Abstract
Dietary information is lacking in most of small to mid-sized carnivores due to their elusive predatory behaviour and versatile feeding habits. The leopard cat (LPC; Prionailurus bengalensis) and the Asiatic golden cat (AGC; Catopuma temminckii) are two important yet increasingly endangered carnivore species in the temperate mountain forest ecosystem in Southwest China, a global biodiversity hotspot and a significant reservoir of China’s endemic species. We investigated the vertebrate prey of the two sympatric felids using faecal DNA and a next-generation sequencing (NGS)/metabarcoding approach. Forty vertebrate prey taxa were identified from 93 LPC and 10 AGC faecal samples; 37 taxa were found in the LPC diet, and 20 were detected in the AGC diet. Prey included 27 mammalian taxa, 11 birds, one lizard and one fish, with 73% (29/40) of the taxa assigned to the species level. Rodents and pikas were the most dominant LPC prey categories, whereas rodents, pheasant, fowl and ungulates were the main AGC prey. We also analysed the seasonal and altitudinal variations in the LPC diet. Our results provide the most comprehensive dietary data for these felids and valuable information for their conservation planning.
Knowledge of food web interactions and predator–prey relationships is essential for understanding the ecosystem function of a species and designing conservation strategies1. As apex predator populations have declined drastically worldwide as a result of habitat loss and eradication by humans2,3, small to mid-sized carnivores that often occupied the middle-rank in the predator hierarchy (i.e. mesopredators) have ascended to the top of the food chain and function as apex predators in a range of ecosystems4. Mesopredators tend to have more diverse and flexible diets compared to those of large carnivores5; hence, their ascendency in the tropic hierarchy can lead to profound changes in the food web structure and affect virtually all other organisms in the ecosystem through cascading effects4,6. Studies of mesopredator dietary habits and variations provide critical information for identifying the structure and dynamics of trophic interactions, evaluating the ecosystem services provided by predator species, and making informed wildlife management and conservation decisions.
The temperate forests of the Minshan mountains in Sichuan Province are in the northernmost range of the biodiversity hotspot of the Mountains of Southwest China7. The original predator assemblage may have included tiger (Panthera tigris), leopard (Panthera pardus), clouded leopard (Neofelis nebulosa), dhole (Cuon alpinus) and the wolf (Canis lupus), which have all been locally extirpated or are functionally extinct8. Mammalian carnivores inhabiting the area include giant pandas (Ailuropoda melanoleuca), Asiatic black bears (Ursus thibetanus), hog badgers (Arctonyx collaris), Chinese ferret badgers (Melogale moschata), yellow-throated martens (Martes flavigula), Siberian weasels (Mustela sibirica), masked palm civets (Paguma larvata), leopard cats (LPC; Prionailurus bengalensis), and Asiatic golden cats (AGC; Catopuma temminckii)9,10. LPC and AGC are the only known extant members of the family Felidae and key predators in this ecosystem because of their highly carnivorous diets, versatile hunting skills, and adaptability to a wide range of habitat types11. LPC and AGC occur in moderate to high population densities relative to other carnivores in this ecosystem based on camera-trapping surveys9,12 and molecular species identification of faecal samples (see Materials and Methods). However, very little data are available regarding the ecological functions of these cats or their trophic interactions with other species.
LPC is a small felid with an adult body weight range of 1.7–7.1 kg13. It is one of the most common wild cats with a wide distribution across Asia and is found in a variety of habitats14. The population status of the LPC varies in different regions. The species is listed as least concern on the IUCN Red List15, although some local populations and subspecies are endangered16. In China, LPC was extensively harvested for fur trade prior to the early 1990 s, and its populations in the South and Southwest China may have suffered significant reductions17. Current threats to the species are mainly habitat loss and fragmentation due to anthropogenic land use8. No systematic population survey of the LPC has been conducted in China. Although LPC is detected at a relatively high frequency by camera-trapping surveys in the study area18, the population status of the species is unclear. Analyses of faeces and stomach contents show that the main LPC prey items are rodents (mostly murids), supplemented with other small mammals, such as squirrels and shrews, birds, reptiles, amphibians and fish, across its range19,20,21,22,23,24,25,26. Little data are available on their dietary habits in most regions of China.
AGC is a medium-sized felid (adult body weight: 9–16 kg) mostly distributed in Southeast Asia and South and Central China, primarily found in forest habitats17,27. Due to habitat loss and declining prey abundance, AGC populations are decreasing in most of its range28 and this species is listed as near threatened on the IUCN Red List15. AGC population status in China is unknown. In the study area, AGC occurs at a much lower density than LPC, according to camera-trapping records9. Data on AGC dietary habits are scarce. Morphological analysis of faeces or stomach contents of AGC in Thailand and Malaysia have identified small mammals, such as rodents and ground squirrels, small ungulates, birds, reptiles and amphibians21,29,30. However, AGC diets in China remain uninvestigated.
Conventional dietary analysis methods are primarily based on microscopic examination of hard parts of food remains in faeces or stomach/gut contents. These methods rely highly on undigested portions of prey, expert knowledge of their morphology, are generally time-consuming and often unable to provide precise identification of species31. Molecular identification of species based on sequence analysis of polymerase chain reaction (PCR) amplified fragments of a standardised DNA region (i.e. DNA barcoding32) from faecal or gut samples provides a reliable and effective alternative to dietary analysis in a wide range of species. This type of analysis requires no a priori information on prey composition, is powerful for resolving closely-related species and highly complex diets, is highly efficient for processing large-scale samples and the results can be rechecked and refined at a later time with updated sequence information31,33. Recent technological advances in next-generation sequencing (NGS) combined with metabarcoding have made DNA-based dietary analysis even more efficient and affable34.
We previously analysed the LPC diet in this area using faecal DNA and a traditional cloning-sequencing approach and identified 16 vertebrate taxa in 25 faecal samples35. However, due to the small sample size and low throughput sequencing technique, the results are unlikely to represent the complete dietary profile of the species, nor demonstrate the seasonal or regional variations. The goals of this study were (1) to investigate vertebrate prey compositions of LPC and AGC and analyse potential seasonal and altitudinal differences in the diet in the mountain forests of Southwest China using an NGS and metabarcoding-based approach and (2) to inform conservation planning and management actions based on the dietary data.
Materials and Methods
Study site and faecal sample collection
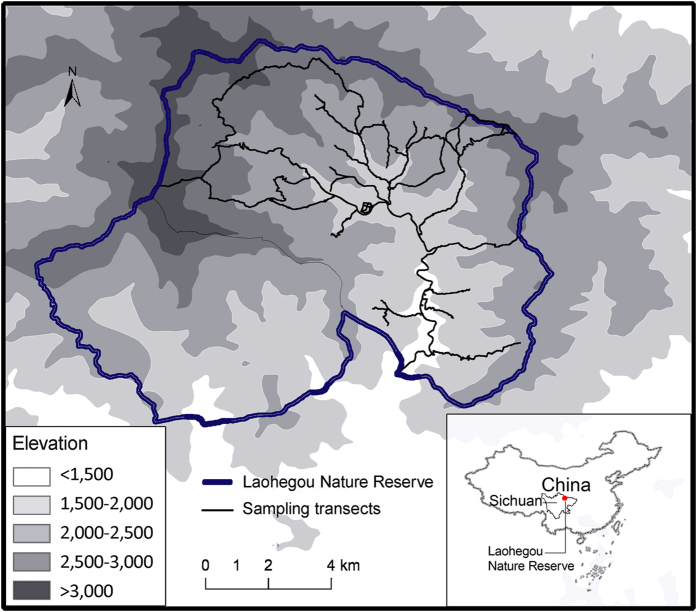
The study site was in the Laohegou Nature Reserve (104°32′42″–104°45′25″ E, 32°25′52″–32°36′22″N, altitude: 1,200–3,500 m; Fig. 1) located in northern Sichuan Province, China. Mean annual rainfall ranges from 950 to 1,300 mm, and mean monthly temperature varies from −4 °C to 12 °C. The Reserve has documented occurrences of 21 mammalian species (excluding small-bodied species, such as rodents, shrews and bats), 188 bird species, 18 reptilian species, 15 amphibian species and five fish species36.
