Abstract
Objective
To describe SLE disease activity patterns in the Hopkins Lupus Cohort.
Methods
Disease activity was studied in 1886 patients followed-up for 1–28 years. Disease activity patterns were defined using (1) Physician Global Assessment (PGA) and (2) modified SLE Disease Activity Index (M-SLEDAI) as follows: long quiescent (LQ), M-SLEDAI=0/PGA=0 at all visits; relapsing-remitting (RR), periods of activity (M-SLEDAI>0/PGA>0) interspersed with inactivity (M-SLEDAI=0/PGA=0); chronic active (CA), M-SLEDAI>0/PGA>0 at all visits. The pattern of first 3 consecutive follow-up years was determined in 916 patients as: persistent LQ (pLQ), persistent RR (pRR) and persistent CA (pCA), LQ, RR and CA pattern in each of the 3 years, respectively; mixed, at least two different pattern types were identified.
Results
The RR pattern accounted for the greatest proportion of follow-up time both by M-SLEDAI and PGA, representing 53.8% and 49.9% of total patient-years, respectively. The second most frequent pattern was LQ based on M-SLEDAI (30.7%) and CA based on PGA (40.4%). For the first 3-year intervals, the mixed pattern type was the most common (56.6%). The pRR was the second most frequent (M-SLEDAI 33.3%, PGA 26.5%), while pLQ (M-SLEDAI 6.4%, PGA 0.7%) and pCA were less frequent (M-SLEDAI 3.7%, PGA 16.3%).
Conclusions
The RR pattern was the most prevalent pattern. LQ was achieved in a subset of patients, using the M-SLEDAI. However, the PGA captured mild activity missed on the M-SLEDAI in these patients. Over a 3-year perspective, less than half of patients maintained their original pattern.
Keywords: Disease Activity, Systemic Lupus Erythematosus, Disease Activity Patterns
Introduction
SLE is characterised by an extreme variability of disease expression, both between individuals and within individuals, over time. Persistent disease activity over time is one of the major causes of both morbidity and mortality in patients with SLE, being associated with organ damage as well as higher use of corticosteroids and immunosuppressive drugs.1–3 It has been suggested that the inclination for flare or remission in the initial years of disease is predictive of long-term outcome, with those achieving remission earlier having a more favourable disease course.4–6
Barr et al described three major patterns of SLE disease activity over time, as defined by the Physician Global Assessment (PGA) and the modified SLE Disease Activity Index—modified to remove serology (M-SLEDAI). The prospective study of 204 consecutive patients with SLE from the Hopkins Lupus Cohort reported that the chronically active (CA) disease activity pattern was the most frequent pattern observed, followed by the second most common relapsing-remitting (RR) pattern type. The long quiescent (LQ) pattern, which indicated disease that had remained clinically silent for at least 1 year, accounted for the smallest proportion of follow-up time.7
The objective of the present study was to discern and describe SLE disease activity patterns over time by analysing 28 years of accumulated data from the Hopkins Lupus Cohort.
Methods
Patient population
Demographical, clinical and laboratory data were collected and analysed for 2386 patients enrolled in the Hopkins Lupus Cohort from 1987 to 2014. The cohort included all patients at the Hopkins Lupus Center who had a clinical diagnosis of SLE. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. All patients gave informed consent. Demographical data included age, sex, race, disease duration, socioeconomic status at baseline, which was defined as the time of inclusion in the registry. Data on M-SLEDAI,8 Lupus Activity Index9 and PGA were prospectively collected every 3 months and at additional visits, which occurred when clinically indicated. Laboratory data included information on complete blood count, antinuclear antibodies, antidouble-stranded DNA (anti-dsDNA) antibodies and complement levels. Patients with at least 1 year of regular follow-up (≥3 annual visits) were included in the present analysis. All patients met the American College of Rheumatology10 or Systemic Lupus International Collaborating Clinics11 classification criteria for SLE.
