the most common haematological adverse reactions associated with rituximab, and most often appear several weeks after rituximab infusion. In one analysis conducted on 77 courses of rituximab,5 delayed-onset cytopenias were documented in 29.8% of the cases, including neutropenia (27.3%) at a median of 10 weeks, thrombocytopenia (10.4%) at a median of 4 weeks, and anaemia (5.2%) at a median of 5 weeks. Another study reported on cytopenias in 53 patients, most of whom received rituximab for non-Hodgkins’ lymphoma.29 In this series eight episodes of grade 4, neutropenia were detected between 1 and 5 months after rituximab infusion; five of these occurred when rituximab was used as single agent and the other three when rituximab was used in combination with other agents.29 In our case, the patient experienced a dramatic drop in total white cell count within 5 hours of infusion, with rituximab as a single agent.
In contrast, reports of cytopenias with coagulopathy and temporal association with rituximab infusion are uncommon in the literature. Indeed, the acute, postinfusion thrombocytopenia associated with laboratory evidence of coagulopathy and fibrinolysis (rapid rise in D-dimer and prothrombin time, and drop in fibrinogen) presented in our case seems quite rare (reported only in four cases;10 15 21 22 and is suggestive of DIC or a DIC-like abnormality, despite the absence of schistocytes on peripheral blood smear. Further, there are no reported cases of these abnormalities as sequelae to a haemodynamically significant cytokine-release syndrome with clinical manifestations of bleeding in a patient with pre-B-ALL. The presence of these attributes together makes the patient in our case unique. Only the case reported by Kotsianidis et al is similar when one considers the chronological progression of the adverse events and the nature of the coagulation abnormalities, including the lack of schistocytes, but this case did not involve haemodynamically significant cytokine-release syndrome, mucocutaneous bleeding or pre-B-ALL.15
The mechanism underlying the coagulopathy associated with rituximab may not be concluded from the case report per se. We hypothesise, however, a few mechanisms based on observations from our case report as well as review of the literature. A rituximab infusion associated with a severe cytokine-release syndrome with rapid-onset cytopenias and a DIC-like syndrome, like the one seen in our patient, may reflect a relationship between profound cytokine release and activation of the complement cascade.14 Consider that a postinfusion drop in total white cell count is a common, expected occurrence with rituximab, likely due to the binding by rituximab to CD20 positive B lymphocytes, activating the complement cascade and cell lysis. Whether the extent of complement-mediated lymphocyte destruction is proportional to the severity of a preceding cytokine-release syndrome would be a matter for further study. Only three reported cases of rituximab-associated fibrinolysis before ours were accompanied by cytokine-release syndrome while the fourth was not; which suggests that the two phenomena might not be mechanistically linked. Another theory would be related to the burden of disease whereby rituximab causes rapid and dramatic tumour destruction regardless of complement activity. The massive destruction products lead to coagulopathy in a DIC-like picture. The observation that our patient well tolerated subsequent infusions of rituximab without developing this coagulopathy might be as well related to the decreased burden of disease after initial infusion.
Learning points.
This is a case report of a patient who developed acute haematological abnormalities including thrombocytopenia as well as fibrinolysis and coagulation abnormalities very similar to disseminated intravascular coagulation (DIC) shortly after the first infusion of rituximab.
Rituximab-associated coagulopathy in a DIC-like fashion seems to be a very rare phenomenon.
It is extremely important to recognise this phenomenon early as part of the differential diagnosis of thrombocytopenia and DIC following rituximab administration.
We recommend laboratory monitoring shortly after rituximab infusion and thereafter to detect these abnormalities and initiate supportive treatment early.
Footnotes
Contributors: HR has substantial contributions to the conception and design of the report, the acquisition, analysis and interpretation of data. HR also performed drafting of the work and revised it critically. HR also provided final approval of the version published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SN has contribution into the design of the paper, drafting the paper in its different sections and revising the paper critically. IFG has substantial contribution to the conception of the idea and designing and drafting of the paper in its different sections, and also in revising the paper critically over multiple review drafts.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.US Department of Health and Human Services 2010. Common terminology criteria for adverse events v4. 03.