Figure 1. Map of the study site in the Laohegou Nature Reserve for the leopard cat and Asiatic golden cat dietary analyses in northern Sichuan, China.
Map was generated in ArcGIS v10.1 (ESRI, Relands, CA, USA) and modified with Adobe Photoshop CS5 software (Adobe Systems Inc., San Jose, CA, USA).
We collected faecal samples in the Reserve from March 2013 to November 2014. The majority of samples were collected in spring (March–May) and autumn (September–November). We were unable to obtain samples during the summer season (July–August) or winter months (December–February) due to weather conditions. Mammalian faeces were collected along fixed transects at altitudes of 1,250–3,200 m (Fig. 1) during weekly patrols, submerged in 95% ethanol and stored at −20 °C.
DNA extraction
We used the 2CTAB/PCI method to extract DNA from faecal samples37. We included approximately 100 mg of the external surface in each DNA extraction for molecular identification of species. Faecal samples that were confirmed to be from LPC or AGC were homogenised individually, and 100 mg of the homogenised faeces was used in DNA extraction for the molecular dietary analysis.
Molecular species identification
We designed the 16S-F/16S-R primer pair to amplify a ~350 bp fragment of the mitochondrial 16 S rRNA gene to unambiguously assign all known predator species in the study area35. The LPC and AGC sequences of this fragment differed at 15 nucleotides (GenBank Accession numbers LPC: JN392459.1 and AGC: KP202267.1). The PCR conditions and sequencing of the products were described previously35. The species was assigned when faecal DNA amplicons matched the sequences in the GenBank database with 98–100% identity.
We successfully amplified DNA and identified species in 253 (73%) of the 345 field-collected faecal samples, of which 141 were from LPC and 24 were from AGC. Other commonly occurring species included takins (Budorcas taxicolor, n = 31), Siberian weasels (n = 18), tufted deer (Elaphodus cephalophus; n = 9), Chinese gorals (Nemorhaedus griseus; n = 8), wild boars (Sus scrofa; n = 8), Chinese muntjac (Muntiacus reevesi; n = 4), Chinese rock squirrels (Sciurotamias davidianus, n = 3), hog badgers (n = 2), Malayan porcupines (Hystrix brachyura, n = 2), Asiatic black bears (n = 1), yellow-throated martens (n = 1), and complex-toothed flying squirrels (Trogopterus xanthipes, n = 1).
We selected LPC and AGC faecal samples to be included in subsequent dietary analyses based on DNA quality and sample location information. Samples that failed to yield sufficient DNA for PCR amplification of food items were excluded. We also eliminated faecal samples collected in close proximity (<50 m), which may represent pellets of a single defecation. A total of 94 LPC and 11 AGC faecal samples met the selection criteria and were included in the NGS run.
PCR for diet analysis
To amplify DNA of vertebrate species in the faecal DNA extracts by PCR, we used the 12SV5-F/12SV5-R primers targeting a ~100 bp fragment of the mitochondrial 12 S rRNA gene, which has been demonstrated to have high resolution power for identifying the genus and species across most vertebrate taxa38. A blocking oligonucleotide (PrioB)39 designed to specifically inhibit amplification of the LPC sequences was also included in the PCR. We tested the blocking efficiency of PrioB with AGC samples and found that it also limited amplification of the AGC sequences at high efficiency (Appendix S1). The PrioB sequence diverged from small mammals (pikas, rodents and shrews), ungulates and birds that commonly occur in the study area (Appendix S2) and should not prevent amplification of these vertebrate groups.
PCR amplifications were conducted in a total volume of 20 μL, including 1 × EasyTaq PCR SuperMix (TransGen Biotech, Beijing, China), 0.2 μM 12SV5-F/R primers, 4 μM PrioB, 0.4 mg/mL bovine serum albumin and 20–40 ng DNA extract. The PCR program started with an initial denaturation step of 5 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 60 °C and no elongation. The 12SV5-F and 12SV5-R primers were tagged at the 5′ end with nine nucleotides that started with CC followed by seven variable nucleotides40. These tags differed by at least three nucleotides among the tags; hence, allowing a unique tag for each PCR to sort sequences according to samples following NGS. Seven negative PCR controls were also included in the amplifications to check for contamination.
Next-generation sequencing
PCR products were purified using a PCR purification kit (EasyPure PCR Purification Kit, TransGen Biotech). DNA concentrations were determined by agarose gel electrophoresis and using a NanoDrop 2000 spectrophotometer (ThermoScientific, Waltham, MA, USA) and were mixed in equimolar concentrations. Sequencing was carried out on the Illumina HiSeq 2500 (Illumina Inc., San Diego, CA, USA) at the NGS facility of the Biodynamic Optical Imaging Centre, Peking University, using paired-end sequencing following the manufacturer’s instructions. A total of 100 nucleotides were sequenced on each end of the DNA fragment.
NGS data analysis
The sequence reads were first checked using the FastQC program to evaluate data quality, and low quality reads were filtered with the NGSQCToolkit v2.33.
We used the OBITools program41 to analyse the sequence reads. The direct and reverse sequences were aligned using the illuminapairedend program, and aligned sequences with a quality score <40 were removed using the obigrep program. Sequences with perfectly matched tags and a maximum of two mismatches in primers were identified using the ngsfilter program and kept for further analysis. Identical sequences were clustered into unique sequences using the obiuniq program. Sequences <80 bp or with a total count in the whole dataset <1,000 were removed using the obigrep program. PCR and sequencing errors were detected using the obiclean program42. A reference database was prepared by extracting vertebrate 12 S gene sequences from GenBank with the 12SV5 primers using the ecoPCR program43. Taxonomic identification was conducted using the ecoTag program44 based on sequence similarity in this reference database. A unique taxon was assigned to each sequence. Sequence alignment and automatically assigned taxa were checked manually. We excluded sequences with <94% identity to the query sequence to increase accuracy of the taxonomic assignments. We removed obvious human contaminants, sequences of the two felids and low-frequency sequences (<0.1% or 50 reads in the sample or less than the count of the sequence in the negative control PCRs) that were the likely result of cross-contamination and/or tag jumps45. The unique sequences were further collapsed into discrete taxa using a 2% sequence divergence threshold and relative abundance of the sequences. We refined the automatic taxonomic assignments with species distribution data from the study area14,36,46.