Disease activity measures
In this study, disease activity was defined in two ways by two different instruments which were completed at each visit by the senior author. The PGA, reflecting the clinician's judgement of the disease activity, was scored, prior to reviewing the complement and anti-DNA antibody results, on a 3 cm visual analogue scale, with anchors at 0 for no disease activity, 1 mild, 2 moderate and 3 severe active disease.12 The M-SLEDAI, a validated and reliable instrument for disease activity omitting the serology items (complement levels, anti-dsDNA antibodies), was used in the present analysis in order to allow comparison with PGA.
M-SLEDAI was scored, based on the presence or absence of disease manifestations during the 10 days prior to the visit.
Disease activity patterns
For each year of a patient's follow-up the disease activity pattern was classified into one of three different patterns based on those identified in the study by Barr et al,7 namely:
LQ: The LQ pattern indicates disease that has remained quiescent for at least 1 year. This minimum duration was selected to be consistent with previous criteria for remission. The definition for LQ is fulfilled if M-SLEDAI=0/PGA=0 at all visits.
Chronic active (CA): The CA pattern is characterised by disease that was constantly active for at least 1 year. The CA pattern was represented by 1-year interval when M-SLEDAI>0/PGA >0 at each visit.
RR: The RR pattern describes disease where periods of disease activity (M-SLEDAI>0/PGA>0) interspersed with periods of disease inactivity (M-SLEDAI=0/PGA=0) on one or more visits during a 1-year period.
New pattern subgroups were defined based on the major patterns seen for each patient in succession.
Persistent LQ (pLQ): The pLQ indicates that LQ pattern is present in three consecutive follow-up years.
Persistent CA (pCA): The pCA pattern is described with three consecutive CA years. (Even a mild disease activity present at each patient-visit over a 3-year interval was sufficient to maintain pCA.)
Persistent relapsing-remitting (pRR): The pRR is characterised by three consecutive RR years. At least one flare per year, and three flares per 3 years were required to meet this definition.
Mixed: The mixed group indicates at least two different pattern types (CA, RR or LQ) during a 3-year follow-up interval.
Disease activity at yearly intervals (first year) for each patient and at overall patient-years was readily classified into one of the three major pattern groups as defined above (see online supplementary figure S1). At a next step, 3-year period of three consecutive follow-up years were formed and new subgroups were defined based on the original patterns seen for each patient in succession. The 3-year period pattern for each patient was determined, by classifying each 3-year period into one of the four subgroups as defined above (see online supplementary figure S1). Patients included in this analysis were those with ≥3 years of follow-up. The frequency of the different pattern groups (LQ, RR, CA) in first year of follow-up for each patient and overall patient-years and pattern subgroups (pLQ, pRR, pCA, mixed) in each 3-year period were studied.
lupus-2016-000192supp_figure.pdf (115.5KB, pdf)
Consecutive 1-year and 3-year periods of each patient were studied sequentially over the entire follow-up time, providing information about changes in pattern distribution, over time.
Statistical analysis
The entire follow-up time of all patients was analysed as patients-years. Disease activity patterns of overall patient-years and absolute number of patterns at first and the first three consecutive years (3-year period) were classified according to the above pattern definitions and were expressed as frequencies. Spearman's correlation was applied to estimate the percentage of agreement between SLEDAI and PGA. Appropriate parametric statistical tests were used for the analysis of data. All continuous data were examined for normal distribution by skewness. Normally distributed variables are expressed as mean with SD. Numerical variables with no normal distribution such age, disease duration or length of education, are presented as median values with range at baseline (=date of study entry). Categorical variables are expressed as frequencies. All statistical analyses were performed with IBM SPSS Statistics V.22.
Results
A total of 1886 patients were identified and contributed a total of 10 792 patient-years of follow-up. As expected, there was a marked female predominance in the study population (92.5%) and a large proportion of African-American patients (38.5%). Disease duration was very diverse, ranging between 0 and 48 years (median 2 years). The most frequent clinical disease manifestations were arthritis, alopecia, photosensitivity and mucosal ulcers. Hydroxychloroquine was the most commonly prescribed medication in the cohort. Almost 87% of patients were treated with corticosteroids at some time during follow-up. The overall baseline demographic and clinical characteristics of the patients are summarised in tables 1 and 2.
Table 1.
