Abstract
Cryptococcus neoformans and C. gattii cause invasive fungal disease, with meningitis being the most common manifestation of central nervous system (CNS) disease. Encapsulated cryptococcomas occur rarely, predominantly in immunocompetent hosts, usually related to C. gattii. Our patient was an immunocompetent man who presented with headache and a large cystic CNS lesion thought to be glioblastoma. Biopsy of a concomitant lung lesion confirmed cryptococcoma and empiric antifungal therapy was started for presumed CNS cryptococcoma. Antifungal therapy failed to shrink the CNS lesion, and surgical excision confirmed C. gattii CNS cryptococcoma. Following surgery he had complete resolution of symptoms. This case highlights that cryptococcoma cannot be distinguished from tumour on clinical or imaging findings. A combined medical and surgical approach is optimal for the management of large or surgically accessible cryptococcomas, as antifungal therapy alone is unlikely to penetrate large lesions sufficiently to lead to a cure.
Background
The diagnosis and treatment of central nervous system (CNS) cryptococcoma is more complicated than that of meningitis. Cryptococcomas are radiologically non-specific, may be mistaken for malignant lesions, and the diagnosis may only be made on histology after resection. It is important to consider cryptococcoma in the differential for malignant and mass lesions of the CNS, particularly in areas in which Cryptococcus gattii is endemic.
C. neoformans and C. gattii are encapsulated yeasts that can cause invasive fungal infection in humans, with the lung and CNS the most common sites of infection. C. neoformans classically causes meningitis in the immunosuppressed host, whereas C. gattii predominantly causes disease in immunocompetent hosts and can lead to the formation of large pulmonary and CNS cryptococcomas.1–3 C. neoformans is found worldwide. C. gattii, which causes most cryptococcomas, was thought to be geographically restricted to areas such as Australia, Papua New Guinea, Southeast Asia, Africa, South America, Southern California and Vancouver Island but more discriminating identification suggests that it too is worldwide.4–6
Although there are no clinical trials on which to base timing (early vs delayed) of surgical resection of cryptococcomas, prolonged courses of antifungal agents are usually recommended. Morbidity remains high and long-term neurological sequelae are more common in C. gattii-related cryptococcomas than in the C. neoformans meningoencephalitis, although mortality is higher with the latter.3 7
Case presentation
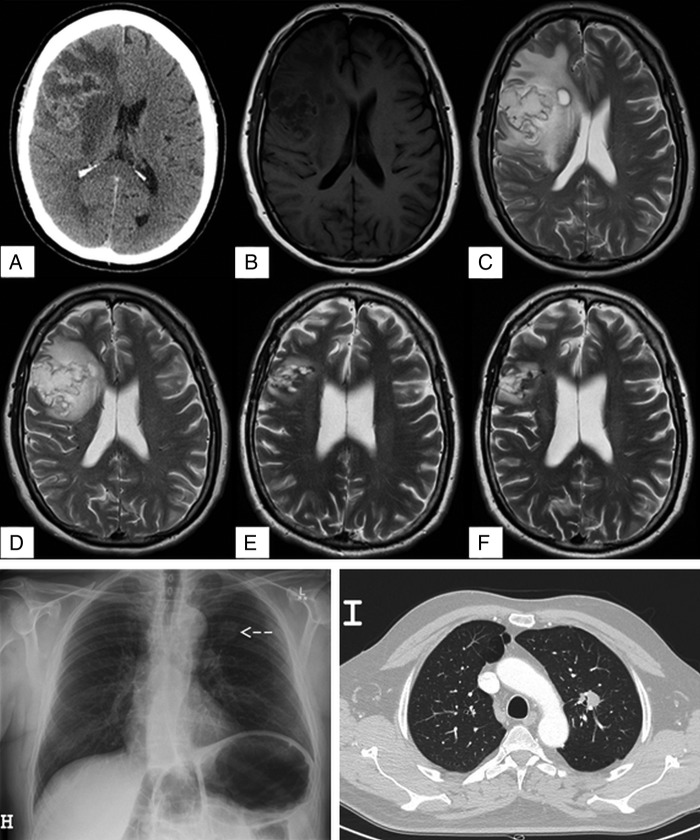
A 55-year-old man presented to his general practitioner with a 1-month history of headaches. The headaches were severe, radiating from the occiput to the frontal regions of his head with no associated systemic symptoms. His medical history included controlled essential hypertension and gout, but no history of diabetes or immunocompromise. He was an ex-smoker and drank alcohol sparingly. A CT scan revealed a large right frontoparietal lesion (figure 1A), suggestive of a CNS malignancy. He was started on 16 mg of dexamethasone daily in divided doses and referred for neurosurgical evaluation.