We used the following criteria for taxonomic assignments: (1) if the query sequence matched a single locally occurring species in the databases with ≥98% identity, a species was assigned; (2) if the query matched more than one species with ≥98% identity, a species was assigned based on knowledge of the distribution or the lowest taxonomic level that included all of the species was assigned if more than one species occurred locally; and (3) if the query matched the reference sequence with a maximum identity of <98% but ≥94%, the query was assigned to the lowest taxonomic level that included all locally occurring species with the highest identity scores. If a single species showed the highest identity but was not known to inhabit the area, the most closely related local species within the same genus was assigned. Locally occurring species were identified using guides to the mammals14 and birds46 of China, and a survey of species in the Laohegou Nature Reserve36 that included (but was not limited to) mid- to large-sized mammals, birds, amphibians, and fish. To avoid misidentification of taxa due to insufficient local species records, we kept to conservative taxonomic assignments and only excluded species that showed no occurrence in all of Southwest China.
Diet analysis
The LPC and AGC diets were quantified in two ways:
(1) As a percent frequency of occurrence in faecal samples (%FC):
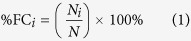 |
where Ni is the number of faecal samples containing the ith food resource, and N is the total number of faecal samples containing food;
(2) As a proportion of occurrence of an individual food resource (%TX):
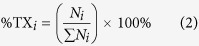 |
The %TX of all food resources summed to one, but the sum of %FC of the food resources could be larger than one due to the presence of multiple food items in the faecal sample.
Calculation of the dietary parameters including the standardised Levins’ measure of niche breadth (BA), Shannon’s diversity index (H), Peilou’s J and Pianka’s measure of niche overlap (Ojk) followed the equations shown in Appendix S3.
We analyzed the altitudinal variation in the LPC diet by dividing the entire altitudinal range (1250–2300) into four classes: low (<1,500 m), lower-middle (1,500–2,000 m), higher-middle (2,000–2,500 m), and high (>2,500 m). The definition of altitude classes was mainly based on vegetation type, as the distribution of forest types shows a strong altitudinal pattern. Mainly evergreen forests are observed at <1,500 m, evergreen and deciduous mixed forests occur at 1,500–2,500 m, and deciduous and coniferous mixed forests and coniferous forests occur at 2,500–3,200 m. Since vegetation composition and structure also exhibits gradual changes along altitude gradients within each vegetation zone, we further divided the middle zone (1,500–2,500 m) into lower- (1,500–2,000 m) and higher- (2,000–2,500 m) classes to achieve relatively even ranges of the altitude classes.
We used the Wilcoxon signed-rank test to evaluate the seasonal difference of the main food categories in the LPC diet. Statistical significances of altitudinal variation in the LPC diet were assessed for each main prey taxon across the altitude classes with Fisher’s exact tests. We estimated the 95% Wilson score intervals of the frequency of occurrence for the prey orders with the “Hmisc” package47 in R software48.
Statistical analyses were conducted using R software48. All comparisons were two-tailed, with the significance level set to 0.05.
Results
NGS data analysis
The sequencing run generated 4.05 Gb of sequence data (40.1 million raw sequences), which was assembled to produce 18.6 million properly assembled (paired) sequences. After removing low quality sequences, primer or tag sequences and unidentified reads, we collapsed the sequences into 398,588 unique sequences. A further step to eliminate short (<80 bp) or low-occurrence (<1,000) sequences resulted in 638 sequences, which were reduced to 205 sequences after removing PCR and sequencing errors. The high-quality sequences were further reduced by removing low (<94%) identity, low per-sample frequency and non-prey sequences and were collapsed into 40 discrete taxa. Based on the species distribution data, we assigned 29 taxa (73%) to species, four to genera, five to family or subfamily and two to order (Table 1). Of the 94 LPC and 11 AGC PCR products, 93 and 10 produced sequence reads, respectively and were included in the following analyses.
Table 1. Prey taxa identified in the diets of the leopard cat (LPC) and Asiatic golden cat (AGC) in northern Sichuan, China.
| Accession no. | Putative taxon | No. of occurrence in LPC faeces (n = 93) | No. of occurrence in AGC faeces (n = 10) | Sequences with maximum identity in GenBank | |||
|---|---|---|---|---|---|---|---|
| Scientific name | common name | species | Maximum identity | Accession no. | |||
| Rodentia | |||||||
| KU254241- KU254242 | Niviventer confucianus | Chinese white-bellied rat | 58 | 2 | Niviventer confucianus | 100 | KJ152220.1 |
| KU254243- KU254245 | Apodemus draco | South China field mouse | 41 | 0 | Apodemus draco | 99 | HQ333255.1 |
| KU254246- KU254250 | Eothenomys chinensis | Sichuan Chinese vole | 35 | 1 | Eothenomys chinensis | 96 | FJ483847.1 |
| KU254251- KU254254 | Niviventer excelsior | Large white-bellied rat | 25 | 1 | Niviventer excelsior | 100 | JQ927552.1 |
| KU254255- KU254256 | Eothenomys melanogaster | Père David’s vole | 18 | 0 | Eothenomys melanogaster | 99 | KP997311.1 |
| KU254259 | Apodemus chevrieri | Chevrier’s field mouse | 5 | 0 | Apodemus chevrieri | 100 | HQ896683.1 |
| KU254260 | Leopoldamys edwardsi | Edwards’s long-tailed giant rat | 5 | 0 | Leopoldamys edwardsi | 99 | KM434322.1 |
| KU254261 | Trogopterus xanthipes | Complex-toothed flying squirrel | 2 | 1 | Trogopterus xanthipes | 100 | AY227546.1 |
| KU254257- KU254258 | Rhizomys sinensis | Chinese bamboo rat | 1 | 4 | Rhizomys sinensis | 100 | AF326254.1 |