Baseline (=time at cohort entry) demographics and disease characteristics of patients included in the SLE cohort
| Demographics and other characteristics | N patients with available data | |
|---|---|---|
| Age at baseline, years | 35 (10–89) | 1884 |
| Disease duration, years | 2 (0–48) | 1884 |
| SLEDAI at baseline | 2 (0–31) | 1886 |
| M-SLEDAI at baseline | 1 (0–27) | 1886 |
| PGA at baseline | 0.5 (0–3) | 1882 |
| Female sex, n (%) | 1744 (92.5) | 1886 |
| Race | ||
| African-American, n (%) | 727 (38.5) | 1886 |
| Caucasian, n (%) | 1019 (54) | 1886 |
| Other, n (%) | 140 (7.4) | 1886 |
| Education, years | 14 (0–28) | 1847 |
| Smoking at baseline | 285 (15.2) | 1886 |
M-SLEDAI, modified SLE Disease Activity Index; PGA, Physician Global Assessment; SLEDAI, SLE Disease Activity Index.
Table 2.
Clinical manifestations presented at least one time during the observational period
| Clinical manifestations (ever) | n (%) | N available data |
|---|---|---|
| Lupus-specific fever | 706 (37.5) | 1884 |
| Lymphadenopathy | 610 (32.4) | 1881 |
| Malar rash | 961 (51) | 1884 |
| Discoid rash | 384 (20.4) | 1881 |
| Subacute cutaneous lupus | 103 (5.5) | 1882 |
| Livedo reticularis | 528 (28.1) | 1881 |
| Photosensitivity | 1006 (53.4) | 1884 |
| Mucosal ulcers | 978 (51.9) | 1883 |
| Alopecia | 1044 (55.5) | 1882 |
| Arthritis | 1382 (73.5) | 1880 |
| Vasculitis cutaneous | 282 (15) | 1883 |
| Pleurisy | 854 (45.4) | 1882 |
| Pericarditis | 443 (23.6) | 1879 |
| Seizures | 187 (9.9) | 1885 |
| Psychosis | 64 (3.4) | 1885 |
| Nephrotic syndrome | 350 (18.8) | 1860 |
| Renal insufficiency* | 385 (20.4) | 1883 |
| Renal failure† | 144 (7.7) | 1881 |
| Raynaud's disease | 982 (52.2) | 1881 |
*Renal insufficiency defined as creatinine ≥1.5 mg/dL or <75% function due to lupus.
†Renal failure defined as requiring dialysis or transplant.
Patterns at overall patient-years
Table 3 summarises the proportion of follow-up time spent in each of the three disease activity patterns. In the present study, the RR pattern accounted for the greatest proportion of follow-up time both as measured by PGA and M-SLEDAI, representing 49.9% and 53.8% of total person-years, respectively. The second most frequent pattern was CA using PGA (40.4%) and LQ (30.7%) using M-SLEDAI (table 3).
Table 3.
Disease activity patterns at overall patient-years
| RR n (%) |
LQ n (%) |
CA n (%) |
|
|---|---|---|---|
| M-SLEDAI | 5805 53.8% |
3315 30.7% |
1672 15.5% |
| PGA | 5387 49.9% |
1038 9.6% |
4365 40.4% |
CA, chronic active; LQ, long quiescent; M-SLEDAI, modified SLE Disease Activity Index; PGA, Physician Global Assessment; RR, relapsing-remitting.
Patterns at first year and first 3 years
A total of 1886 patients had available first year data and were classified to patterns of disease activity. Table 4 shows the number of patients in each pattern by M-SLEDAI and PGA. RR was the most common pattern. A total of 916 patients had a first 3-year block. The mixed pattern, indicating at least two different pattern types, was the most common both by M-SLEDAI and PGA, representing almost 57% of total 3-year period (table 4). The aforementioned major patterns of disease activity, defined within 1-year period, often change over time, as more than half of the patients switched their pattern within a 3-year long observation period as well as within the year intervals (pRR 33.3% based on M-SLEDAI; 26.5% based on PGA). The pLQ and pCA patterns were less frequent patterns. According to M-SLEDAI, the pCA was the least frequent pattern (3.7%), while a small subgroup maintained long quiescence for 3 years (pLQ 6.4%). On the contrary, the least frequent pattern based on PGA was the pLQ (0.7%), whereas around 16% presented chronic activity over the first 3 years (table 4).
