Abstract
Objectives
To determine the impact of baseline rheumatoid factor (RF) and anticyclic citrullinated peptide (anti-CCP) status on the clinical efficacy of intravenous abatacept in biologic-naïve patients with rheumatoid arthritis (RA) enrolled in the real-world ACTION study.
Methods
Clinical outcomes (European League Against Rheumatism (EULAR) response, mean Clinical Disease Activity Index (CDAI) and Boolean remission) at 6 months were compared by baseline RF and anti-CCP status.
Results
Of 672 biologic-naïve patients, RF status was reported in 577 (86%) (412 (71%) positive) and anti-CCP status in 552 (82%) (364 (66%) positive); of 511 patients for whom data were available, 308/511 (60%) were double positive and 127/511 (25%) were double negative. Clinical outcomes were improved with RF-positive or anti-CCP-positive versus RF-negative/anti-CCP-negative status—good or moderate EULAR response: RF: 84.6 vs 72.9%, p=0.012; anti-CCP: 85.2 vs 74.2%, p=0.015; mean CDAI (calculated): RF: 10.8 vs 15.3, p<0.001; anti-CCP: 10.9 vs 14.3, p=0.002; and Boolean remission: RF: 13.3 vs 4.0%, p=0.008; anti-CCP: 12.5 vs 6.3%, p=0.096. Clinical outcomes were also improved with single or double RF-positive/anti-CCP-positive versus double-negative status.
Conclusions
In biologic-naïve patients with RA, RF-positive and/or anti-CCP-positive status is associated with greater efficacy of intravenous abatacept than seronegative status.
Trial registration number
Keywords: Ant-CCP, Rheumatoid Arthritis, Rheumatoid Factor
Key messages.
What is already known about this subject?
Clinical response to some antitumour necrosis factor agents appears to be unaffected by either rheumatoid factor (RF) or anticyclic citrullinated peptide (CCP) antibody status at baseline.
Seropositivity is associated with improved clinical outcomes with abatacept.
What does this study add?
This study demonstrated that RF and/or anti-CCP seropositivity is associated with improved clinical response to abatacept in biologic-naïve patients with rheumatoid arthritis.
How might this impact on clinical practice?
Testing serostatus at baseline could be used to identify patients most likely to benefit from abatacept treatment.
Introduction
Abatacept, a selective T-cell costimulation modulator, is approved worldwide for the treatment of patients with moderate-to-severe rheumatoid arthritis (RA).1 2 The identification of patients most likely to derive clinical benefit from individual disease-modifying antirheumatic drugs (DMARDs) is an important consideration in therapy selection.
The detection of rheumatoid factor (RF) and/or anticitrullinated protein antibody (ACPA; tested as anticyclic citrullinated peptide (anti-CCP)) forms part of the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for the definitive diagnosis of RA in clinical practice.3 RF and/or ACPA are associated with radiographic progression of joint damage and are poor prognostic factors for RA.4 5 Clinical response to antitumour necrosis factor (anti-TNF) agents appears to be unaffected by either RF or anti-CCP antibody status at baseline, as reflected in a meta-analysis, although some studies show differences in anti-TNF efficacy by serostatus.6 7 In contrast, greater clinical benefit of treatment in seropositive versus seronegative patients has been shown with abatacept6 8 9 and rituximab.10
The ACTION study is a 2-year, non-interventional, international (Europe and Canada) prospective study of patients who initiated intravenous abatacept therapy during routine clinical practice from May 2008 to December 2013.11 A prespecified, 6-month analysis was performed separately on biologic-naïve patients and on those with prior biological failure, stratified by baseline RF and anti-CCP serostatus in order to evaluate the clinical response to treatment with abatacept. Previously published real-world abatacept data included cohorts with prior biological failure;9 11 12 however, no real-world data have been published in biologic-naïve cohorts, which is the focus of the current paper.
Methods
In the ACTION study,11 patients were enrolled in three distinct periods (Cohorts A, B and C), either prospectively at initiation of intravenous abatacept, or retrospectively within 3 months following the first administration of intravenous abatacept, according to the local requirements for non-registrational studies (see online supplementary figure S1).