| KU254262 | Sciurotamias davidianus | Chinese rock squirrel | 1 | 1 | Sciurotamias davidianus | 99 | AY227554.1 |
| KU254264 | Apodemus peninsulae | Korean field mouse | 1 | 0 | Apodemus peninsulae | 98 | AJ311142.1 |
| KU254265 | Hystrix brachyura | Malayan porcupine | 1 | 0 | Hystrix brachyura | 100 | AY012117.1 |
| KU254263 | Dremomys pernyi | Perny’s long-nosed squirrel | 0 | 1 | Dremomys rufigenis* | 97 | KC447304.1 |
| Lagomorpha | |||||||
| KU254235- KU254240 | Ochotona spp. | Pikas | 71 | 3 | Ochotona curzoniae | 99 | KM225729.1 |
| Carnivora | |||||||
| KU254278 | Carnivora (?) | ? | 27 | 2 | Mustela erminea | 94 | KM091450.1 |
| KU254279 | Felidae (?) | ? | 1 | 0 | Panthera tigris* | 94 | EU983280.1 |
| Galliformes | |||||||
| KU254287 | Tragopan temminckii | Temminck’s tragopan | 15 | 6 | Tragopan temminckii | 100 | FJ752427.1 |
| KU254288 | Gallus gallus | Red jungle fowl | 7 | 2 | Gallus gallus | 100 | KM096864.1 |
| KU254289 | Chrysolophus pictus | Golden pheasant | 3 | 0 | Chrysolophus pictus | 100 | KC749452.1 |
| KU254290 | Bambusicola thoracicus | Chinese bamboo partridge | 1 | 0 | Bambusicola thoracicus | 98 | KC749450.1 |
| Passeriformes | |||||||
| KU254280 | Passeriformes | ? | 8 | 1 | Hirundo rustica/Hirundo daurica/Leiothrix lutea | 99 | KP148840.1/KJ499911.1/JQ423933.1 |
| KU254283 | Timaliidae | Old World babblers | 4 | 0 | Alcippe morrisonia/Garrulax ocellatus | 100 | AY167412.1/AF484898.1 |
| KU254281 | Prinia inornata | Plain prinia | 4 | 1 | Prinia subflava* | 100 | AY136564.1 |
| KU254282 | Turdidae | Thrushes | 2 | 1 | Luscinia cyanura/Phoenicurus auroreus | 99 | KF997864.1/KF997863.1 |
| KU254284 | Alcippe chrysotis | Golden-breasted fulvetta | 2 | 0 | Alcippe chrysotis | 100 | AF484888.1 |
| KU254285 | Yuhina spp. | Yuhinas | 2 | 0 | Yuhina nigrimenta | 95 | AF376919.1 |
| Soricomorpha | |||||||
| KU254266 | Episoriculus macrurus | Long-tailed mountain shrew | 7 | 1 | Episoriculus macrurus | 99 | GU981048.1 |
| KU254267 | Sorex spp. | Shrews | 7 | 0 | Sorex cylindricauda/Sorex minutus | 99 | KF696672.1/EF027290.1 |
| KU254268 | Scaptonyx fusicaudus | Long-tailed mole | 3 | 0 | Scaptonyx fusicaudus | 98 | AB106232.1 |
| KU254269 | Uropsilus soricipes | Chinese shrew mole | 3 | 0 | Uropsilus soricipes | 100 | JQ658979.1 |
| KU254270 | Soricinae | Red-toothed shrews | 1 | 0 | Cryptotis parva*/Blarina brevicauda* | 96 | JN393209.1/S73801.1 |
| Artiodactyla | |||||||
| KU254274 | Sus scrofa | Wild boar | 9 | 2 | Sus scrofa | 100 | KM275217.1 |
| KU254275 | Budorcas taxicolor | Takin | 3 | 2 | Budorcas taxicolor | 100 | FJ006534.1 |
| KU254276 | Naemorhedus griseus | Chinese goral | 2 | 0 | Naemorhedus griseus | 100 | KF500173.1 |
| KU254277 | Moschus berezovskii | Forest musk deer | 0 | 1 | Moschus berezovskii | 100 | EU043465.1 |
| Primates | |||||||
| KU254271 | Rhinopithecus roxellana | Golden snub-nosed monkey | 2 | 1 | Rhinopithecus roxellana | 100 | JQ821835.1 |
| KU254272- KU254273 | Macaca mulatta | Rhesus macaque | 0 | 1 | Macaca mulatta | 98 | AJ842854.1 |
| Anseriformes | |||||||
| KU254291 | Anas spp. | Ducks | 2 | 0 | Anas crecca/Anas platyrhynchos/Anas acuta/Anas poecilorhyncha/Anas clypeata/Anas querquedula | 100 | KC466567.1/KJ833587.1/KF312717.1/KF751616.1/KF469288.1/AF173691.1 |
| Lacertiformes | |||||||
| KU254292 | Plestiodon capito | Yellow-striped skink | 1 | 0 | Plestiodon elegans* | 97 | KM017746.1 |
| Cypriniformes | |||||||
| KU254293 | Cyprinidae | Carps | 1 | 0 | Ctenopharyngodon idella/Hypophthalmichthys molitrix | 100 | JQ231115.1/JQ231114.1 |
Putative taxa were identified with the aid of species distribution information. Sequences with maximum identity only show locally occurring species; a species is indicated by an asterisk (*) if the species with maximum sequence identity is not known to inhabit the study area. Question marks (?) indicate that taxonomic identification requires further information.
Dietary composition and diversity
Among the total 40 prey taxa, 37 were consumed by LPC and 20 by AGC, with 17 taxa shared by both species. The number of prey taxa per faecal sample varied from one to seven with a mean of 4.1 (SD 1.7) and 3.6 (SD 1.8) for LPC and AGC, respectively. The LPC diet contained 24 mammalian taxa (six orders), 11 bird taxa (three orders), one lizard and one fish, whereas the AGC diet was comprised of 15 mammalian taxa (six orders) and five bird taxa (two orders) (Table 1). The dietary niche analysis at the taxon level showed that LPC had a narrower diet breadth than that of the AGC, as indicated by Levins’ standardised measure (BA: LPC = 0.27; AGC = 0.64). Dietary diversity of the two species was similar (Shannon diversity index, H: LPC = 2.78; AGC = 2.80), and diet evenness was relatively high for both species (Peilou’s J: LPC = 0.77; AGC = 0.93).
The most frequently occurring taxon in the LPC diet was pikas (Ochotona spp.), which was present in 76% of the faecal samples, followed by several rodents, including the Chinese white-bellied rat (Niviventer confucianus; %FC = 62%), South China field mouse (Apodemus draco; 44%) and Sichuan Chinese vole (Eothenomys chinensis; 40%). Another four taxa were detected in >10% of the LPC samples: undetermined carnivorous species, 29%; large white-bellied rat, Niviventer excelsior, 27%; Père David’s vole, Eothenomys melanogaster, 19%; and Temminck’s tragopan, Tragopan temminckii, 16% (Table 1). Species in the order Rodentia were the most common prey of the LPC when the samples were analysed by the proportion of occurrence of individual prey taxa (%TX = 51%), followed by Lagomorpha (18%) (Fig. 2a).
Figure 2.
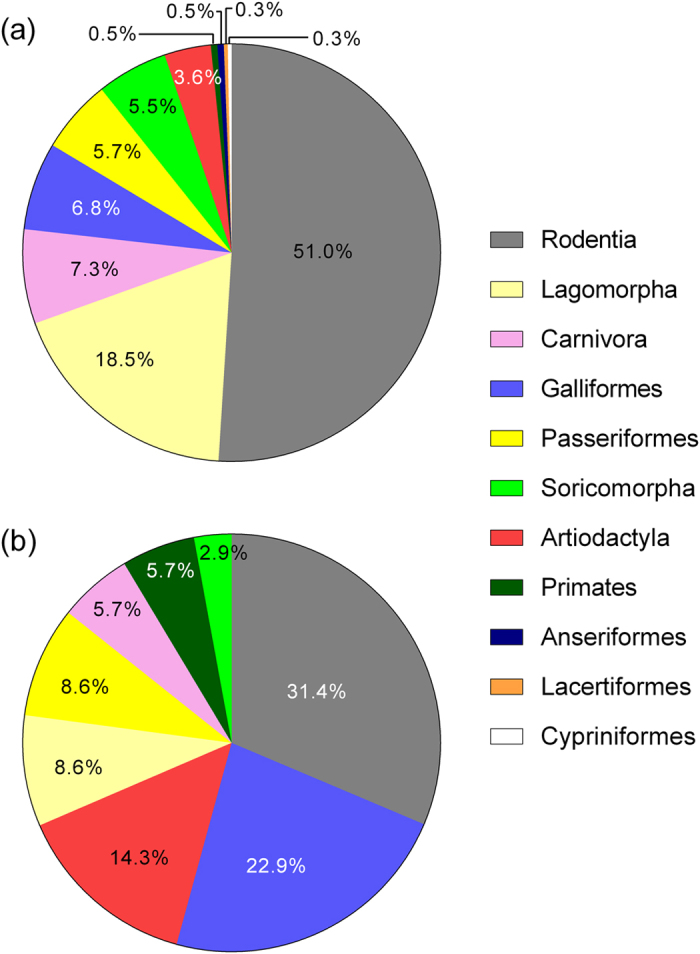
Dietary composition by the prey orders identified in the diets of (a) the leopard cat (n = 93) and (b) Asiatic golden cat (n = 10) in northern Sichuan, China. Diet composition by prey order is presented as a proportion of occurrence (%TX).