Table 4.
Frequency of disease activity patterns during the first year of observation and during the first three consecutive years (3-year block)
| First year block (N=1886) |
||||
|---|---|---|---|---|
| RR n (%) |
LQ n (%) |
CA n (%) |
Median (min-max) n (%) |
|
| M-SLEDAI | 1244 66% |
367 19.5% |
275 14.6% |
|
| PGA | 1069 56.7% |
104 5.5% |
712 37.8% |
|
| First 3-year block (N=916) | ||||
| pRR n (%) |
pLQ n (%) |
pCA n (%) |
Mixed n (%) |
|
| M-SLEDAI | 305 33.3% |
59 6.4% |
34 3.7% |
518 56.6% |
| PGA | 243 26.5% |
6 0.7% |
149 16.3% |
518 56.6% |
CA, chronic active; LQ, long quiescent; M-SLEDAI, modified SLE Disease Activity Index; pCA, persistent chronic active; PGA, Physician Global Assessment; pLQ, persistent long quiescent; pRR, persistent relapsing-remitting; RR, relapsing-remitting.
Pattern switch
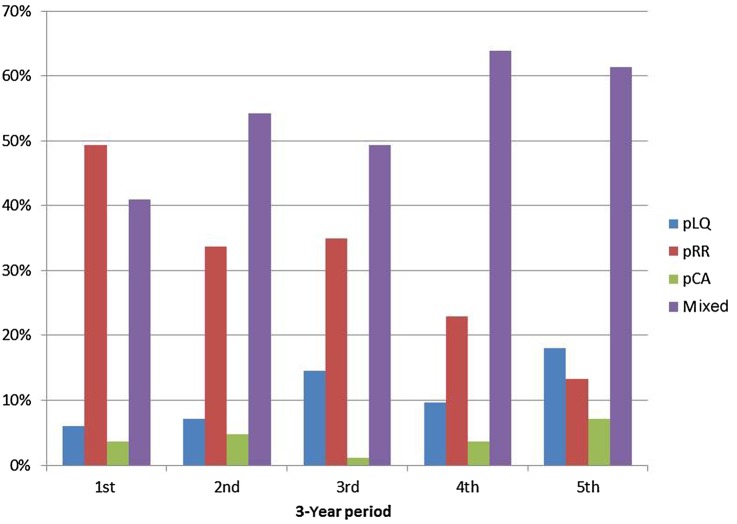
Disease activity was frequently observed to switch from one pattern to another during follow-up. The most common transition type was between the CA and RR patterns. Over a 3-year perspective, almost 45% of the patients maintained their disease activity pattern according to both the M-SLEDAI and PGA. Of the 518 patients in the mixed pattern according to M-SLEDAI, 42.3% improved (most moved from RR to LQ, 30.7%, a smaller percentage of 9.7% moved from CA to RR and 1.9% moved from CA to LQ) while 26.2% deteriorated (12.5% moved from LQ to RR and 12.9% moved from RR to CA); 31.5% of patients returned at third year of follow-up to the former pattern of the first year (table 5). When examining the frequency of patterns over 15 consecutive years for a subset of patients (N=94), it became even more obvious that around half of patients switch between patterns (figure 1). The frequency of LQ exhibits an increasing trend while the frequency of CA pattern is quite stable (figure 1).
Table 5.
Types of pattern switch within the mixed pattern group based on M-SLEDAI for the first three consecutive years
| Improvement | % (n) | Deterioration | % (n) |
|---|---|---|---|
| RR→LQ | 30.7% (159) | LQ→RR | 12.5 (65) |
| CA→LQ | 1.9% (10) | LQ→CA | 0.8% (4) |
| CA→RR | 9.7% (50) | RR→CA | 12.9% (67) |
| Total | 42.3% | Total | 26.2% |
CA, chronic active; LQ, long quiescent; RR, relapsing-remitting; M-SLEDAI, modified SLE Disease Activity Index.
Figure 1.