Figure 1.
(A) CT brain with contrast, axial view, showing large complex cystic mass in the right frontal lobe with midline shift consistent with primary brain neoplasm. (B) T1-weighted MRI brain, axial view. (C) T2-weighted MRI brain, axial view, showing heterogeneous predominantly cystic mass (4×5×4.8 cm) centred in the right frontal lobe with peripheral enhancement, vasogenic oedema, and 5 mm of midline shift. (D) T2-weighted MRI after 24 days of amphotericin B plus flucytosine showing decrease in oedema and mass effect, but no change in the size of the lesion. (E) T2-weighted MRI 3 months postresection of large cryptococcoma on medical therapy with oral fluconazole. (F) T2-weighted MRI brain 10 months postresection showing residual small gliotic cavity, without enhancement or other evidence of any residual disease. (H) Chest radiograph showing a left upper lobe lesion indicated by the arrow. (I) CT of the chest showing a 22 mm spiculated soft tissue density nodule in the left upper lobe.
Clinical examination revealed papilloedema of the right optic disc and left pronator drift. The remainder of the examination was normal. Laboratory investigations were normal with no evidence of leucocytosis. MRI demonstrated a 4×5×4.8 cm heterogeneous predominantly cystic mass in the right frontoparietal region with peripheral enhancement and surrounding oedema. There was moderate mass effect with effacement of the right ventricle and 5 mm midline shift to the left side (figure 1B, C). The remainder of the brain parenchyma was normal. These findings were consistent with a primary CNS malignancy, most in keeping with a glioblastoma, and surgical resection of this lesion was planned.
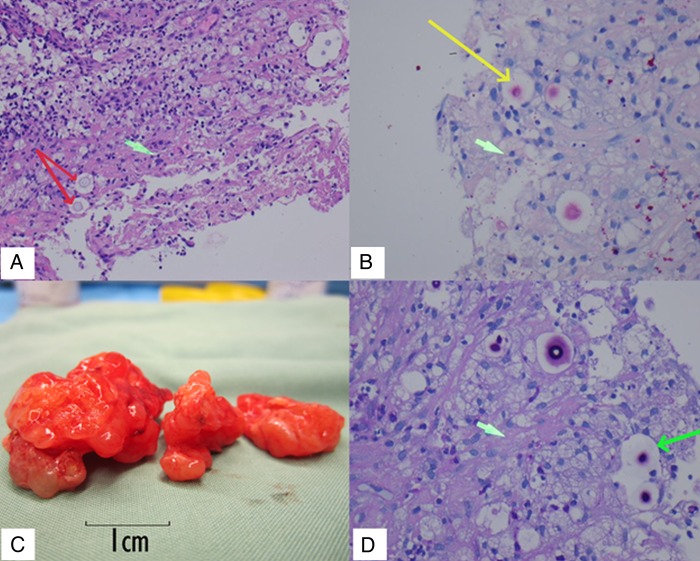
Preoperative chest radiography and CT scan demonstrated a mass lesion in the left upper lobe (figure 1H, I). Bronchoscopic biopsy and washings demonstrated encapsulated yeast morphologically resembling Cryptococcus; however, these were unable to be cultured (figure 2A, B). Serum cryptococcal antigen was found to be reactive at 1:512. Cerebrospinal fluid (CSF) cryptococcal antigen was 1:8 with normal glucose (4.2 mmol/L) and protein (370 mg/L). Blood and CSF cultures were negative. The patient was HIV negative with normal lymphocyte subsets. In light of these findings, the primary differential diagnosis became cerebral and pulmonary cryptococcoma.
Figure 2.