The most common AGC prey taxa were Temminck’s tragopan (%FC = 60%), the Chinese bamboo rat (Rhizomys sinensis; 40%), and pikas (30%). Other taxa occurring in 10% of the AGC samples included the Chinese white-bellied rat (20%) and undetermined carnivorous species (20%) (Table 1). Species in the orders Rodentia (%TX = 31%) and Galliformes (23%) were the most dominant prey of the AGC by proportion of occurrence (Fig. 2b).
Seasonal and altitudinal variations in the leopard cat diet
LPC consumed 33 and 23 prey taxa in spring (n = 61 faeces) and autumn (n = 32 faeces), respectively. Dietary niche breath in spring (BA = 0.29) was slightly lower than that in autumn (BA = 0.41), whereas the Shannon diversity index values were similar in both seasons (H: spring = 2.75; autumn = 2.59). The LPC diet composition varied little between spring and autumn, as indicated by very high and significant dietary overlap (Ojk = 0.92, p < 0.001) analysed at the taxon level, and similar frequencies of occurrence (%FC) of the seven most common prey orders (Wilcoxon signed-rank test: Z = −0.338, n = 7, p = 0.735; Fig. 3).
Figure 3. Seasonal variations in diet composition by the prey orders identified in the leopard cat diet in northern Sichuan, China. Spring: n = 61; autumn: n = 32.
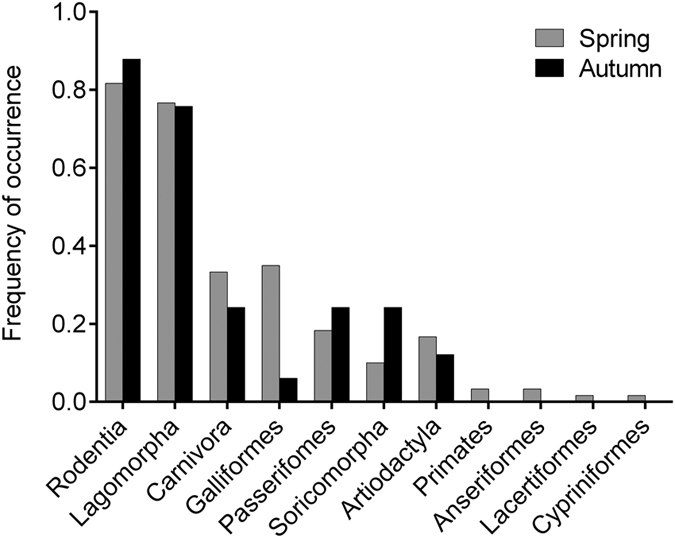
Diet composition is presented as a percent frequency of occurrence (%FC).
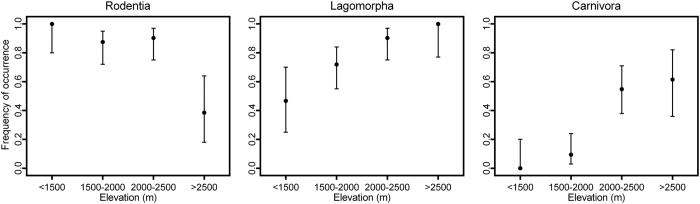
The LPC samples were collected at altitudes of 1,250–3,200 m. Species from the seven most common prey orders (Rodentia, Lagomorpha, Carnivora, Galliformes, Passeriformes, Soricomorpha, and Artiodactyla) were detected across the entire altitudinal range (low: <1,500 m; lower-middle: 1,500–2,000 m; higher-middle: 2,000–2,500 m; and high: >2,500 m) (Appendix S4). Fisher’s exact tests showed that the frequency of occurrence (%FC) for Rodentia, Lagomorpha, and Carnivora varied significantly in different altitudinal zones (all p < 0.001), whereas altitudinal variations in the occurrence of Galliformes, Passeriformes, Soricomorpha, and Artiodactyla were insignificant. Calculation of 95% confidence intervals for Rodentia, Lagomorpha, and Carnivora indicated distinct distribution patterns for different prey orders (Fig. 4). For example, the frequency of occurrence of rodents was high at low and middle altitudes and reduced by half at altitudes >2,500 m, whereas the occurrence of pikas and carnivores increased with altitude (Fig. 4). At the taxon level, various prey taxa also exhibited different patterns of occurrence (Fig. 5).
Figure 4. Altitudinal variation in the prey order Rodentia, Lagomorpha, and Carnivora in the leopard cat diet in northern Sichuan, China.
<1,500 m: n = 15; 1,500–2,000 m: n = 32; 2,000–2,500 m: n = 31; >2,500 m: n = 13. Observed values of frequency of occurrence (%FC) and the 95% Wilson score intervals are shown.
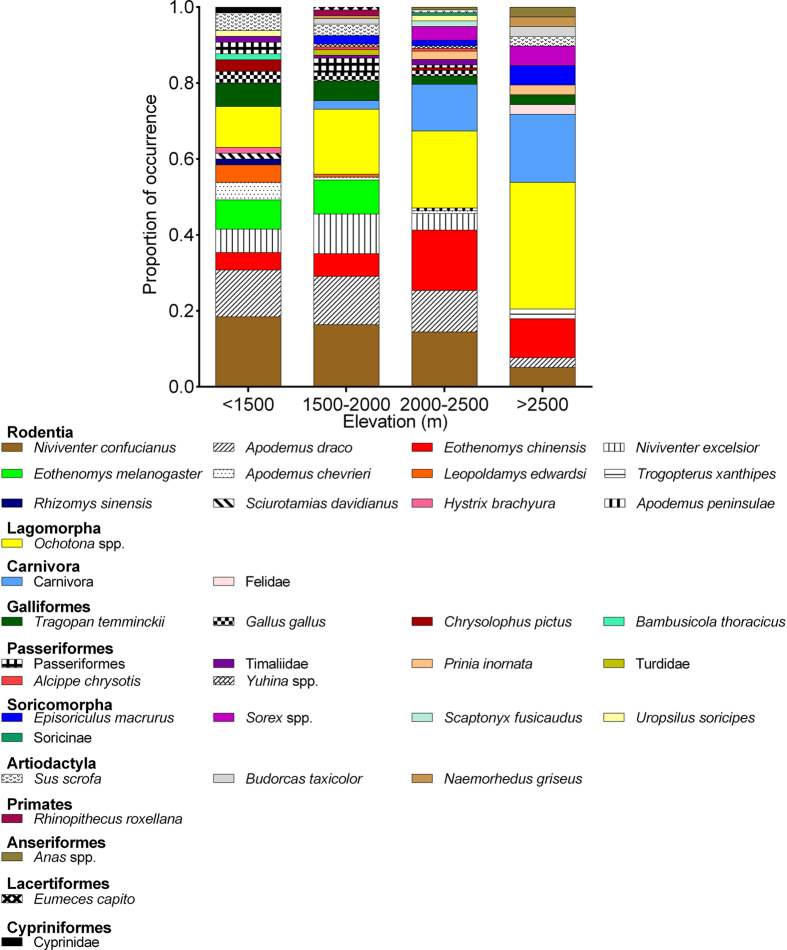
Figure 5. Altitudinal variations in diet composition by the prey taxa identified in the leopard cat diet in northern Sichuan, China.