This analysis included only biologic-naïve patients from Cohorts A and B for whom baseline RF and anti-CCP data were available. All patients were aged ≥18 years and had an established diagnosis of moderate-to-severe RA according to the 1987 ACR Revised Classification Criteria.13
Comparisons of baseline demographics and patient characteristics by RF and anti-CCP status (separately and combined) between Cohorts A and B were conducted using the χ2 test or Fisher's exact test for small patient numbers.
At 6 months, EULAR response criteria based on Disease Activity Score in 28 joints (DAS28; erythrocyte sedimentation rate (ESR), otherwise C reactive protein (CRP)), were compared ‘as observed’ across baseline serostatus groups. In order to adjust the comparison for baseline characteristics, we performed a multivariate logistic model to assess the impact of RF/anti-CCP double seropositivity status on EULAR response (defined as binary outcome: good/moderate vs no response), adjusting for factors identified as clinically relevant and significantly different at baseline.
Finally, several other clinical outcomes were also compared ‘as observed’ across serostatus groups. These included Clinical Disease Activity Index (CDAI), Boolean remission rates and Health Assessment Questionnaire-Disability Index (HAQ-DI). Unadjusted comparisons were conducted using analysis of variance on ranks for quantitative variables and Fisher's exact test for qualitative variables.
Results
Patients in Cohorts A and B had comparable baseline characteristics and were subsequently pooled. In total, 672/2359 enrolled patients were biologic naïve (122 and 550 in Cohorts A and B, respectively), and data for these patients will be presented here. In biologic-naïve patients, RF status was reported in 577/672 (86%) patients and 412/577 (71%) were RF positive; anti-CCP antibody status was reported in 552/672 (82%) patients and 364/552 (66%) were anti-CCP positive. Of the biologic-naïve patients for whom both baseline RF and anti-CCP data were available, 308/511 (60%) were both RF and anti-CCP positive and 127/511 (25%) were both RF and anti-CCP negative; 50/511 (10%) were single RF positive only and 26/511 (5%) were single anti-CCP positive only.
At baseline, biologic-naïve patients who were RF or anti-CCP positive versus negative and biologic-naïve patients who were double positive versus double negative, had significantly longer mean disease duration, significantly more erosive disease and were significantly more likely to be receiving concomitant corticosteroids (table 1). Biologic-naïve patients who were RF positive versus negative were significantly less likely to have specific comorbidities (metabolism and nutrition disorders, and nervous system disorders). Biologic-naïve patients who were RF and anti-CCP double positive versus double negative had a significantly lower body mass index (BMI).
Table 1.
Baseline patient demographics and disease characteristics by RF and anti-CCP serostatus
| Characteristic | RF positive (n=412) | RF negative (n=165) | p Value positive vs negative | Anti-CCP positive (n=364) | Anti-CCP negative (n=188) | p Value positive vs negative | RF and anti-CCP double positive (n=308) |
RF and anti-CCP double negative (n=127) | p Value double positive vs double negative |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 59.1 (12.7) | 61.1 (12.8) | 0.099 | 59.8 (12.4) | 59.7 (13.3) | 0.829 | 59.2 (12.6) | 60.8 (13.1) | 0.270 |