(A) Lung biopsy, H&E stain, ×100 magnification, red arrows indicating individual Cryptococci with surrounding capsular space. Green arrow indicates possible early stage of granuloma formation. (B) Lung biopsy, mucicarmine stain, ×200 magnification. Yellow arrow indicates Cryptococcus stained pink with large capsular space around it. Green arrow indicates a foamy macrophage (C) Macroscopic cryptococcoma resection. (D) Central nervous system cryptococcoma tissue, Periodic acid-Schiff (PAS) stain, ×400 magnification. Long green arrow shows Cryptococcus with staining capsular material. Short green arrow indicates gliosis.
Investigations
The patient had CT and MRI brain scans, chest X-ray and CT chest to look for possible lung malignancy. Bronchoscopy was performed to biopsy a lung nodule that identified Cryptococcus. A lumbar puncture was performed and CSF and serum were tested for cryptococcal antigen that confirmed infection.
Differential diagnosis
The differential diagnoses included pulmonary and CNS cryptococcoma, pulmonary cryptococcoma and CNS glioblastoma multiforme, or primary lung malignancy with CNS metastasis. Biopsy of the lung excluded malignancy and suggested Cryptococcus. Excision of the cryptococcoma confirmed C. gattii as the pathogen.
Treatment
Dexamethasone (to reduce brain oedema when malignancy was suspected) was weaned over 9 days and antifungal induction treatment was started with lipid complex amphotericin B (Ablecet 450 mg (5 mg/kg/day), given over 12 hours, intravenous) in combination with 5-flucytosine (5-FC), 1500 mg per oral four times per day (66 mg/kg/day). Interval MRI performed at 1 month revealed a marked decrease in oedema and mass effect, but there was no change in the size of the lesion (figure 1D). Given the minimal radiographic improvement, uncertainty regarding antifungal penetration into the lesion, as well as lack of a microbiologically confirmed diagnosis to guide therapy, the decision for surgical resection was made.
Surgical resection confirmed the diagnosis of C. gattii cryptococcoma (figure 2C, D, see video). The mean inhibitory concentrations (MIC) of the fungal isolate were 0.5 mg/L for 5-FC, 0.5 mg/L for amphotericin and 4 mg/L for fluconazole. Amphotericin and 5-FC were withheld 24 hours prior to surgery due to renal insufficiency. Intraoperative CSF samples indicated therapeutic levels of 5-FC (28 mg/L, or 56 times the MIC); intralesion drug levels were not able to be measured. Amphotericin B was not detected in CSF in keeping with previous experimental studies.8
Video 1.
Intraoperative video: the cryptococcoma is shiny and gelatinous in appearance but relatively firm and strengthened by internal fibrous strands. It is well encapsulated and non-adherent, with rare bridging arteries.
Outcome and follow-up
Immediately postoperatively the patient experienced mild left upper and lower limb weakness, which resolved within 4 days, as well as mild left-sided facial weakness, which gradually resolved over 2 months. The lipid complex amphotericin and 5-FC were continued for 10 days postoperatively (total 34 days; 15.3 g amphotericin). Oral fluconazole therapy at a dose of 800 mg daily was continued for 9 months, after which therapy was stopped. Follow-up MRIs carried out 3 and 10 months postresection showed a residual small gliotic cavity, without enhancement or evidence of any residual disease (figure 1E, F). He had incidentally developed mild primary hyperparathyroidism thought to be unrelated to this condition.
Discussion
The encapsulated yeasts C. neoformans and C. gattii cause invasive fungal infections referred to as cryptococcosis. The lung and CNS are the most common sites of infection. Most reported cases of cryptococcosis worldwide are caused by C. neoformans. However, improved diagnostic discrimination indicates that C. gattii has a similar worldwide distribution.4–6 Whereas C. neoformans usually causes disease in patients with T-cell immunodeficiency, such as those with AIDS, C. gattii is more likely to be associated with disease in immunocompetent hosts.1–3 CNS disease may present with acute or subacute onset of meningitis, meningoencephalitis, space occupying lesions, or hydrocephalus. CNS mass lesions or cryptococcomas are significantly associated with C. gattii, particularly in immunocompetent hosts.1–3 9 10
Diagnosis of cryptococcal CNS disease can be delayed in immunocompetent patients where the index of suspicion is low, and because symptoms are often non-specific and subacute. Serum cryptococcal antigen testing is a good screening test, though less sensitive in HIV-negative patients.11 Examination of CSF can reveal abnormalities such as high opening pressure, positive India ink stain and the presence of detectable cryptococcal capsular polysaccharide antigen. Detection of cryptococcal antigen in CSF is very sensitive and specific for the diagnosis of cryptococcal meningitis.11 Glucose is usually low, and protein elevated. Very high lumbar CSF protein may indicate obstruction to CSF flow known as Froin's syndrome.12 Cryptococcus can often be cultured from the CSF. In our case, CSF glucose and protein were found to be normal, Cryptococcus was not able to be cultured from the CSF, India ink stain was negative and the cryptococcal antigen in the CSF was detected at a low titre of 1:8 suggesting a sequestered encapsulated infection.