< 1,500 m: n = 15; 1,500–2,000 m: n = 32; 2,000–2,500 m: n = 31; >2,500 m: n = 13. Diet composition is presented as a proportion of occurrence (%TX).
Discussion
Diets of the two felids
We identified 37 vertebrate taxa in the LPC diet. The main LPC prey categories in the present study generally agree with those of previous studies at the ordinal level but vary in specific species composition. Studies across the LPC range generally show that small rodents, particularly Rattus and Mus spp., are dominant prey items20,23,39. However, we found that Niviventer, Apodemus and Eothenomys spp. were the most common rodents in the LPC diet but no Rattus or Mus spp. were detected. No systematic population survey on small mammals has been conducted in the Laohegou Nature Reserve. Although no Mus spp. have been found in the study area, two Rattus species, R. nitidus and R. norvegicus are reported to occur in evergreen broadleaf forests (<1600 m) of the adjacent Tangjiahe Nature Reserve at densities similar to or higher than Niviventer spp.49, raising questions about the prey selectivity of LPC. Additionally, pikas have been reported as LPC prey in our study area, but not in other regions, even where they are common food of other similar-sized carnivores50. Prey composition and availability along altitudinal gradients have been demonstrated to shape the diets of several carnivorous species51,52. Studies in Tangjiahe Nature Reserve showed that Niviventer and Apodemus spp. were the most abundant groups below 2,500 m in various forest types, but their numbers diminished quickly above this altitude; pikas were not detected below 1,800 m, occurred at a low number at 1,800–2,500 m, yet prevailed above 2,500 m in Alpine meadows and scrub habitats53. The altitudinal gradient in the occurrence of rodents and pikas is roughly correlated with these distribution data, despite that pikas were also frequent in the LPC diet at mid-elevations (1,500–2,500 m) in our analysis, suggesting a versatile generalist feeding tactic that may be influenced more by prey availability than a specific food preference. The above interpretation is based on the assumption that prey remains in faeces reflect predation occurring in the same altitudinal zone. However, LPC can move across large distances in short periods of time21, potentially compromising the association between faecal sampling and predation location. The impacts of altitude on LPC prey composition bear further investigation. Additionally, we detected several ungulates in the LPC diet. Thus, this small cat may possibly catch young wild boars and Chinese gorals, which are smaller-bodied ungulates, whereas the occurrence of takins in the diet may most likely be explained as scavenging behaviour. Our analyses also show that LPC consume a wide variety of birds, from small passerines to large ground-dwelling pheasants, demonstrating the predator’s versatile hunting skills.
We identified 20 vertebrate taxa in the AGC diet, representing the most comprehensive dietary analysis reported for this species. Similar to the LPC, AGC also frequently consumed rodents and pikas, but the proportions of larger prey, such as pheasants and ungulates, were higher than those in the LPC diet, possibly reflecting their larger body size compared to that of LPC. Due to the small sample size of AGC, the quantitative dietary analysis results regarding prey diversity remain tentative. AGC appeared to occupy only a portion of the LPC’s range in the study area, as AGC faeces were found only within a relatively narrow altitudinal zone (2,300–2,700 m), whereas LPC faeces were collected throughout the reserve across all altitudes from 1,250 to 3,200 m. Additionally, the AGC population density is likely to be only a fraction of that of LPC in the study area18, which is in accordance with the different numbers of faecal samples collected for the two species. Further investigation of the AGC diet is required to better understand this species’ food web hierarchy and niche interactions with other sympatric carnivores.
Molecular dietary analysis
Most previous studies of the LPC diet were based on microscopic examination of food remains in faeces where generally <10 taxa were identified, often only to the family or higher taxonomic levels20,21,23, demonstrating the superior resolution power of the faecal DNA-based method. The number of vertebrate prey categories identified in the present study (37 taxa) considerably exceeds not only reports based on a traditional cloning-Sanger sequencing approach for the LPC diet in the same area (16 taxa)35 and in South Korea (13 taxa)25, but also a study based on NGS analysis of LPC faeces collected in northern Pakistan (18 taxa)50. Technical advances represented by the NGS approach offer remarkably higher throughput and economy compared to traditional morphological analysis and sequencing methods, demonstrating the high detection sensitivity and assignment power of molecular-metabarcoding/NGS methods. The difference in the number of food taxa between the two NGS-based studies can be explained by the higher species diversity of potential prey in the mountain forests of Southwest China compared to those of northern Pakistan, and the larger sample size of our study (93 vs. 38 faecal samples).
However, species resolution and accuracy using molecular identification are critically dependent on the completeness and quality of the reference databases, and limited coverage of the potential prey can strongly impede species-level identification. We found that the pika sequences in LPC faeces matched the Ochotona spp. sequences with 94–99% identity, suggesting that there might be more than one pika species in the diet. Of the eight Ochotona spp. potentially present in the study area, including O. cuzoniae, O. cansus, O. erythrotis, O. gloveri, O. huangensis, O. macrotis, O. thibetana and O. thomasi14, only the 12 S rRNA sequence of O. cuzoniae is available in the GenBank database. Therefore, we only assigned this taxon to the genus. Pikas were the most frequently occurring food item at the individual taxon level; yet when analysed at the ordinal level, they showed a lower proportion of occurrence in the diet than rodents due to the lack of species-level resolution within the genus Ochotona. One promise of the molecular dietary analysis is that taxonomic assignments can be refined as reference databases improve.
The species resolution of molecular identification approaches is also restricted by the extent of sequence divergence among closely-related species and can show varying degrees of discriminating power for different taxa. The DNA barcode used in our study demonstrated high species resolution in most mammalian groups, yet low power differentiating avian species (particularly babblers and ducks). Limited species resolution can be particularly challenging in fine-scale dietary analysis when the study area has high species diversity, such as ours, where nearly 200 bird species have been recorded36. Thus, a combination of DNA barcodes targeting specific food groups has been recommended to improve species-level identification54.
Ecosystem function of mesopredators and conservation implications
Removal of apex predators from an ecosystem can lead to loss of top-down control and subsequent outbreaks of mesopredator populations, an effect known as “mesopredator release55”, which may result in declines in prey populations and eventual “ecological meltdown56”. Despite overall extirpation of former apex predators in many mountain forests in Southwest China, neither explosive growth of mesopredator populations, nor depletion of the main prey groups have been observed18,36. Habitat loss and fragmentation, hunting by humans, intra-guild competition and limited prey resources may account for the lack of apparent increase in mesopredators in this ecosystem. Ecological processes in such a complex ecosystem can be multivariate and more intricate, and the dynamics and consequences following natural and human disturbances may not fully agree with predications often deduced from analyses of comparatively simple ecosystems56. Therefore, a closer examination of the many environmental, biological (intraspecific and interspecific) and anthropogenic factors is required in a particular ecosystem to gain a better understanding of the population dynamics and to predict the patterns of ecological response.