| BMI, kg/m2 | 26.6 (5.3) | 27.7 (5.6) | 0.016 | 26.8 (5.6) | 27.5 (5.3) | 0.057 | 26.3 (5.3) | 27.3 (5.3) | 0.042 |
| RA duration, years | 7.8 (8.5) | 5.5 (7.2) | <0.001 | 7.7 (8.5) | 5.4 (6.1) | 0.005 | 7.9 (8.7) | 5.2 (5.9) | 0.002 |
| CRP, mg/L | 18.6 (28.1) | 12.1 (21.1) | <0.001 | 17.2 (25.7) | 14.5 (23.4) | 0.002 | 17.7 (26.5) | 11.9 (21.5) | <0.001 |
| ESR, mm/hour | 35.7 (24.3) | 26.9 (21.6) | <0.001 | 34.8 (23.6) | 28.3 (22.1) | 0.001 | 35.3 (24.0) | 25.9 (21.7) | <0.001 |
| DAS28 (CRP) (derived) | 4.8 (1.0) | 4.8 (1.1) | 0.978 | 4.7 (1.0) | 4.9 (1.1) | 0.113 | 4.7 (1.0) | 4.8 (1.1) | 0.475 |
| CDAI | 26.20 (12.82) | 28.22 (14.73) | 0.240 | 27.43 (13.45) | 28.10 (14.27) | 0.818 | 27.45 (13.49) | 30.10 (15.47) | 0.351 |
| HAQ-DI | 1.4 (0.7) | 1.4 (0.7) | 0.463 | 1.4 (0.7) | 1.4 (0.7) | 0.880 | 1.4 (0.7) | 1.4 (0.6) | 0.874 |
| Radiographic erosion, % | 62.8 | 46.5 | 0.001 | 61.4 | 50.3 | 0.019 | 62.5 | 47.5 | 0.006 |
| Concurrent non-biological DMARDs, % | 0.183 | ||||||||
| MTX only | 68.9 | 59.9 | 69.4 | 62.1 | 70.5 | 61.9 | |||
| MTX+other non-biological DMARDs | 11.7 | 17.5 | 0.117 | 11.3 | 15.0 | 0.267 | 9.4 | 15.2 | 0.183 |
| Other non-biological DMARDs | 19.4 | 22.6 | 19.3 | 22.9 | 20.1 | 22.9 | |||
| Concurrent corticosteroid, % | 71.8 | 62.4 | 0.029 | 73.1 | 59.6 | 0.001 | 72.1 | 59.8 | 0.017 |
| Mean dose, mg/day | 7.55 (7.10) | 8.00 (8.15) | 0.895 | 7.31 (4.26) | 8.23 (8.13) | 0.708 | 7.32 (4.31) | 8.57 (9.35) | 0.786 |
| Tobacco use, % | 17.7 | 9.1 | 0.01 | 15.9 | 12.8 | 0.377 | 16.2 | 9.4 | 0.071 |
| Comorbidities, % | |||||||||
| Metabolism/nutrition disorders | 26.2 | 35.2 | 0.042 | 29.1 | 33.5 | 0.330 | 27.6 | 36.2 | 0.085 |
| Nervous system disorders | 1.2 | 4.8 | 0.013 | 3.0 | 2.7 | 1.0 | 1.6 | 3.1 | 0.294 |
Data are mean (SD) unless otherwise specified.
Numbers in bold indicate differences of statistical significance.
BMI, body mass index; CCP, cyclic citrullinated peptide; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor.
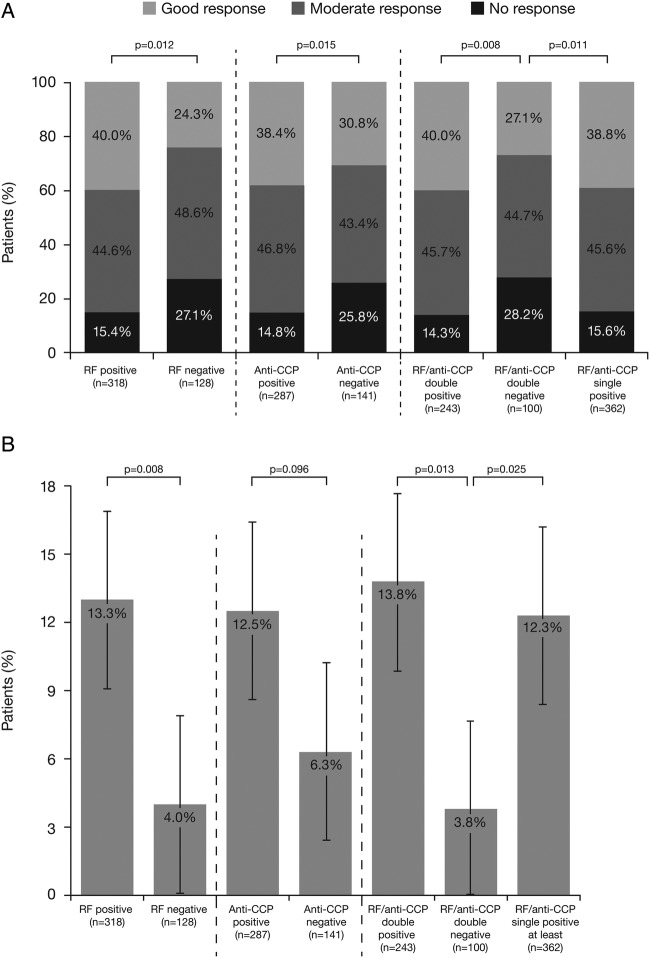
At 6 months, in biologic-naïve patients, the following subgroups were compared: patients who were single RF or anti-CCP positive (n=318 and n=287, respectively) versus single negative (n=128 and n=141, respectively); patients who were at least single positive (RF and/or anti-CCP; n=362) versus double negative (n=100); patients who were single RF positive only (n=34) and single anti-CCP positive only (n=20) versus double negative (n=100), or patients who were double positive (n=243) versus double negative (n=100). A good or moderate EULAR response was observed in a significantly greater proportion of RF-positive versus RF-negative patients (84.6 vs 72.9%; p=0.012), in a significantly greater proportion of anti-CCP-positive versus anti-CCP-negative patients (85.2 vs 74.2%; p=0.015) and in a significantly greater proportion of at least single or double RF-positive/anti-CCP-positive patients versus double RF-negative/anti-CCP-negative patients (84.4 and 85.7 vs 71.8%; p=0.011 and p=0.008, respectively; figure 1A). The significant difference between double RF-postive/anti-CCP-positive and double RF-negative/anti-CCP-negative groups was maintained following adjustment for baseline characteristics (OR (95% CI) 2.50 (1.24 to 5.02); p=0.010). The following significant variables were introduced into the model: disease duration, BMI, coprescribed corticosteroids, CRP, ESR, radiographic erosion and tobacco use (table 1).