Although neuroimaging is frequently normal in patients with cryptococcal meningitis, radiological abnormalities can include cryptococcomas, pseudocysts and obstructing hydrocephalus.13–18 Cryptococcomas are more common in immunocompetent patients, and have been found in up to 69% of immunocompetent patients with C. gattii.1 2 19 All of the very large cryptococcomas described to date are thought to be due to C. gattii.2 Although MRI is the most sensitive neuroimaging modality for cryptococcal disease cryptococcomas can appear indistinguishable from other CNS lesions such as pyogenic abscesses, toxoplasma, lymphoma, primary CNS malignancy, tuberculomas and metastatic malignancy.20–22 Cryptococcomas in immunocompetent patients are misdiagnosed in over half of the cases, usually as brain tumours, and diagnosis may only be recognised after surgical resection or biopsy.14 22 MRI features of cryptococcoma have been described to include hypointensity in T1-weighted images and hyperintensity in T2-weighted images with peripheral oedema and ring-shaped enhancement after gadolinium in the majority of cases with cystic lesions.17 18 22 MR spectroscopy may, in the future, assist with radiological diagnosis of CNS cryptococcal disease.23–28 Interpretation of neuroimaging abnormalities after treatment is difficult because some degree of MRI changes may persist a number of years after successful treatment.29
The goal of therapy is sterilisation of CSF to achieve neurological recovery. Treatment of CNS cryptococcal infection includes antifungal treatment and relief of elevated intracranial pressure, and, in the case of large (>3 cm) cryptococcomas, debulking of the lesion.7 Guidelines for patients with cerebral cryptococcomas recommend antifungal induction therapy for at least 6 weeks with amphotericin (amphotericin D (0.7–1 mg/kg/day, intravenous) or liposomal amphotericin B (3–4 mg/kg/day, intravenous) or lipid complex amphotericin (5 mg/kg/day, intravenous)) with 5-FC (100 mg/kg/day orally in four divided doses).7 30 31 Induction should be followed by consolidation and maintenance therapy with 400–800 mg per day of oral fluconazole (if susceptible) for between 6 and 18 months. A longer course of treatment may be required in cases of C. gattii.2 3 Corticosteroids to reduce oedema and mass effect of cryptococcoma has not been evaluated systematically.7
Our experience suggests that early surgery in cryptococcomas may significantly shorten the duration of required antifungal therapy and should be considered as part of first-line therapy in all cases which are amenable to surgical resection.
Patient's perspective.
Initial journey
On 1 January 2013 I woke post-New Year's Eve with an outrageous headache including heightened sense of hearing. I mentioned to my wife that the ocean was very loud that morning.
The discomfort continued and worsened until I attended my general practitioner who requested a CT. By 19:00 on Friday night, I was admitted to the neurosurgical ward with a suspected brain tumour. This was a devastating event for my wife and children, though I tried to remain somewhat optimistic. I was prepared for surgery which included a chest X-ray, and then a preoperative bronchoscopy.
Brain surgery was scheduled for the following Monday and I was permitted (encouraged) to spend the weekend with my family. The reality of brain tumour surgery became very raw that Friday afternoon travelling home. On my way home, however, I received a phone call from a resident doctor advising that after the bronchoscopy result: they now suspected Cryptococcus of the brain instead of a cancer! This was a fantastic relief together with a belief that surgery was also off the agenda.
I was requested to return to the hospital on the Sunday for further tests including a spinal fluid tap. It was the intern's first lumber puncture; I can still feel his hands shaking against my back!
