Abstract
Duodenocolic fistula (DCF) is a rare complication of colon cancer with only 70 cases reported since its first description in 1862. Owing to its rarity, current knowledge on DCF still relies on single case reports. We present 2 cases of DCF from a hepatic flexure adenocarcinoma demonstrated initially by endoscopy. 2 adult male patients were admitted due to a 2–3-month history of right-upper quadrant pain, vomiting, diarrhoea and a palpable right upper quadrant mass. In both cases, a circumferential, friable mass was noted on upper endoscopy at the second portion of the duodenum, leading to the ascending colon. A similar-looking lesion was also noted on colonoscopy. Biopsies in both cases confirmed colonic adenocarcinoma. Owing to the advanced nature of the disease, en bloc resection was not achieved. Instead, tube jejunostomy and loop ileostomy were created. Both patients were discharged tolerating feeding with improvement in symptoms.
Background
Duodenocolic fistulae (DCF) commonly occur through localised perforations of the colon with subsequent infiltration into adjacent organs. As a complication of a malignant condition such as colon carcinoma, DCF is very rare, with only 70 cases sporadically reported since its first description by Haldane in 1862.1–3 It commonly presents with very non-specific gastrointestinal symptoms. Hence, early recognition of this disease entity is of utmost importance to facilitate immediate endoscopic interventions for tissue sampling, histological confirmation and subsequent curative surgery. En bloc resection of the tumour–fistula complex constitutes the definitive management of DCF. However, for patients with advanced lesions, palliative surgery may offer the best solution.
Case presentation
Case 1
A 33-year-old man who underwent right hemicolectomy for ascending colon adenocarcinoma stage III (T4aN1M0) was lost to follow-up after only two cycles of adjuvant chemotherapy (5-fluorouracil, leucovorin, oxaliplatin). Six months postsurgery, he presented with a 2-month history of postprandial vomiting, watery diarrhoea, weight loss and generalised body weakness. The patient on admission was normotensive, tachycardic (blood pressure 100/60 mm Hg, heart rate 100–110 bpm), weak looking and cachectic. Physical examination revealed a palpable left supraclavicular lymph node; soft, scaphoid abdomen with a 20 cm×18 cm palpable hard, fixed, tender, predominantly right upper quadrant mass extending to the epigastrium. No bruit or succussion splash was noted.
Case 2
A 62-year-old man with a history of 6-month treatment of pulmonary tuberculosis was admitted due to a 3-month history of right upper quadrant pain, episodes of melena, diarrhoea, generalised body weakness, pallor and weight loss (50% in 3 months). On admission, the patient was normotensive, tachycardic (BP 90/60 mm Hg, HR 120), pale and cachectic. His abdomen was flat, soft, non-tender but with a 10 cm×10 cm palpable right subcostal mass. Digital rectal examination showed external haemorrhoids and yellow stools per examining finger.
Investigations
Case 1
Initial laboratories showed normocytic, normochromic anaemia (hemoglobin 74 g/L), leucocytosis (16.7×109/L) with neutrophilic predominance (90%), hypokalaemia (3.0 mmol/L), hypoalbuminaemia (24 g/L) and an elevated carcinoembryonic antigen (CEA, 14.62 ng/mL). The rest of the blood chemistries were unremarkable. He was started on antibiotics, intravenous fluids with electrolyte correction and transfused with two units packed red blood cells until endoscopy was performed.
Esophagogastroduodenoscopy (EGD) revealed a fungating, circumferential, friable mass with exudates starting at the second portion of the duodenum. Distal to the tumour, abrupt change to colonic mucosa was noted (figure 1). A colonoscopy was performed on the same day, which revealed a similar-looking fungating mass at 65 from the anal verge (figure 2). Histopathological examination of specimens from both procedures was adenocarcinoma (tumour recurrence) (figure 3).
Figure 1.