Figure 1.
(A) EULAR response based on DAS28 (ESR, otherwise CRP) and (B) Boolean remission, at 6 months in patients treated with abatacept as a first-line biologic by RF or anti-CCP seropositivity* alone or combined. p Value for likelihood of a good/moderate EULAR response versus no response based on DAS28 (ESR, otherwise CRP). Error bars represent 95% CI. *Derived DAS28 based on core components. CCP, cyclic citrullinated peptide; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; RF, rheumatoid factor.
Good EULAR response rates were numerically higher in seropositive versus seronegative patients (40.0 vs 24.3% (single RF-positive vs single RF-negative); 38.4 vs 30.8% (single anti-CCP-positive vs single anti-CCP-negative); 39.9 vs 27.1% (RF/anti-CCP double positive vs double negative); and 38.8 vs 27.1% (single RF/anti-CCP positive vs double negative)).
No significant differences in the proportion of patients with good or moderate EULAR response were found between single RF-positive/anti-CCP-negative or single anti-CCP-positive/RF-negative versus double-negative biologic-naïve patients (p=0.325 and p=0.389, respectively). Consistently, single RF-positive/anti-CCP-negative versus single anti-CCP-positive/RF-negative patients did not show a significant difference in attaining good or moderate EULAR response (p=1.000; see online supplementary figure S2).
Mean CDAI score (calculated) was significantly lower for RF-positive versus RF-negative patients (10.8 vs 15.3; p<0.001), for anti-CCP-positive versus anti-CCP-negative patients (10.9 vs 14.3; p=0.002), and for at least single or double RF/anti-CCP-positive versus double-negative patients (11.1 and 10.5 vs 14.5; p=0.003 and p=0.001, respectively).
Boolean remission rates were significantly higher for RF-positive versus RF-negative patients (13.3 vs 4.0%; p=0.008), numerically higher for anti-CCP-positive versus anti-CCP-negative patients (12.5 vs 6.3%; p=0.096) and significantly higher for at least single or double RF/anti-CCP-positive versus double-negative patients (12.3 and 13.8 vs 3.8%; p=0.025 and p=0.013, respectively; figure 1B).
Improvements in HAQ-DI with abatacept treatment were comparable regardless of baseline serostatus. Mean (SD) HAQ-DI score at 6 months was 0.87 (0.68) versus 1.01 (0.69) for single RF-positive versus single RF-negative patients (p=0.072); 0.88 (0.72) versus 0.98 (0.63) for single anti-CCP-positive versus single anti-CCP-negative patients (p=0.071); and 0.87 (0.69) versus 0.98 (0.59) for RF/anti-CCP double-positive versus double-negative patients (p=0.114).