Frequency of different patterns over 15 consecutive years by examining ‘3-year period’ according to SLE Disease Activity Index, starting the illustration with the first 3 years and ending it with the last 3 years of follow-up. pLQ; persistent long quiescent; pRR; persistent relapsing-remitting; pCA, persistent chronic active.
Agreement between instruments
The overall agreement between the two instruments was very good at first and third year of follow-up (r=0.59 for both). The most common discrepancy between instruments was that the PGA demonstrated CA while the M-SLEDAI indicated LQ patterns. The M-SLEDAI was more likely to depict the LQ pattern than was the PGA. The results suggest that considerably more patient-years can be classified into the LQ and the pLQ pattern groups according to M-SLEDAI, compared with PGA measures.
Discussion
The fluctuating nature of disease activity over time in SLE has been recognised for many years. Recently, the concept of remission gained great interest when evaluating disease activity and treat-to-target strategies in several autoimmune rheumatic diseases. Unfortunately, the definition of remission in SLE still remains elusive due to the heterogeneity of the disease and the variety of available activity scores.13–15 Cohort studies have shown that long-term durable remission, defined either as complete or as partial (clinical remission with serologic activity), is a rare occurrence reported in <10% of patients.6 7 16–21 However, it has to be pointed out that inconsistent definitions of clinically quiescent disease and length of follow-up could have contributed to the large variability of results among studies assessing disease activity patterns and their effects on disease outcome.2 6 13 20 22 23
We based the present study on the observations of Barr et al,7 who first published results on 204 patients from the Hopkins Lupus Cohort, describing three major patterns of disease activity over time, as defined by the PGA and M-SLEDAI. The study observed disease activity within 1-year interval and evaluated the proportion of total follow-up time represented by each pattern. They reported that the CA pattern was the most prevalent pattern for both the PGA and M-SLEDAI (58% and 40%, respectively), followed by the RR type (PGA 26%, M-SLEDAI 35%), while the most favourable long quiescence disease course was the least commonly achieved (PGA 16%, M-SLEDAI 25%). Average disease activity during RR periods tended to be mild, while that during CA periods was more likely to be moderately severe.7
Steiman et al6 reported that 2.4% of 1616 patients of the Toronto Cohort achieved a 5-year long prolonged remission with a mean duration of 11.5 years, while taking no medications. Another 2.1% of the study population exhibited prolonged remission with the help of contemporary steroids and/or immunosuppressive therapy. Seventy-one per cent had RR disease, 28.9% had monophasic illness and none had CA disease prior to remission. It is worth mentioning that in the above study 5 years of remission were required and the authors used SLEDAI to define remission.
Zen et al15 defined prolonged remission in lupus using SLEDAI-2000 by 5 years of continuous disease quiescence, and published observations on an Italian cohort of 224 Caucasian patients with SLE. During the 5-year follow-up interval, 7.1% of the patients achieved prolonged clinical remission with no serologic disease activity, while 14.7% exhibited prolonged clinical remission with serologically active disease. A further 15.6% of the patients were able to fulfil the criteria of prolonged clinical remission, but only with the assistance of concomitant corticosteroid treatment. They also reported that prolonged remission was associated with a better outcome in terms of damage accrual.
In this study, the three major patterns of SLE disease activity as originally identified by Barr et al were confirmed. Disease activity followed longitudinally, using either the PGA or the M-SLEDAI, was readily classified into one of three patterns: CA, RR and LQ as described through this study. The RR was the most prevalent pattern, and accounted for the greatest proportion of follow-up time. The CA pattern was the second most frequent pattern observed using the PGA score. Complete long quiescence was achieved in a subset of patient-years by PGA (9.6%), which however accounted for the smallest proportion of the observation time. The frequency of pLQ pattern was remarkably lower at first 3-year period compared with the first year, indicating that the maintenance of complete remission for a 3-year long interval was relatively rare. However, due to our strict criteria for remission, defined at both 1-patient-year and 3-patient-year intervals (each patient-visit had to display PGA/M-SLEDAI=0 over the given observation period), the fulfilment of the long quiescence disease pattern was a rare outcome.