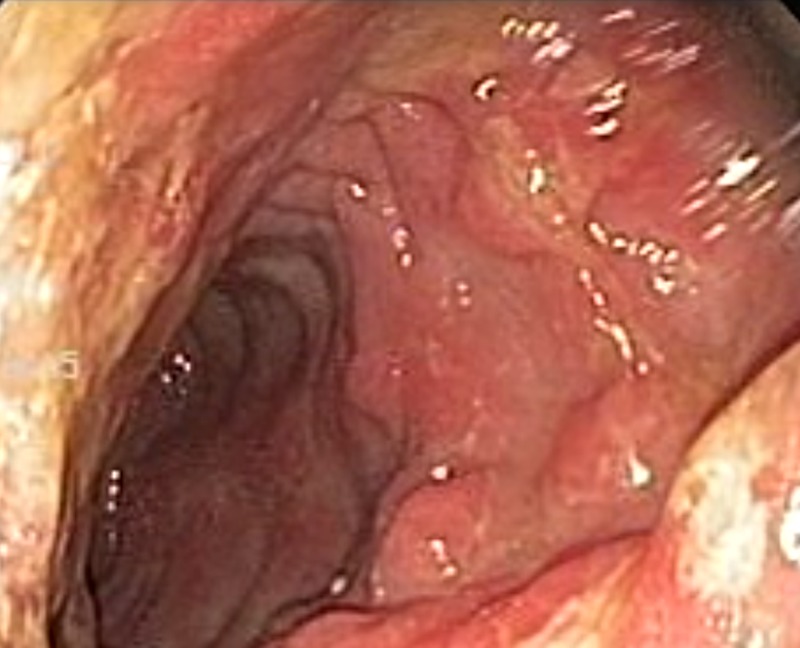
Endoscopic (EGD) picture of a fungating, circumferential, mass starting at the second portion of the duodenum occupying ∼50% of the lumen and what seemed to be colonic mucosa distally.
Figure 2.
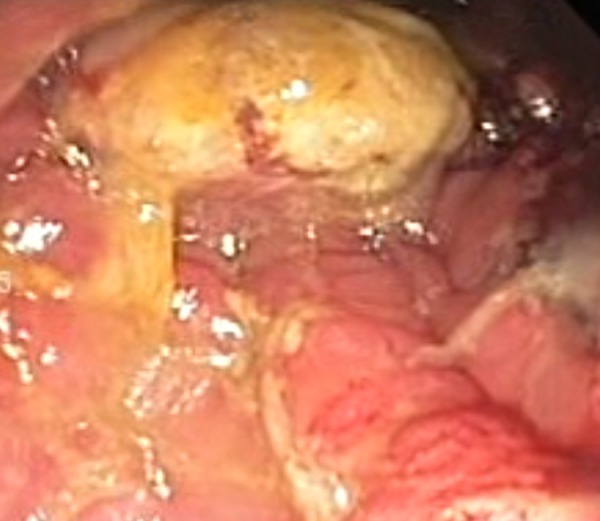
Colonoscopic picture of a similar-looking fungating mass with areas of necrosis at level 65 from the anal verge.
Figure 3.
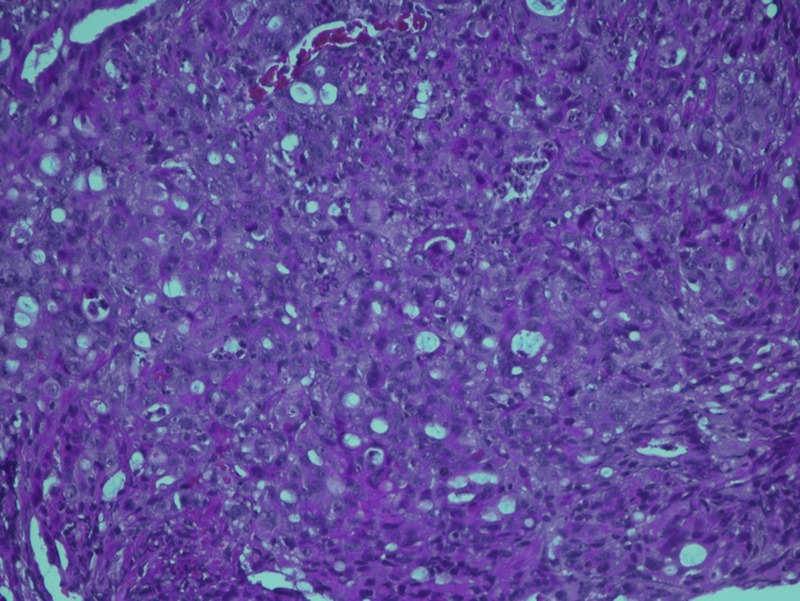
High power view of the histopathology slide showing colonic adenocarcinoma.