Discussion
In this 6-month analysis of the ACTION study, abatacept demonstrated clinical efficacy in biologic-naïve patients with RA, irrespective of baseline serostatus. Consistent with previous findings in patients with prior biological failure, a significantly superior clinical efficacy of abatacept was found in patients who were seropositive versus seronegative at baseline, even when stringent remission criteria were employed. Interestingly, this significant difference in the proportion of patients with good or moderate EULAR response was found between those who were RF positive or anti-CCP positive versus negative, and between those who were double positive versus double negative; this result was maintained following adjustment for baseline characteristics. However, when patients who were RF positive/anti-CCP negative or anti-CCP positive/RF negative were compared with patients who were double negative, these differences did not reach significance, probably due to the low number of patients with available data (n=18 in the single anti-CCP-positive group). A trend in favour of single RF-positive or anti-CCP-positive patients versus single-negative patients was observed for the HAQ-DI data only. These comparisons could help to identify the patients most likely to benefit from abatacept therapy.
This analysis demonstrated a positive association between seropositivity and clinical response to abatacept, consistent with the findings from clinical trials (AMPLE6 and AVERT14). Data on the predictive value of RF or anti-CCP seropositivity on patient response to other biological agents are limited. Although seropositivity has similarly been linked with improved clinical response to rituximab,10 a recent meta-analysis failed to identify any association between RF or anti-CCP seropositivity and clinical response to anti-TNFs;7 however, in the AMPLE study, a superior clinical response was found at group level in seropositive versus seronegative patients in the abatacept and adalimumab groups, although a ‘dose–response’ in favour of higher ACPA titres was noticed only in the abatacept group.6 It has been postulated that an association between seropositivity and response to abatacept could be attributable to the specific mode of action of abatacept and its modulation of the interactions between T and B cells. Compared with patients who are seronegative, seropositive patients may represent a more homogeneous patient population, which is more likely to respond to a therapy targeting RA pathophysiology. Patients in whom B cells play a major part in RA disease activity may experience improved efficacy of abatacept through the inhibition of antigen presentation to the T cells by anti-CCP and RF producing B cells.9 Furthermore, animal studies show that the activation of B cells themselves may be reduced through a decrease in differentiation of helper T cells with abatacept treatment.15
This was a prespecified analysis of a prospective observational study and consequently limitations included physician referral and channelling bias, and the lack of an active comparator. Tests were conducted using the local laboratory procedures and an assessment of patient serostatus was made by the physician as per routine clinical practice. The data on the scales and kits used were not collected. In addition, this was a 6-month (interim) analysis only and no RF and anti-CCP titres were available for more detailed analyses to be performed.
In summary, RF-seropositive and/or anti-CCP-seropositive versus RF-seronegative/anti-CCP-seronegative status at baseline was associated with improved clinical efficacy of intravenous abatacept in biologic-naïve patients with RA, irrespective of concurrent prognostic factors of disease progression. Testing patient serostatus at baseline could be used to identify patients most likely to benefit from abatacept treatment in clinical practice.
Acknowledgments
The authors would like to thank all physicians who participated in the ACTION study. The clinical research organisations involved in the ACTION study were: Inventiv Health Clinical, Winicker Norimed, TFS Trial Form Support S.r.l. and Archemin BVBA. Statistical analyses support was provided by Stat Process. Professional medical writing and editorial assistance was provided by Linda Brown at Caudex and was funded by Bristol-Myers Squibb.
Footnotes
Contributors: RA designed the analysis and analysed and interpreted the data. H-GN designed the analysis and acquisition of data, and analysed and interpreted the data. XM collected data, and analysed and interpreted the data. MG designed the analysis and acquisition of data. H-ML designed the analysis and acquisition of data, and analysed and interpreted the data. AC collected data. MC designed the analysis and acquisition of data, and analysed and interpreted the data. CP designed the analysis and acquisition of data, and analysed and interpreted the data. CR designed the analysis and acquisition of data, and analysed and interpreted the data. MLB designed the analysis and acquisition of data, and analysed and interpreted the data.
Funding: This study was sponsored by Bristol-Myers Squibb.