There was a sense of joy and relief by the ward staff that I was not one of the many patients on the floor whose outcomes remained bleak with the scourge of brain cancer—I had dodged the bullet! I was quickly reminded that I had a large lump of fungus in my brain with high risks requiring significant treatment which included a long course of intravenous medicine, tablets, and eventually I still needed brain surgery. I recall asking the consultant neurosurgeon preoperative if she thought it was only cryptococcoma rather than tumour, but she was non-committal. “We will see,” she said. And I asked her again a couple of days postoperative, she replied it was only fungus with no tumour. Those were great words.
After 30 L bags of Abelcet, gasquillion pills, losing 8 kg of weight, and spending 46 days in the hospital, my hospital journey finally came to an end. Be it so humble there is no place like home.
It is quite surprising how weak the human body becomes after surgery and hospitalisation, I had no energy, and the simplest tasks were exhausting. At times I thought this weakness may be permanent but the body does recover, the weight is back on (plus more unfortunately).
The treatment, care and respect I received from hospital were simply outstanding. They saved my life and I thank them very, very much.
Learning points.
Cryptococcus gattii can cause central nervous system (CNS) mass lesions that are indistinguishable from primary CNS malignancy in immunocompetent patients.
Cryptococcal antigen testing of CNS and serum should be considered in the diagnostic workup of CNS mass lesions.
A combined medical and surgical approach for CNS cryptococcoma may be optimal to shorten duration of antifungal therapy, provide definitive diagnosis and improve outcome.
Footnotes
Contributors: KBU wrote the first draft and completed an extensive literature review as well as participating in the acute and ongoing medical care of the patient. JWJC assisted in the literature review and care of the patient when in neurosurgery. RJ was the consultant neurosurgeon who performed the surgery and filmed the attached procedure. MLW edited the final draft and was involved in the ongoing medical management and follow-up of the patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chen S, Sorrell T, Nimmo G et al. . Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 2000;31:499–508. 10.1086/313992 [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DH, Sorrell TC, Allworth AM et al. . Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 1995;20:611–16. 10.1093/clinids/20.3.611 [DOI] [PubMed] [Google Scholar]
- 3.Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis 1995;21:28–34; discussion 5-6 10.1093/clinids/21.1.28 [DOI] [PubMed] [Google Scholar]
- 4.Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol 1984;120:123–30. [DOI] [PubMed] [Google Scholar]
- 5.Galanis E, Macdougall L, Kidd S et al. . British Columbia Cryptococcus gattii Working G. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis 2010;16:251–7. 10.3201/eid1602.090900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer DJ, Chaturvedi V. Projecting global occurrence of Cryptococcus gattii. Emerg Infect Dis 2010;16:14–20. 10.3201/eid1601.090369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect JR, Dismukes WE, Dromer F et al. . Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010;50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capilla J, Clemons KV, Sobel RA et al. . Efficacy of amphotericin B lipid complex in a rabbit model of coccidioidal meningitis. J Antimicrob Chemother 2007;60:673–6. 10.1093/jac/dkm264 [DOI] [PubMed] [Google Scholar]
- 9.Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol 2001;39:155–68. 10.1080/mmy.39.2.155.168 [DOI] [PubMed] [Google Scholar]
- 10.Peachey PR, Gubbins PO, Martin RE. The association between cryptococcal variety and immunocompetent and immunocompromised hosts. Pharmacotherapy 1998;18:255–64. [PubMed] [Google Scholar]