Triple contrast CT scan of the abdomen was performed revealing an ill-defined, fungating, heterogeneously enhancing mass in the right hemi-abdomen. It appeared to communicate with the transverse colon at the hepatic flexure at the region of the right lower quadrant, approximately measuring 12.0 cm×9.3 cm×10.5 cm (CC×W×AP) (figure 4).
Figure 4.
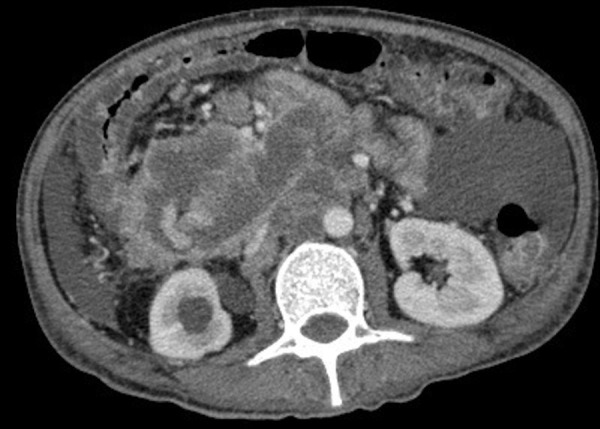
Contrast-enhanced CT scan showing a fungating, heterogeneously enhancing mass communicating with the transverse colon at the hepatic flexure in the right hemi-abdomen.
Case 2
Initial laboratory tests showed severe anaemia (hgb 46 g/L), hypokalaemia (2.9) and hypoalbuminaemia (32). CEA (17.81 ng/mL) and carbohydrate antigen (CA) 19–9 (>1200) were both elevated. The rest of the laboratory tests were unremarkable. He was transfused with six units packed RBC with repeat haemoglobin of 120 g/L.
EGD was then performed which revealed a luminal narrowing of up to 70% starting at the second portion of the duodenum (figure 5). On insinuation, a circumferential, friable, nodular mass was noted through a tract which seemed to lead to the ascending colon (figure 6). Colonoscopy was performed showing a similar-looking mass at the area of the hepatic flexure. Multiple biopsies of the mass were taken from both procedures and sent for histopathological examination and TB PCR. Results from both sites revealed well-differentiated adenocarcinoma (figure 7), and both specimens were Mycobacterium tuberculosis (TB) polymerase chain reaction (PCR) negative.
Figure 5.
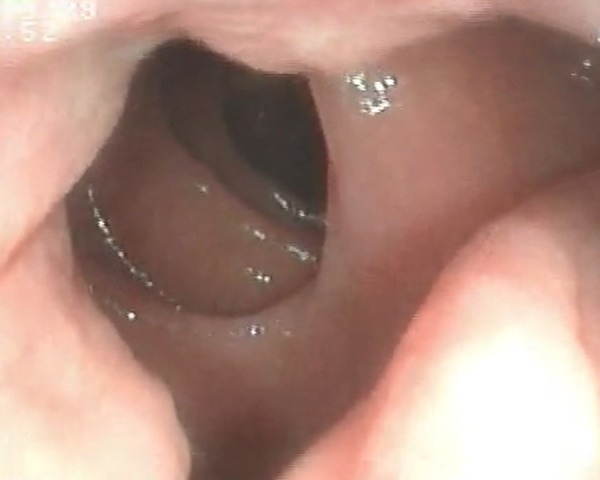
Endoscopic (EGD) picture of a circumferential, nodular mass with areas of exudates occupying up to 70% of the lumen.
Figure 6.