Competing interests: RA has received research grants and consulting fees and is on a speaker bureau for Bristol-Myers Squibb. HGN is a consultant for Bristol-Myers Squibb, Abbott, Chugai, UCB, Essex, Wyeth, Pfizer, MSD, Novartis and Roche and is on speaker bureaus for Bristol-Myers Squibb, Abbott, Chugai, UCB, Essex, Wyeth, Pfizer, MSD, Novartis and Roche. MG has no competing interests to disclose. XM is on speaker bureaus for Bristol-Myers Squibb, GSK, Pfizer and UCB. H-ML is a consultant for AbbVie, Bristol-Myers Squibb, Roche-Chugai, UCB, MSD, GSK, SOBI, Medac, Novartis, Janssen-Cilag, AstraZeneca, Pfizer and Actelion, and is on speaker bureaus for AbbVie, Bristol-Myers Squibb, Roche-Chugai, UCB, MSD, GSK, SOBI, Medac, Novartis, Janssen-Cilag, AstraZeneca, Pfizer and Actelion. AC has received research grants from UCB and Pfizer, and consultant fees from AbbVie, Bristol-Myers Squibb, Lilly, MSD, Novartis, Pfizer and Roche. MC is an employee of Bristol-Myers Squibb. CP is a consultant for Bristol-Myers Squibb. CR is a shareholder and employee of Bristol-Myers Squibb. MLB is a shareholder and employee of Bristol-Myers Squibb.
Ethics approval: The study protocol and patient enrolment were approved by ethics committees and regulatory agencies in accordance with each country's requirements. The central ethics committee that first approved the study on 31 January 2008 was the Munich, Bavaria, Germany, central ethics committee. For each country, local ethics committee approvals were also obtained as required. The ACTION study was conducted in accordance with the Declaration of Helsinki and was consistent with the International Conference on Harmonisation Good Clinical Practice Guideline16 and Good Epidemiological Practice Guidelines.17
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Orencia (250 mg powder for concentrate for solution for infusion) Summary of Product Characteristics. http://www.medicines.org.uk/emc/medicine/19714/SPC/ (accessed 10 Sep 2015).
- 2.Orencia prescribing information. http://packageinserts.bms.com/pi/pi_orencia.pdf (accessed 18 May 2016).
- 3.Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 4.Unger M, Alasti F, Studenic P et al. Are changes in autoantibody levels reflecting change in prognosis of rheumatoid arthritis? Arthritis Rheumatol 2015;67(Suppl 10):1–4046. [Google Scholar]
- 5.van der Helm-van Mil AH, Verpoort KN, Breedveld FC et al. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–58. 10.1186/ar1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolove J, Schiff M, Fleischmann R et al. Impact of baseline anti-cyclic citrullinated peptide 2 antibody titre on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis 2015;74:983–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv Q, Yin Y, Li X et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFα agent treatment in patients with rheumatoid arthritis: a meta-analysis. PLoS ONE 2014;9:e89442 10.1371/journal.pone.0089442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottenberg JE, Ravaud P, Cantagrel A et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis 2012;71:1815–19. 10.1136/annrheumdis-2011-201109 [DOI] [PubMed] [Google Scholar]
- 9.Gottenberg JE, Courvoisier DS, Hernandez MV et al. Brief report: association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: results from the pan-European registry analysis. Ann Rheum Dis 2016;68:1346–52. 10.1002/art.39595 [DOI] [PubMed] [Google Scholar]
- 10.Isaacs JD, Cohen SB, Emery P et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis 2013;72:329–36. 10.1136/annrheumdis-2011-201117 [DOI] [PubMed] [Google Scholar]
- 11.Nüßlein HG, Alten R, Galeazzi M et al. Real-world effectiveness of abatacept for rheumatoid arthritis treatment in European and Canadian populations: a 6-month interim analysis of the 2-year, observational, prospective ACTION study. BMC Musculoskelet Disord 2014;15:14 10.1186/1471-2474-15-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harigai M, Ishiguro N, Inokuma S et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 14.Huizinga TWJ, Connolly SE, Johnsen A et al. Effect of anti-cyclic citrullinated peptide 2 immunoglobulin M serostatus on efficacy outcomes following treatment with abatacept plus methotrexate in the AVERT trial. Ann Rheum Dis 2015;74(Suppl 2):234.24106048 [Google Scholar]
- 15.Platt AM, Gibson VB, Patakas A et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol 2010;185:1558–67. 10.4049/jimmunol.1001311 [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration, Department of Health and Human Services. International Conference on Harmonisation; Good Clinical Practice: Consolidated Guideline; Availability. Federal Register 1997;62:25692–709. [Google Scholar]
- 17.Olsen J. Good Epidemiological Practice (GEP): IEA Guidelines for Proper Conduct in Epidemiological Research. http://ieaweb.org/good-epidemiological-practice-gep/ (accessed 25 May 2016).