The relatively high frequency of the mixed pattern at first 3-year period suggests that the major patterns of disease activity, defined within 1-year period, are most often not stable over time. Over a 3-year perspective, more than half the patients switched disease activity pattern at least once, while only 45% of the patients maintained their pattern within a 3-year long follow-up period. The most prevalent transition type was a switch from RR to LQ pattern (30.7%). The second most common pattern-switch was from RR to CA (12.9%) and from LQ to RR (12.5%).
It has been suggested that fatigue, due to its subjective nature, may be a confounding factor for the correct evaluation of disease activity and therefore should be omitted when defining remission in SLE. In this study, fatigue occurred mostly in conjunction with other objective symptoms, scored by the M-SLEDAI. Interestingly, only 2.3% of all patient-visits exhibited lupus-related fatigue without any other symptoms or signs of disease activity based on both the SLEDAI and PGA. However, this observation should be interpreted with caution, since fatigue was only scored when it was defined as SLE-related (ie, responsive to steroids).
In the present study, the overall agreement between the two instruments was very good at baseline. However, a marked discrepancy was observed between the PGA and M-SLEDAI regarding the identification of the LQ pattern. This is expected as the SLEDAI can miss mild degrees of lupus activity. M-SLEDAI might have missed mild disease activity at the scoring of certain organ manifestations (such as arthralgia, thrombocytopenia >100 000 platelets/mm3, haemolytic anaemia, etc), which could lead to the overestimation of the frequency of LQ patterns. The results suggest that it was easier to achieve remission defined by the M-SLEDAI compared with the PGA. The M-SLEDAI was more likely to depict the LQ, whereas the PGA was more likely to demonstrate the CA pattern.
One of the strengths of this observational study is the large size of the cohort, the fairly long follow-up period of the participants, the tight and regular follow-up visits and the homogeneity of the patient group. There are however limitations that have to be addressed. The definitions for the patterns were arbitrary and may not be universally applicable. Nevertheless, these definitions were reliable and easy to use as conceived by all within our group. The strict criteria for defining long quiescence and chronically active disease may have resulted in more patient-years being defined as RR at 1-year period, and as mixed at 3-year interval. With this approach, clear patterns have been identified. Another potential limitation is that the patients from Baltimore with an African-American and Caucasian predominance may not represent patients with SLE in other geographic areas. The relative frequency of the patterns may have also been influenced by the time interval between scheduled patient-visits. For instance, weekly or monthly visits might yield different disease activity patterns. Patients with fluctuating disease activity may skip a visit at the clinic when they are feeling well, which could lead to the underestimation of the frequency of LQ and RR and the overestimation of CA. Finally, we acknowledge that the classification and evaluation of SLE disease activity were performed irrespectively of the individual disease manifestations, organ involvements and treatment strategies. This descriptive analysis was able to capture the considerable burden of global SLE disease activity, in terms of persistence over time. These results have clinical relevance and may be applicable in optimising treatment strategies with the principle of ‘treat to target’ in patients with SLE.
In summary, three major patterns of SLE disease activity were identified: RR, CA and LQ. Despite discrepancies between the PGA and the M-SLEDAI, both instruments identified the RR pattern as the most prevalent pattern type. pLQ disease course was achieved only in a small subset of patients. Over a 3-year observation period, almost half the patients maintained their disease activity pattern. A transition to a more favourable pattern occurred more frequently than a transition to a less favourable pattern.
Footnotes
Contributors: MP acquired the data in the cohort. MP, RFvV, NG, IG, LM and KC contributed to study design, data analysis, interpretation of data and preparation of the manuscript. All authors approved the final version of the manuscript.
Funding: The Hopkins Lupus Cohort is supported by National Institutes of Health AR 43727.
Competing interests: None declared.