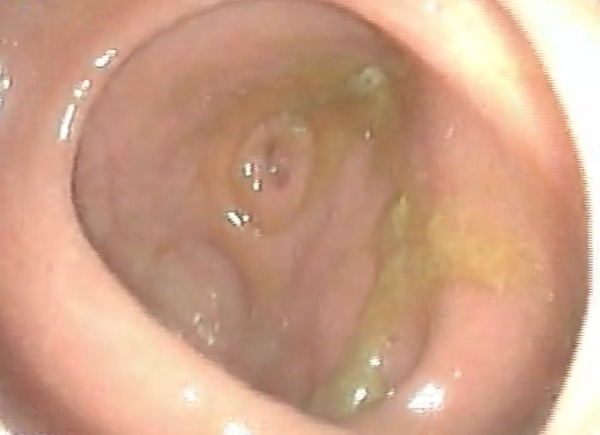
Distal to the mass, a tract was noted on EGD, which seemed to lead to the ascending colon and the cecum where the appendiceal lumen was appreciated.
Figure 7.
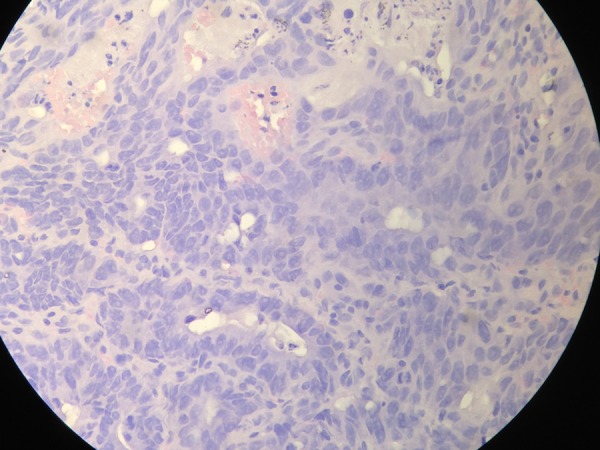
High power view of the histopathology slide showing well-differentiated adenocarcinoma.
Triple contrast CT scan also reported an ill-defined complex mass in the right upper quadrant of the abdomen with diffuse wall thickening of the stomach and ascending and transverse colon (figure 8). Superiorly, this mass cannot be clearly delineated with the second and third portion of the duodenum, starting at the level of the ampulla, which appears to infiltrate the periampullary region and pancreatic head.
Figure 8.
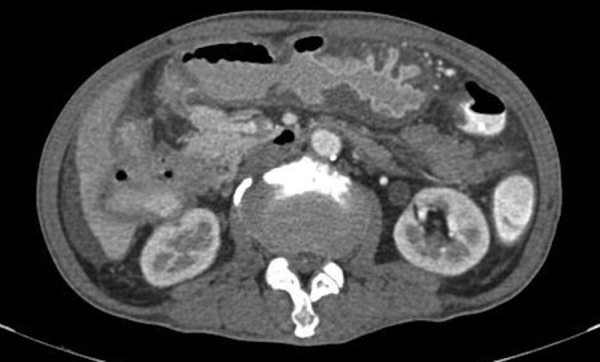
Contrast-enhanced CT scan of the abdomen showing an ill-defined complex mass in the right upper quadrant.
Outcome and follow-up
Case 1
Owing to the advanced nature of the disease, en bloc resection was not achieved. Instead, a mini laparotomy was performed and tube jejunostomy and loop ileostomy were created to facilitate feeding. The patient was referred to the medical oncology service and sent home with plans for palliative care.
Case 2
The patient was scheduled for curative surgery. However, intraoperatively, the hepatic flexure mass was noted to be adherent to the antrum and the right liver lobe precluding curative resection. Hence, tube jejunostomy and ileostomy were performed. The patient was referred to medical oncology for chemotherapy and discharged tolerating feeding with significant improvement from baseline symptoms, particularly diarrhoea and abdominal pain.