- 11.Tanner DC, Weinstein MP, Fedorciw B et al. . Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol 1994;32:1680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenfield JG. Original papers: on Froin's syndrome, and its relation to allied conditions in the cerebrospinal fluid. J Neurol Psychopathol 1921;2:105–41. 10.1136/jnnp.s1-2.6.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumari R, Raval M, Dhun A. Cryptococcal choroid plexitis: rare imaging findings of central nervous system cryptococcal infection in an immunocompetent individual. Br J Radiol 2010;83:e14–17. 10.1259/bjr/50945216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awasthi M, Patankar T, Shah P et al. . Cerebral cryptococcosis: atypical appearances on CT. Br J Radiol 2001;74:83–5. 10.1259/bjr.74.877.740083 [DOI] [PubMed] [Google Scholar]
- 15.Popovich MJ, Arthur RH, Helmer E. CT of intracranial cryptococcosis. AJR Am J Roentgenol 1990;154:603–6. 10.2214/ajr.154.3.2106227 [DOI] [PubMed] [Google Scholar]
- 16.Ho TL, Lee HJ, Lee KW et al. . Diffusion-weighted and conventional magnetic resonance imaging in cerebral cryptococcoma. Acta Radiol 2005;46:411–14. 10.1080/02841850510021201 [DOI] [PubMed] [Google Scholar]
- 17.Narai H, Manabe Y, Deguchi K et al. . Serial MRI findings in patient with chronic cryptococcus meningo-encephalitis. Neurol Res 2001;23:810–12. 10.1179/016164101101199397 [DOI] [PubMed] [Google Scholar]
- 18.Dubey A, Patwardhan RV, Sampth S et al. . Intracranial fungal granuloma: analysis of 40 patients and review of the literature. Surg Neurol 2005;63:254–60; discussion 60 10.1016/j.surneu.2004.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Perfect JR. Cryptococcus neoformans. In: Gerald L, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th edn 2010:3287–303. [Google Scholar]
- 20.Kovoor JM, Mahadevan A, Narayan JP et al. . Cryptococcal choroid plexitis as a mass lesion: MR imaging and histopathologic correlation. AJNR Am J Neuroradiol 2002;23:273–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Gologorsky Y, DeLaMora P, Souweidane MM et al. . Cerebellar cryptococcoma in an immunocompetent child. Case report. J Neurosurg 2007;107(Suppl 4):314–17. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, You C, Liu Q et al. . Central nervous system cryptococcoma in immunocompetent patients: a short review illustrated by a new case. Acta Neurochir (Wien) 2010;152:129–36. 10.1007/s00701-009-0311-8 [DOI] [PubMed] [Google Scholar]
- 23.Dusak A, Hakyemez B, Kocaeli H et al. . Magnetic resonance spectroscopy findings of pyogenic, tuberculous, and Cryptococcus intracranial abscesses. Neurochem Res 2012;37:233–7. 10.1007/s11064-011-0622-z [DOI] [PubMed] [Google Scholar]
- 24.Himmelreich U, Allen C, Dowd S et al. . Identification of metabolites of importance in the pathogenesis of pulmonary cryptococcoma using nuclear magnetic resonance spectroscopy. Microbes Infect 2003;5:285–90. 10.1016/S1286-4579(03)00028-5 [DOI] [PubMed] [Google Scholar]
- 25.Himmelreich U, Dzendrowskyj TE, Allen C et al. . Cryptococcomas distinguished from gliomas with MR spectroscopy: an experimental rat and cell culture study. Radiology 2001;220:122–8. 10.1148/radiology.220.1.r01jl25122 [DOI] [PubMed] [Google Scholar]
- 26.Nagar VA, Ye J, Xu M et al. . Multivoxel MR spectroscopic imaging—distinguishing intracranial tumours from non-neoplastic disease. Ann Acad Med Singapore 2007;36:309–13. [PubMed] [Google Scholar]
- 27.Dzendrowskyj TE, Dolenko B, Sorrell TC et al. . Diagnosis of cerebral cryptococcoma using a computerized analysis of 1H NMR spectra in an animal model. Diagn Microbiol Infect Dis 2005;52:101–5. 10.1016/j.diagmicrobio.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 28.Gupta RK, Jobanputra KJ, Yadav A. MR spectroscopy in brain infections. Neuroimaging Clin N Am 2013;23:475–98. 10.1016/j.nic.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Hospenthal DR, Bennett JE. Persistence of cryptococcomas on neuroimaging. Clin Infect Dis 2000;31:1303–6. 10.1086/317434 [DOI] [PubMed] [Google Scholar]
- 30.Brouwer AE, Rajanuwong A, Chierakul W et al. . Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 2004;363:1764–7. 10.1016/S0140-6736(04)16301-0 [DOI] [PubMed] [Google Scholar]
- 31.Chen SC, Australasian Society for Infectious Diseases (ASID) Mycoses Interest Group. Cryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin B. J Antimicrob Chemother 2002;49(Suppl 1):57–61. 10.1093/jac/49.suppl_1.57 [DOI] [PubMed] [Google Scholar]