- 2.Rituxan [package insert]. South San Francisco, CA: Genentech, Inc., 2011. [Google Scholar]
- 3.Kasi PM, Tawbi HA, Oddis CV et al. Clinical review: serious adverse events associated with the use of rituximab-a critical care perspective. Critical Care 2012;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaneo C, Spedini P, Casari S et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma 2006;47:1013–17. 10.1080/10428190500473113 [DOI] [PubMed] [Google Scholar]
- 6.Rigamonti C, Volta C, Colombi S et al. Severe thrombocytopenia and clinical bleeding associated with rituximab infusion in a lymphoma patient with massive splenomegaly without leukemic invasion. Leukemia 2001;15:186. [DOI] [PubMed] [Google Scholar]
- 7.Shah C, Grethlein SJ. Case report of rituximab-induced thrombocytopenia. Am J Hematol 2004;75:263 10.1002/ajh.20028 [DOI] [PubMed] [Google Scholar]
- 8.Pamuk GE, Donmez S, Turgut B et al. Rituximab-induced acute thrombocytopenia in a patient with prolymphocytic leukemia. Am J Hematol 2005;78:81 10.1002/ajh.20218 [DOI] [PubMed] [Google Scholar]
- 9.Otrock ZK, Mahfouz RA, Oghlakian GO et al. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica 2005;90(Suppl):ECR23. [PubMed] [Google Scholar]
- 10.Thachil J, Mukherje K, Woodcock B. Rituximab-induced haemorrhagic thrombocytopenia in a patient with hairy cell leukaemia. Br J Haematol 2006;135:273–4. 10.1111/j.1365-2141.2006.06299.x [DOI] [PubMed] [Google Scholar]
- 11.Rosado M, Chao H, Rose M. Severe acute thrombocytopenia following rituximab therapy. Leuk Lymphoma 2007;48:2239–40. 10.1080/10428190701625099 [DOI] [PubMed] [Google Scholar]
- 12.Yi JH, Kim SJ, Ahn HK et al. Rituximab-induced acute thrombocytopenia: a case report and review of the literature. Med Oncol 2009;26:45–8. 10.1007/s12032-008-9079-6 [DOI] [PubMed] [Google Scholar]
- 13.Dhand S, Bahrain H. Rituximab-induced severe acute thrombocytopenia: a case report and review of literature. Cancer Invest 2008;26:913–15. 10.1080/07357900802010509 [DOI] [PubMed] [Google Scholar]
- 14.Ram R, Bonstein L, Gafter-Gvili A et al. Rituximab-associated acute thrombocytopenia: an under-diagnosed phenomenon. Am J Hematol 2009;84:247–50. 10.1002/ajh.21372 [DOI] [PubMed] [Google Scholar]
- 15.Kotsianidis I, Goutzouvelidis A, Anastasiades A et al. Severe thrombocytopenia and fibrinolysis mimicking disseminated intravascular coagulation after rituximab infusion. Am J Hematol 2010;85:146 10.1002/ajh.21597 [DOI] [PubMed] [Google Scholar]
- 16.Adiyodi J, Thachil J, Hawkins S et al. Thrombocytopenia with rituximab treatment—splenomegaly as the risk factor. Ann Hematol 2010;89:95–6. 10.1007/s00277-009-0768-9 [DOI] [PubMed] [Google Scholar]
- 17.Parajuli R, Hire E, Shah BK. Rituximab-induced acute severe thrombocytopenia. Br J Haematol 2010;149:804 10.1111/j.1365-2141.2010.08119.x [DOI] [PubMed] [Google Scholar]
- 18.El-Osta H, Nair B. Rituximab-induced acute thrombocytopenia: an underappreciated entity. Leuk Lymphoma 2013;54:2736–7. [DOI] [PubMed] [Google Scholar]
- 19.Sadashiv SK, Rao R, Fazal S et al. Rituximab-induced acute severe thrombocytopenia: a case series in patients with mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2013;13:602–5. [DOI] [PubMed] [Google Scholar]
- 20.Giezen TJ, Mantel-Teeuwisse AK, ten Berg MJ et al. Rituximab-induced thrombocytopenia: a cohort study. Eur J Haematol 2012;89:256–66. 10.1111/j.1600-0609.2012.01808.x [DOI] [PubMed] [Google Scholar]
- 21.Pilorge S, Park S, Dreyfus F et al. Rituximab-induced life-threatening coagulopathy occurring in a patient with Waldenström macroglobulinemia treated with fludarabine, cyclophosphamide, and rituximab combination. Leuk Lymphoma 2010;51:2288–90. 10.3109/10428194.2010.523127 [DOI] [PubMed] [Google Scholar]
- 22.Novak J, Mocikova H, Pavlicek P et al. Rituximab-induced coagulopathy. Leuk Lymphoma 2012;53:2299–301. 10.3109/10428194.2012.682313 [DOI] [PubMed] [Google Scholar]
- 23.Keystone E, Fleischmann R, Emery P et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 2007;56:3896–908. 10.1002/art.23059 [DOI] [PubMed] [Google Scholar]
- 24.Avilés A, Nambo MJ, Neri N et al. Dose dense (CEOP-14) vs dose dense and rituximab (CEOP-14+R) in high-risk diffuse large cell lymphoma. Med Oncol 2007;24:85–9. [DOI] [PubMed] [Google Scholar]
- 25.Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 2010;14:E10 10.1188/10.CJON.E10-E21 [DOI] [PubMed] [Google Scholar]
- 26.Byrd JC, Murphy T, Howard RS et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 2001;19:2153–64. 10.1200/jco.2001.19.8.2153 [DOI] [PubMed] [Google Scholar]
- 27.Winkler U, Jensen M, Manzke O et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999;94:2217–24. [PubMed] [Google Scholar]
- 28.Byrd JC, Waselenko JK, Maneatis TJ et al. Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: association with increased infusion-related side effects and rapid blood tumor clearance. J Clin Oncol 1999;17:791 10.1200/jco.1999.17.3.791 [DOI] [PubMed] [Google Scholar]
- 29.Chaiwatanatorn K, Lee N, Grigg A et al. Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol 2003;121:913–18. [DOI] [PubMed] [Google Scholar]