Our results indicate that rodents and pikas constitute the majority of the felids’ diets (particularly the LPC) in the forest ecosystem of Southwest China. These small mammals are generally considered pests, as they degrade forests and grasslands and have been targets of control by poisoning and execution in many regions of China57,58. However, small mammals assist with seed dispersal and improve plant diversity59, provide important prey for many carnivores and predatory birds60,61 and may serve as keystone species to enhance ecosystem functioning62,63. Pest control by poisoning has been demonstrated to be ineffective and often incurs undesirable consequences on the environment and untargeted species64. In contrast, conserving predator communities is an ecologically safe, economic and sustainable strategy to control small mammalian populations and maintain ecosystem equilibrium, stability and resilience65.
In conclusion, our results reveal the highly diverse dietary habits and versatile foraging skills of two felids. The other mammalian carnivores (e.g., Asiatic black bears, hog badgers, Chinese ferret badgers, yellow-throated martens, and masked palm civets) in this ecosystem are largely omnivorous, but LPC and AGC are highly carnivorous and may play pivotal roles as predators66. Large proportions of the LPC and AGC diets were composed of pikas and rodents, suggesting that these predators play an important role controlling their populations in the mountain forests of Southwest China. Therefore, caution should be taken in pest control as it may pose serious threats to predators and ecosystem sustainability. The faecal DNA and NGS-based approach enabled a very large-scale dietary analysis and fine species resolution. The remarkable taxonomic discriminating power offered by the molecular approach was particularly powerful to disentangle the food web structure in a highly complex ecosystem such as our study area, where closely related and morphologically similar prey species coexist.
Additional Information
How to cite this article: Xiong, M. et al. Molecular dietary analysis of two sympatric felids in the Mountains of Southwest China biodiversity hotspot and conservation implications. Sci. Rep. 7, 41909; doi: 10.1038/srep41909 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank X. Chen, Z. Xiang, G. Jiang, C. Luo, C. Liang, H. Du and C. Shu from the Laohegou and Wanglang Nature Reserves for their help with the fieldwork. This study was funded by The Nature Conservancy China, the Sichuan Nature Conservation Foundation, and the National Project 985 Research Fund of Peking University to M. Y.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.Y., D.W., S.L. and R.W. conceived the study. M.Y. and D.W designed the study. H.B., M.X. and D.Z. collected the samples. M.X. developed the methods and performed the experiments. M.X., M.Y. and X.S. analysed the data. M.Y. wrote the manuscript with input from all authors.
References
- Sheppard S. K. & Harwood J. D. Advances in molecular ecology: tracking trophic links through predator-prey foodwebs. Funct. Ecol. 19, 751–762 (2005). [Google Scholar]
- Estes J. A. et al. Trophic downgrading of planet earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- Ripple W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- Prugh L. R. et al. The rise of the mesopredator. BioScience 59, 779–791 (2009). [Google Scholar]
- Roemer G. W., Gompper M. E. & Valkengurgh B. V. The ecological role of the mammalian mesocarnivore. BioScience 59, 165–173 (2009). [Google Scholar]
- Brashares J. S., Prugh L. R., Stoner C. J. & Epps C. W. In Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature (eds Terborgh J. & Estes J. A.) (Island Press, 2010). [Google Scholar]
- Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B. & Kent J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- Jutzeler E. et al. Cat News Special Issue: Cats in China. (2010). [Google Scholar]
- Li S., McShea W. J., Wang D., Lu Z. & Gu X. Gauging the impact of management expertise on the distribution of large mammals across protected areas. Diversity Distrib. 18, 1166–1176 (2012). [Google Scholar]
- Li S., Wang D., Bu H., Liu X. & Jin T. Camera-trapping survey on the mammal diversity of Laohegou Nature Reserve, Sichuan Province. Acta Theriol. Sinica. 36, 282–291 (2016). [Google Scholar]
- Mills L. S., Soule M. E. & Doak D. F. The keystone-species concept in ecology and conservation. BioScience 43, 291–224 (1993). [Google Scholar]
- Bu H. et al. Spatial co-occurrence and activity patterns of mesocarnivores in the temperate forest of southwest China. PLoS ONE 11, e0164271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunquist M. E. & Sunquist F. C. In Handbook of the Mammals of the World. Vol. 1. Carnivores (edsWilson D. E. & Mittermeier R. A.) (Lynx Edicions, 2009). [Google Scholar]
- Smith A. T. et al. A Guide to the Mammals of China. (Princeton University Press, 2008). [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2016.1. < www.iucnredlist.org>. Downloaded on 07 July 2016 (2016).
- Sanderson J. et al. Prionailurus bengalensis. The IUCN Red List of Threatened Species. Version 2016.1. < www.iucnredlist.org> Downloaded on 07 July 2016 (2016).
- Nowell K. & Jackson P. Wild cats, Status Survey and Conservation Action Plan. (IUCN, Gland, Switzerland, 1996). [Google Scholar]
- Li S., Wang D., Lu Z. & McShea W. J. Cats living with pandas: the status of wild felids within giant panda range, China. Cat News 52, 20–23 (2010). [Google Scholar]
- Rabinowitz A. Notes on the behavior and movements of leopard cats, Felis bengalensis, in a dry tropical forest mosaic in Thailand. Biotropica 22, 397–403 (1990). [Google Scholar]
- Fernandez D. A. P. & de Guia A. P. O. Feeding habits of Visayan leopard cats (Prionailurus bengalensis rabori) in sugarcane fields of Negros Occidental, Philippines. Asia Life Sciences 20, 143–154 (2011). [Google Scholar]
- Grassman L. I., Tewes M. E., Silvy N. J. & Kreetiyutanont K. Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. Journal of Zoology (London) 266, 45–54 (2005). [Google Scholar]
- Rajaratnam R., Sunquist M., Rajaratnam L. & Ambu L. Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J. Trop. Ecol. 23, 209–217 (2007). [Google Scholar]
- Tatara M. & Doi T. Comparative analyses on food-habits of Japanses marten, Siberian weasel and leopard cat in the Tsushima Islands, Japan. Ecol. Res. 9, 99–107 (1994). [Google Scholar]
- Watanabe S. Factors affecting the distribution of the leopard cat Prionailurus bengalensis on East Asian islands. Mammal Stud. 34, 201–207 (2009). [Google Scholar]
- Lee O., Lee S., Nam D.-H. & Lee H. Y. Molecular analysis for investigating dietary habits: genetic screening of prey items in scat and stomach contents of leopard cats Prionailurus bengalensis euptilurus. Zool. Stud. 52, 45 (2013). [Google Scholar]
- Lee O., Lee S., Nam D.-H. & Lee H. Y. Food habits of the leopard cat (Prionailurus bengalensis euptilurus) in Korea. Mammal Stud. 39, 43–46 (2014). [Google Scholar]
- Sunquist M. E. & Sunquist F. C. Wild Cats of the World. (University of Chicago Press, 2002). [Google Scholar]
- Sanderson J. et al. Catopuma temminckii. The IUCN Red List of Threatened Species. Version 2016.1 < www.iucnredlist.org> Downloaded on 07 July 2016 (2016).