Discussion
DCF commonly occur through localised perforations of the colon with subsequent infiltration into adjacent organs. Fistula formation may be due to a benign cause, usually associated with inflammatory diseases such as Crohn's disease,4 tuberculosis,4 gallstone disease5 and occurring as a complication of surgery or radiotherapy.6
As a complication of a malignant condition such as colon carcinoma, DCF is very rare,6 with only 70 cases sporadically reported since its first description by Haldane in 1862.1–3 In a report on the surgical management of right-sided colon cancer, DCF was observed in only 2 of 1400 cases.7 A study by Hershenson described only 1 case among 8100 autopsies.8 Furthermore in 1977, Welch et al9 documented the incidence of DCF to occur only in 1 of 900 colorectal carcinomas in the USA. Most cases were commonly reported to occur from complicated primary carcinomas of the hepatic flexure, rather than an upper gastrointestinal malignancy.4
Patients with DCF typically present with colicky abdominal pain, intractable diarrhoea, vomiting, malnutrition and weight loss.4 8 As in our case, persistent diarrhoea was the most prominent symptom owing to the colonisation of the duodenum with colonic flora from fecal contamination.8 This phenomenon often leads to gastroenteritis and bacterial overgrowth.3 Abdominal discomfort and vomiting often precedes diarrhoea in DCF.8 Vague upper quadrant aching or severe central pain is present in almost 60% of cases while vomiting, with or without fecal eructation, is present in 50% of cases.10
CEA and CA 19–9 are common tumour markers used in the surveillance of colorectal cancer. CEA serves as an intercellular adhesion molecule promoting aggregation of cancer cells of colorectal origin and is said to be increased in 60–85% of patients with colorectal cancer.11–13 CA 19–9 also acts as cancer cell adhesion molecules that preferentially bind to the endothelium as well as to areas of metastases. Although more popularly used in pancreaticobiliary cancers, CA 19–9 has now been used more frequently as an adjunct to monitor colorectal cancer, especially in cases where CEA is normal and the likelihood of metastases is high.14 As seen in the second case, a strikingly higher CA 19–9 compared to CEA was observed. This may be explained by the more widespread disease of the second case on presentation as well as the possibility of tumour infiltration to the pancreas adjacent to the second portion of the duodenum.
Endoscopic evaluation is essential for diagnosis as it permits direct visualisation of the mass as well as the contrasting mucosa of both intestinal segments. However, compared to an EGD, colonoscopy has been reported to be less helpful towards demonstrating a fistula because of difficulty in intubation of an inflamed bowel segment or because of failure to reach the level of the fistula.9 In both patients, EGD and colonoscopy proved to be useful. An upper endoscopy was performed initially showing a suspicious fistulous tract originating in the duodenum, which, on further advancement, showed an abrupt change in mucosal pattern signifying the colon. Colonoscopy performed after the EGD also demonstrated a similar looking mass and mucosal pattern. Histopathological examination of biopsies taken from both procedures revealed similar results, confirming the presence of a fistula.
Radiological studies are useful to delineate the fistula most especially in malignant cases where images provide additional information on disease staging. Such imaging includes CT scan and MRI, which may prove valuable in determining extent of local invasion as well as assisting in preoperative planning.6
Treatment options for malignant DCF largely depend on the status of the patient. Definitive surgical treatment can be offered to patients with localised disease and those who are reasonably fit to tolerate the procedure.3 Surgical options include extended right hemicolectomy for removing the colonic portion of the tumour, hemicolectomy with duodenum reconstruction3 and hemicolectomy followed by pancreaticoduodenectomy3 with en bloc resection of both duodenal and colonic segments of the malignant fistula.6 15 16
However, owing to the advanced nature of disease on diagnosis—as in our case, curative resection may not be feasible. Management of these patients is mainly supportive and directed primarily towards nutritional provision, correction of dehydration and prevention of electrolyte imbalance.3 Surgical palliation with ileotransverse colostomy with gastrojejunostomy has been reported to successfully relieve symptoms of malignant DCF.4 7 17 18 However, survival in these patients is usually less than a year.4 19
Learning points.
Malignant duodenocolic fistula (DCF) is an unusual complication of colon cancer.
It commonly presents with non-specific gastrointestinal symptoms such as vomiting, diarrhoea and abdominal pain.
Diagnosis may require endoscopic interventions for tissue sampling and histologic confirmation to facilitate prompt curative surgical planning.
For patients with advanced lesions, palliative surgery may offer the best solution, often relieving bothersome symptoms of malignant DCF and allowing nutritional provision.