Ethics approval: Johns Hopkins University School of Medicine Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Nossent J, Kiss E, Rozman B et al. . Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus 2010;19:949–56. doi:10.1177/0961203310366572 [DOI] [PubMed] [Google Scholar]
- 2.Zen M, Bassi N, Nalotto L et al. . Disease activity patterns in a monocentric cohort of SLE patients: a seven-year follow-up study. Clin Exp Rheumatol 2012;30:856–63. [PubMed] [Google Scholar]
- 3.Nossent JC. Course and prognostic value of systemic lupus erythematosus disease activity index in black Caribbean patients. Semin Arthritis Rheu 1993;23:16–21. doi:10.1016/S0049-0172(05)80023-X [DOI] [PubMed] [Google Scholar]
- 4.Swaak AJ, Nossent JC, Bronsveld W et al. . Systemic lupus erythematosus. II. Observations on the occurrence of exacerbations in the disease course: Dutch experience with 110 patients studied prospectively. Ann Rheum Dis 1989;48:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drenkard C, Villa AR, Garcia-Padilla C et al. . Remission of systematic lupus erythematosus. Medicine (Baltimore) 1996;75:88–98. doi:10.1097/00005792-199603000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Steiman AJ, Urowitz MB, Ibañez D et al. . Prolonged clinical remission in patients with systemic lupus erythematosus. J Rheumatol 2014;41:1808–16. doi:10.3899/jrheum.131137 [DOI] [PubMed] [Google Scholar]
- 7.Barr SG, Zonana-Nacach A, Magder LS et al. . Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum 1999;42:2682–8. doi:10.1002/1529-0131(199912)42:12<2682::AID-ANR26>3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- 8.Petri M, Kim MY, Kalunian KC et al. . Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. doi:10.1056/NEJMoa051135 [DOI] [PubMed] [Google Scholar]
- 9.Petri M, Hellmann D, Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J Rheumatol 1992;19:53–9. [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF et al. . The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. doi:10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Orbai AM, Alarcón GS et al. . Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. doi:10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam GK, Petri M. Assessment of systemic lupus erythematosus. Clin Exp Rheumatol 2005;23(Suppl 39):S120–32. [PubMed] [Google Scholar]
- 13.Doria A, Gatto M, Zen M et al. . Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmun Rev 2014;13:770–7. doi:10.1016/j.autrev.2014.01.055 [DOI] [PubMed] [Google Scholar]
- 14.Doria A, Gatto M, Iaccarino L et al. . Value and goals of treat-to-target in systemic lupus erythematosus: knowledge and foresight. Lupus 2015;24:507–15. doi:10.1177/0961203314559087 [DOI] [PubMed] [Google Scholar]
- 15.Zen M, Iaccarino L, Gatto M et al. . Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 2015;74:2117–22. doi:10.1136/annrheumdis-2015-207347 [DOI] [PubMed] [Google Scholar]
- 16.Heller CA, Schur PH. Serological and clinical remission in systemic lupus erythematosus. J Rheumatol 1985;12:916–8. [PubMed] [Google Scholar]
- 17.Tozman EC, Urowitz MB, Gladman DD. Prolonged complete remission in previously severe SLE. Ann Rheum Dis 1982;41:39–40. doi:10.1136/ard.41.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urowitz MB, Feletar M, Bruce IN et al. . Prolonged remission in systemic lupus erythematosus. J Rheumatol 2005;32:1467–72. [PubMed] [Google Scholar]
- 19.Steiman AJ, Gladman DD, Ibañez D et al. . Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol 2010;37:1822–7. doi:10.3899/jrheum.100007 [DOI] [PubMed] [Google Scholar]
- 20.Nikpour M, Urowitz MB, Ibañez D et al. . Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Rheum 2009;61:1152–8. doi:10.1002/art.24741 [DOI] [PubMed] [Google Scholar]
- 21.Steiman AJ, Gladman DD, Ibañez D et al. . Outcomes in patients with systemic lupus erythematosus with and without a prolonged serologically active clinically quiescent period. Arthritis Care Res (Hoboken) 2012;64:511–18. doi:10.1002/acr.21568 [DOI] [PubMed] [Google Scholar]
- 22.Walz LeBlanc BA, Gladman DD, Urowitz MB. Serologically active clinically quiescent systemic lupus erythematosus-predictors of clinical flares. J Rheumatol 1994;21:2239–41. [PubMed] [Google Scholar]
- 23.Laustrup H, Voss A, Green A et al. . SLE disease patterns in a Danish population-based lupus cohort: an 8-year prospective study. Lupus 2010;19:239–46. doi:10.1177/0961203309351033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2016-000192supp_figure.pdf (115.5KB, pdf)