- Lim B. L. Distribution and food-habits of the Asiatic golden cat (Catopuma temminckii) in Peninsular Malaysia. Journal of Wildlife and Parks 20, 43–48 (2002). [Google Scholar]
- Kawanishi K. & Sunquist M. E. Food habits and activity patterns of the Asiatic golden cat (Catopuma temminckii) and dhole (Cuon alpinus) in a primary rainforest of Peninsular Malaysia. Mammal Stud. 33, 173–177 (2008). [Google Scholar]
- Pompanon F. et al. Who is eating what: diet assessment using next generation sequencing. Mol. Ecol. 21, 1931–1950 (2012). [DOI] [PubMed] [Google Scholar]
- Valentini A., Pompanon F. & Taberlet P. DNA barcoding for ecologists. Trends in Ecology and Evolution 24, 110–117 (2008). [DOI] [PubMed] [Google Scholar]
- King R. A., Traugott M. & Symondson W. O. C. Molecular analysis of predation: a review of best practice for DNA-based approaches. Mol. Ecol. 17, 947–963 (2008). [DOI] [PubMed] [Google Scholar]
- Thompson K. A. & Newmaster S. G. Molecular taxonomic tools provide more accurate estimates of species richness at less cost than traditional morphology-based taxonomic practices in a vegetation survey. Biodivers. Conserv. 23, 1411–1424 (2014). [Google Scholar]
- Xiong M. et al. Molecular analysis of vertebrates and plants in scats of leopard cats (Prionailurus bengalensis) in southwest China. J. Mammal. 97, 1054–1064 (2016). [Google Scholar]
- Li S. Report on Biodiversity Surveys in the Laohegou Nature Reserve. (2013). [Google Scholar]
- Vallet D., Petit E. J., Gatti S., Levréro F. & Ménard N. A new 2CTAB/PCI method improves DNA amplification success from faeces of Mediterranean (Barbary macaques) and tropical (lowland gorillas) primates. Conserv. Genet. 9, 677–680 (2007). [Google Scholar]
- Riaz T. et al. ecoPrimers: inference of new DNA barcode markers from whole genome sequence analysis. Nucleic Acids Res. 39, e145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad W. et al. Carnivore diet analysis based on next-generation sequencing: application to the leopard cat (Prionailurus bengalensis) in Pakistan. Mol. Ecol. 21, 1951–1965 (2012). [DOI] [PubMed] [Google Scholar]
- Coissac E. In Data Production and Analysis in Population Genomics (eds Pompanon F. & Bonin A.) (Humana Press, 2012). [Google Scholar]
- Boyer F. et al. OBITOOLS: a UNIX-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 16, 176–182 (2016). [DOI] [PubMed] [Google Scholar]
- De Barba M. et al. DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: application to omnivorous diet. Mol. Ecol. Resour. 14, 306–323 (2014). [DOI] [PubMed] [Google Scholar]
- Ficetola G. et al. An In silico approach for the evaluation of DNA barcodes. BMC Genomics 11, 434–443 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegard A. et al. Universal DNA-based methods for assessing the diet of grazing livestock and wildlife from feces. J. Agric. Food. Chem. 57, 5700–5706 (2009). [DOI] [PubMed] [Google Scholar]
- Schnell I. B., Bohmann K. & Gilbert M. T. P. Tag jumps illuminated – reducing sequence-to-sample misidentifications in metabarcoding studies. Mol. Ecol. Resour. 15, 1289–1303 (2015). [DOI] [PubMed] [Google Scholar]
- MacKinnon J. & Phillipps K. A Field Guide to the Birds of China. (Oxford University Press, 2000). [Google Scholar]
- Harrell F. E. Hmisc: Harrell Miscellaneous. R package version 4.0-1. Available: http://CRAN.R-project.org/package=Hmisc. (2016). [Google Scholar]
- Team R. C. R.: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: http://www.R-project.org/. (2016). [Google Scholar]
- Wang Y. et al. Spatial niche of the rodents in summer in Tangjiahe Nature Reserve. Acta Theriol. Sinica 25, 39–44 (2005). [Google Scholar]
- Shehzad W. et al. Prey preference of snow leopard (Panthera uncia) in South Gobi, Mongolia. PLoS ONE 7, e32104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remonti L., Balestrieri A. & Prigioni C. Altitudinal gradient of Eurasian otter (Lutra lutra) food niche in Mediterranean habitats. Can. J. Zool. 87, 285–291 (2009). [Google Scholar]
- Green K. Altitudinal and temporal differences in the food of foxes (Vulpes vulpes) at alpine and subalpine altitudes in the Snowy Mountains. Wildlife Research 30, 245–253 (2003). [Google Scholar]
- Wang Y., Wang X., Hu J. & Chen L. A preliminary research on small mammal community structure at Tangjiahe Natural Reserve, Sichuan Province, China. Acta Theriol. Sinica 23, 39–44 (2003). [Google Scholar]
- Deagle B. E., Kirkwood R. & Jarman S. N. Analysis of Australian fur seal diet by pyrosequencing prey DNA in faeces. Mol. Ecol. 18, 2022–2038 (2009). [DOI] [PubMed] [Google Scholar]
- Soulé M. E. et al. Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Conserv. Biol. 2, 75–91 (1988). [Google Scholar]
- Terborgh J. et al. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (2001). [DOI] [PubMed] [Google Scholar]
- Ma L. Study on the occurrence characteristics of forest pika disaster and its control measures of Mulan Forestry Administration. Hebei Journal of Forestry and Orchard Research 30, 87–89 (2015). [Google Scholar]
- Li J. & Yang G. Integrated methods for control of forest rodents. Journal of Jilin Forestry Science and Technology 43, 61–62 (2014). [Google Scholar]
- Paine C. E. T. & Beck H. Seed predation by neotropical rain forest mammals increases diversity in seedling recruitment. Ecology 88, 3076–3087 (2007). [DOI] [PubMed] [Google Scholar]
- Korpimaki E. Rapid or delayed tracking of multiannual vole cycles by avian predators. J. Anim. Ecol. 63, 619–628 (1994). [Google Scholar]
- Ray J. C. Temporal variation of predation on rodents and shrews by small African forest carnivores. J. Zool. 244, 363–370 (1998). [Google Scholar]
- Smith A. T. & Foggin J. M. The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan plateau. Anim. Conserv. 2, 235–240 (1999). [Google Scholar]
- Delibes-Mateos M., Smith A. T., Slobodchikoff C. N. & Swenson J. E. The paradox of keystone species persecuted as pests: A call for the conservation of abundant small mammals in their native range. Biol. Conserv. 144, 1335–1346 (2011). [Google Scholar]
- Wilson M. C. & Smith A. T. The pika and the watershed: The impact of small mammal poisoning on the ecohydrology of the Qinghai-Tibetan Plateau. AMBIO 44, 16–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz O. J. Predator diversity and trophic interactions. Ecology 88, 2415–2426 (2007). [DOI] [PubMed] [Google Scholar]
- Bu H. Niche relationships and exploration on coexistence mechanisms of sympatric mesocarnivores in Minshan Mountains, Southwest China Ph.D. thesis, Peking University (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.