Footnotes
Contributors: ABGT contributed in acquisition of patient data (history, images, consent) and initial drafting. VCOC and AVD contributed in performance of endoscopy and revision/editing of the initial draft. VPB contributed in final editing and approval of paper for publishing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Iuchtman M, Zer M, Plavnick Y et al. Malignant duodenocolic fistula. The role of extended surgery. J Clin Gastroenterol 1993;16:22–5. 10.1097/00004836-199301000-00007 [DOI] [PubMed] [Google Scholar]
- 2.Haldane D. Case of cancer of caecum accompanied by caeco duodenal and caeco colic fistulae. Edinburgh Med J 1862;7:624–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Giridhar V, Kumar S, Chethan K et al. Successful palliation of diarrhea owing to malignant duodenocolic fistula by octreotide. J Can Chir 2009;52:6. [PMC free article] [PubMed] [Google Scholar]
- 4.Majeed TA, Gaurav A, Shilpa D et al. Malignant coloduodenal fistulas—review of literature and case report. Indian J Surg Oncol 2011;2:205–9. 10.1007/s13193-011-0099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soulsby R, Leung E, Williams N. Malignant colo-duodenal fistula; case report and review of literature. World J Surg Oncol 2006;4:86 10.1186/1477-7819-4-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapey I, Mahmood K, Solkar M. Malignant sigmoidoduodenal fistula. Int J Surg Case Rep 2014;5:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calmenson M, Black B. Surgical management of carcinoma of the right portion of the colon. Surgery 1947;21:476–81. [PubMed] [Google Scholar]
- 8.Hershenson LM, Kirsner JB. Duodeno-colic fistula. Gastroenterology 1951;19:864–73. [PubMed] [Google Scholar]
- 9.Welch JP, Warshaw AL. Malignant duodenocolic fistulas. Am Med J Surg 1977;133:658–61. 10.1016/0002-9610(77)90147-7 [DOI] [PubMed] [Google Scholar]
- 10.Kornfield H, Hogan R. Duodenocolic fistula. West J Med 1971;115:5. [PMC free article] [PubMed] [Google Scholar]
- 11.Filella X, Molina R, Grau JJ et al. Prognostic value of CA 19–9 levels in colorectal cancer. Ann Surg 1992;216:55–9. 10.1097/00000658-199207000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy MJ, Dalen van A, Haglund C et al. Clinical utility of biochemical markers in colorectal cancer. European Group on Tumor Markers (EGTM) guidelines. Eur J Cancer 2003;39:718–27. 10.1016/S0959-8049(02)00811-0 [DOI] [PubMed] [Google Scholar]
- 13.Hanke B, Riedel C, Lampert S et al. CEA and CA 19–9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative fist- line chemotherapy with weekly 24-hour infusion of high-dose 5-fluoracil (5-FU) and folinic acid (FA). Ann Oncol 2001;12:221–6. 10.1023/A:1008378412533 [DOI] [PubMed] [Google Scholar]
- 14.Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A et al. Cacner antigens (CEA and A 19–9) as markers of advanced stage of colorectal carcinoma. Med Arh 2013;67:393–6. 10.5455/medarh.2013.67.393-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuks D, Pessaux P, Tuech JJ et al. Management of patients with carcinoma of the right colon invading the duodenum or pancreatic head. Int J Colorectal Dis 2008;23:477–81. 10.1007/s00384-007-0409-5 [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Schraut W. Diagnosis and treatment of duodenoenteric fistulas complicating Crohn's disease. Arch Surg 1989;124:712–15. 10.1001/archsurg.1989.01410060082017 [DOI] [PubMed] [Google Scholar]
- 17.Zer M, Wolloch Y, Lombrozo R. Palliative treatment of malignant duodenocolic fistulas. World J Surg 1980;4:131–5. 10.1007/BF02393113 [DOI] [PubMed] [Google Scholar]
- 18.Berry S, Fisher J. Biliary and gastrointestinal fistula. In: Schwartz SI, Ellis H, Zimer MJ, eds. Maingot's abdominal operation. 10th edn. Appleton & Lange, 1997:581–625. [Google Scholar]
- 19.Xenos ES, Halverson JD. Duodenocolic fistula: case report and review of literature. J Postgrad Med 1999;45:87–9. [PubMed] [Google Scholar]


